Translate this page into:
Anticancer effects of Morinda lucida and Annona muricata on immunomodulations of Melatonin, tumor necrosis factor-alpha and p53 concentrations in lead acetate-induced toxicity in rats
Address for correspondence: A. A. Akinlolu, Department of Anatomy, Faculty of Basic Medical Sciences, Olabisi Onabanjo University, Ogun State, Nigeria. Phone: +2348062765308. E-mail: adelaja.akinlolu.454@my.csun.edu
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Qassim Uninversity and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objectives:
Lead poisoning accounts for about 0.6% of global burden of disease. Lead-induced toxicity is through confinement of oxidative stress in affected organs. We evaluated the effects of MLF1 (extracted from Morinda lucida leaves) and AMF1 (extracted from Annona muricata leaves) on lipid peroxidation and immunomodulations of Melatonin, tumor necrosis factor-alpha (TNF-α), and p53 proteins in lead acetate (LA)-induced toxicity in rats.
Methods:
Sixty adult female rats were randomly divided into 12 groups (n = 5). Groups 1 and 2 received physiological saline and 100 mg/kg bodyweight of LA, respectively, for 5 weeks. Groups 3–6 received 100 mg/kg bodyweight LA for 2 weeks, followed by treatments with 7.5 and 15 mg/kg bodyweight of MLF1, and 7.5 and 10 mg/kg bodyweight of AMF1, respectively, for 3 weeks. Groups 7–10 received 7.5 and 15 mg/kg bodyweight of MLF1, 7.5 and 10 mg/kg bodyweight of AMF1, respectively, for 5 weeks. Groups 11–12 received co-administrations of 100 mg/kg bodyweight LA with 15 mg/kg bodyweight MLF1 and 10 mg/kg bodyweight of AMF1, respectively, for 5 weeks. Drugs and extracts were administered orally. Consequently, liver histopathology (Hematoxylin and Eosin), sera Melatonin, and TNF-α (enzyme-linked immunosorbent assay [ELISA]) levels were evaluated. Malondialdehyde (MDA) (thiobarbituric acid assay) and p53 (ELISA) levels were evaluated in liver homogenates. Data were statistically analyzed (P ≤ 0.05).
Results:
Results showed normal liver histology in all Groups. Statistical analyses showed significant (P ≤ 0.05) and non-significant decreased levels (P ≥ 0.05) of MDA, TNF-α and p53 in Groups 3-12, compared with Group 2. Furthermore, results showed significant (P ≤ 0.05) and non-significant increased Melatonin levels (P ≥ 0.05) in Groups 4–12 compared with Group 2.
Conclusion:
This study confirmed that MLF1 and AMF1 confer a degree of antioxidant, anticancer and hepato-protetive potentials against LA-induced toxicity in rats.
Keywords
Morinda lucida
Annona muricata
Lead
lipid peroxidation
Melatonin
tumor necrosis factor-alpha
p53
Introduction
Lead (Pb) is widely used in industries, and it is present in the environment water, brass plumbing fixtures, paints, soil, and dust.[1-6] Lead poisoning accounts for about 0.6% of global burden of disease and it occurs due to build up in the body over a period of months or years.[1] Lead poisoning is a major health problem in Zamfara state, North West of Nigeria due to exposure through unsafe artisanal gold ore mining and widespread processing activities across the state. A cross-sectional study in Kawaye village of Anka Local Government Area of Zamfara State in April 2013 reported the prevalence of lead poisoning (Blood Lead Level ≥5 μg/dL) among eligible 307 children as 92.5%, confirming the severity of Lead poisoning in the state.[1] Lead-exposure occurs through inhalation, ingestion, and skin contact with the liver as its most common depository, followed by the kidney. Lead-induced hepatotoxicity is mediated via increased hepatic oxidative stress, hepatic lipid peroxidation, and hepatocytes hyperplasia.[2-6] The ubiquitous environmental presence and pro-carcinogenic potentials of lead make the search for possible plants diets or anticancer compounds that may prevent or counteract lead-induced toxicity quite relevant.
Lipid peroxidation involves lipid degradation, stealing of electrons from the lipid cell membrane, and production of mutagenic reactive aldehydes such as malondialdehye.[7] It results in increased oxidative stress, compromised cell membrane, and cellular damage. Hence, increased level of lipid peroxidation is associated with the developments of cancers and inflammatory conditions.[7] Melatonin is a powerful antioxidant, and it also promotes the stimulation of antioxidant enzymes.[8-10] It inhibits lipid peroxidation, promotes upregulation of cellular glutathione levels, and it reportedly has higher radical scavenging capacity than Vitamins C and E.[8] In addition, melatonin downregulates cyclooxygenase-2, resulting in inhibition of cancer growth and metastasis.[9] It also exhibits anti-inflammatory potential through inhibition of inflammatory cytokines.[10] Melatonin is mainly metabolized in the liver,[8] hence its strong involvement in the resolutions of hepatic oxidative stress and mutagenesis.
Tumor necrosis factor-alpha (TNF-α) is a pro-inflammatory cytokine and its upregulation resulted in induction of necrosis and consequent apoptosis (necroptosis) in animal models.[11-13] TNF-α is involved in the resolution of cancer-associated chronic inflammation; hence, it is a biomarker of interest in the evaluation of tumor progression and cancer cells survival. p53 activates apoptosis and cell cycle arrest to permit repair of damaged DNA. Consequently, cells re-enter the normal cell cycle. However, p53-induced apoptosis functions to eliminate cells with serious DNA damage and inhibit the transfer of damaged DNA to daughter cells. Hence, p53 maintains genomic integrity, and the loss of function mutations in p53 gene is associated with mutagenesis, carcinogenesis, and cancer cells survival.[14-16]
Morinda lucida (ML) is a well grown plant in Nigeria. ML leaf extract has various therapeutic benefits and has been reported to have a strong oral hypoglycemic property[17,18] and antioxidant potentials.[19] Annona muricata (AM) is a member of the Annonaceae family and is a fruit tree with a long history of traditional use across Nigeria. AM has been reported to possess anticancer, anticonvulsant, anti-arthritic, antiparasitic, antimalarial, hepatoprotective, and antidiabetic activities.[20,21] AM leaf extract has also been reported to have antioxidant and anti-lipid peroxidation potentials.[22]
The rat, mouse, and human share about one-third of the genome. Hence, some hormones and genes associated with antioxidant defense mechanism and human cancers, such as Melatonin, TNF-α, and p53 seem to be highly conserved across mammalian evolution and have counterparts in the rat genome.[23,24] Therefore, this study evaluated the effects of fractionated and isolated compounds from ML leaves (MLF1) and AM leaves (AMF1) on lipid peroxidation and immunomodulations of melatonin, TNF-α, and p53 proteins in lead acetate (LA)-induced toxicity, oxidative stress, and mutagenesis in rats to determine their anticancer potentials.
Materials and Methods
Ethical approval
Ethical approval for this study was sought and received from the Ethical Review Committee of the institution where the study was primarily conducted. The ethical approval number is UERC/ASN/2018/1161. This research study was conducted in accordance with the internationally accepted principles for laboratory animal use and care as provided in the European Community guidelines (EEC Directive of 1986; 86/609/EEC) and the US guidelines (NIH publication #85-23, revised in 1985).
Collection, authentication, and deposition of ML and AM leaves
Freshly cut leaves of ML and AM were obtained locally from forest reserves in Ilorin and samples identified and authenticated by a Pharmaceutical Botanist of the Department of Botany, Faculty of Life Sciences, University of Ilorin, Ilorin, Nigeria. ML and AM leaves were deposited at the herbarium of the Department of Botany, Faculty of Life Sciences, University of Ilorin, and assigned Herbarium Identification Numbers UITH/004/1103 and UITH/003/1106 respectively.
Preparations and ethanolic extractions of ML and AM leaves
ML and AM leaves were air-dried at the laboratory unit of the Department of Chemistry, University of Ilorin, Ilorin, Nigeria. The dried leaves of ML and AM were grinded to powder form to enable proper absorption of solvent and weighed using the electronic compact scale. Extraction was carried out using distilled ethanol to remove impurities, and the resultant product was put in a conical flask and heated. Liquid ethanol flowed from the condenser into a container and was continuously recycled to keep the process running. Boiling chips/anti-bumping granules were put in the conical flask to prevent liquid ethanol from “bumping” into the condenser.
The mixture was decanted and then sieved after 24 h. After decantation, another distilled ethanol was added to the sieved ML and AM and left for another 24 h. When the color quality and texture of the dissolved ML and AM in ethanol became evidently low (compared to the previous solutions decanted), the procedure was halted. Ethanol was separated from ML and AM and column chromatography was done to get different fractions of ML and AM.
Column chromatography fractionation of the ethanol extract of ML
The ethanol extract of ML was fractionated in a silica gel open column, using n-hexane, dichloromethane, ethyl acetate, and ethanol in an increasing order of polarity (N-hexane: Dichloromethane [3:1, 3:2, 1:1, 1:2, 1:3]; dichloromethane; dichloromethane: ethylacetate [3:1, 3:2, 1:1, 1:2, 1:3]; ethylacetate; ethylacetate: methanol [3:1, 3:2, 1:1, 1:2, 1:3]; and methanol, to afford 36 eluents of 250 ml each. The resulting eluents were pooled based on the color of the solvents that elute them to give a total of nine combined fractions. The fraction MLF1 which had the best preliminary antioxidant potential out of the 9 fractions was used in this study to evaluate the effects of MLF1 on LA-induced toxicity in rats.
Column chromatography fractionation of the ethanol extract of AM
The ethanol extract of AM was fractionated in a silica gel open column, using n-hexane, dichloromethane, ethyl acetate, and ethanol in an increasing order of polarity (N-hexane: dichloromethane [3:1, 3:2, 1:1, 1:2, 1:3]; dichloromethane; dichloromethane: ethylacetate [3:1, 3:2, 1:1, 1:2, 1;3]; ethylacetate; ethylacetate: methanol [3:1, 3:2, 1:1, 1:2, 1:3]; and methanol, to afford 13 eluents of 250 ml each. The resulting eluents were pooled based on the color of the solvents that elute them to give a total of five combined fractions. The fraction AMF1 which had the best preliminary antioxidant potential out of the five fractions was used in this study to evaluate the effects of AMF1 on LA-induced toxicity in rats.
Animal care and feeding
A total number of 60 female Wistar rats with an average weight of 200 g were used in this study. The rats were acclimatized for 5 days, received water ad libitum and kept in the animal house located in the Faculty of Basic Medical Sciences, College of Health Sciences, University of Ilorin, Nigeria. The animals were fed daily with pelletized grower feed from Ogo-Oluwa Livestock and Aqua Feed enterprise, Kwara State, Ilorin, Nigeria. The grower feed contains 15% crude protein, 7% fat, 10% crude fiber, 1% calcium, 0.35% phosphorus, and 2.55% kcal/kg of metabolized energy as indicated on the pack. The animals were grouped into ten with five animals each in a wire gauzed cage. The animals were kept under a normal room temperature of 37°C and double-crossed ventilation.
Chemicals and reagents
LA was a product of Sigma–Aldrich Japan Co. (Tokyo, Japan) and was purchased from Emed Ejeson enterprises in Ilorin, Kwara State, Nigeria. Normal saline was obtained from MOMROTA pharmaceutical company in Ilorin, Kwara State, Nigeria.
Experimental procedures and drugs administration
Group 1 received physiological saline. Group 2 received 100 mg/kg bodyweight of LA for 5 weeks. Groups 3 and 4 received 100 mg/kg bodyweight LA for 2 weeks, followed by treatments with 7.5 and 15 mg/kg bodyweight of MLF1, respectively, for another 3 weeks. Groups 5 and 6 received 100 mg/kg bodyweight LA for 2 weeks, followed by treatments with 7.5 and 10 mg/kg bodyweight of AMF1, respectively, for another 3 weeks. Groups 7 and 8 received 7.5 and 15 mg/kg bodyweight of MLF1, respectively, for 5 weeks. Groups 9 and 10 received 7.5 and 10 mg/kg bodyweight of AMF1, respectively, for 5 weeks. Group 11 received co-administrations of 15 mg/kg bodyweight MLF1 and 100 mg/kg bodyweight LA for 5 weeks. Group 12 received co-administrations of 10 mg/kg bodyweight of AMF1 and 100 mg/kg bodyweight LA for 5 weeks. All drugs and extract were administered orally. Bodyweights (g) of all rats were measured on day 1 of experimental procedure and at the end of each week.
Animal sacrifice
At the end of experimental procedures, all rats were sacrificed by cervical dislocation.
Histopathological evaluations of the liver
The livers of all rats were excised and a lobe fixed in 10% formal saline of at least 5 times of its volume. The liver tissues were processed for light microscopy using conventional histological procedures. Tissue sections were stained through Hematoxylin and Eosin method as previously described.[25]
Evaluations of lipid peroxidation
The thiobarbituric acid assay (TBARS assay) method was used to quantify Malondialdehyde (MDA) concentrations in liver homogenates of all rats of Control and Experimental Groups as previously described.[7]
Sera melatonin, sera TNF-α, and liver tissues’ p53 proteins concentrations using enzyme linked immunosorbent assay (ELISA)
The thoracic cavity of each rat was exposed and 5 ml blood sample collected through the ventricles of the heart into lithium heparinized bottles. The blood samples were centrifuged and the serum was used for ELISA analyses of concentrations of melatonin and TNF-α protein in all rats of Control and Experimental Groups using ELISA technique. In addition, liver tissues were isolated immediately after animal sacrifice and then subjected to thorough homogenization using porcelain mortar and pestle in ice-cold 0.25M sucrose, in the proportion of 1 g–4 ml of 0.25M sucrose solution. The tissue homogenates were filled up to 5 ml with additional sucrose and collected in a 5 ml serum bottle. Homogenates were thereafter centrifuged at 3000 revolution per minute for 15 min using a centrifuge (Model 90-1). The supernatant was collected with Pasteur pipettes and placed in a freezer at −4°C, and thereafter assayed for concentrations of p53 protein in the liver tissues of all rats of Control and Experimental Groups using ELISA technique.
Statistical analysis
All data obtained were expressed as arithmetic means ± standard error of mean and were subjected to statistical analyses using T-test to compare Group 2 with Groups 1 and 3–12. Differences were tested and considered statistically significant when P ≤ 0.05 using GraphPad Prism software package (Graph Pad Software Inc., San Diego, CA, USA; version 7 for Windows) and Microsoft Excel 2016.
Results
Histopathological evaluations of the liver
Histopathological evaluations showed normal histoarchitectures of the liver in rats of Groups 1–12 [Figures 1-8]. There were normal cellular density and staining characteristics of hepatocytes, hepatic sinusoids, and central veins. The nuclei of hepatocytes were well characterized with no apparent large vacuolations around them.
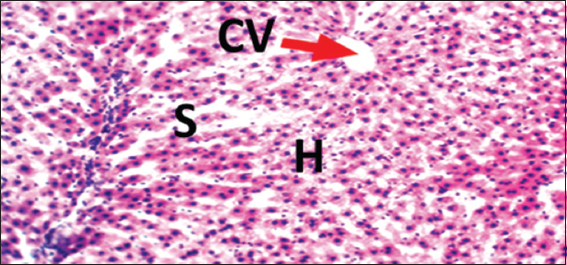
- Photomicrograph of liver of rat of control Group 1, which received normal saline. Hematoxylin and Eosin × 100. H = Hepatocytes, S = Blood sinusoids and CV = Central Vein. Histopathological evaluations showed normal histoarchitecture of the liver components
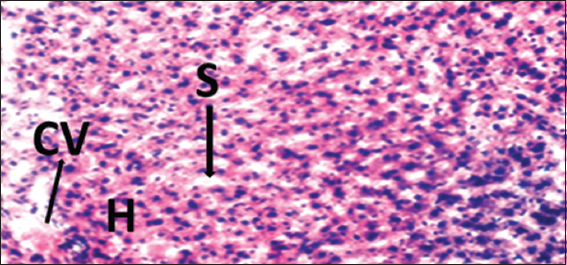
- Photomicrograph of liver of rat of experimental Group 2, which received 100 mg/kg bodyweight of lead acetate only. Hematoxylin and Eosin × 100. H = Hepatocytes, S = Blood sinusoids and CV = Central Vein. Histopathological evaluations showed normal histoarchitecture of the liver components
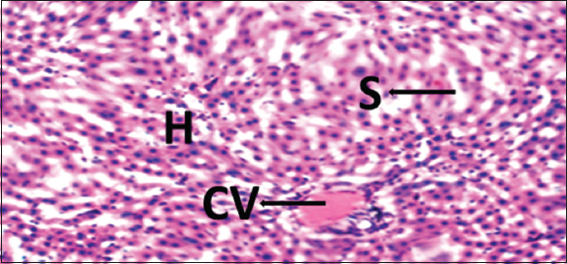
- Photomicrograph of liver of rat of experimental Group 4, which received 100 mg/kg bodyweight lead acetate (2 weeks) + 15 mg/kg bodyweight Morinda lucida (3 weeks) respectively. Hematoxylin and Eosin × 100. H = Hepatocytes, S = Blood sinusoids and CV = Central Vein. Histopathological evaluations showed normal histoarchitecture of the liver components
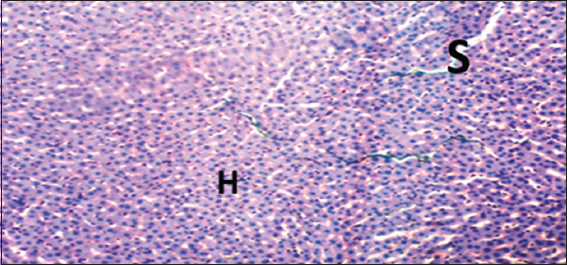
- Photomicrograph of liver of rat of experimental Group 6, which received 100 mg/kg bodyweight lead acetate (2 weeks) + 10 mg/kg bodyweight Annona muricata (3 weeks), respectively. Hematoxylin and Eosin × 100. H = Hepatocytes, S = Blood sinusoids and CV = Central Vein. Histopathological evaluations showed normal histoarchitecture of the liver components
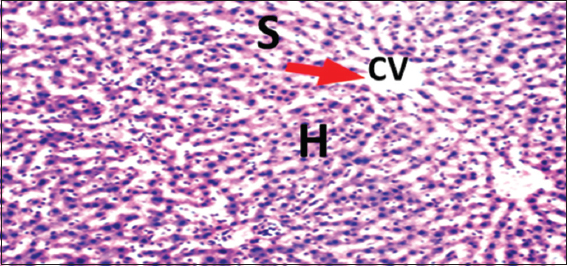
- Photomicrograph of liver of rat of experimental Group 8, which received only 15 mg/kg bodyweight Morinda lucida (3 weeks), respectively. Hematoxylin and Eosin × 100. H = Hepatocytes, S = Blood sinusoids and CV = Central Vein. Histopathological evaluations showed normal histoarchitecture of the liver components
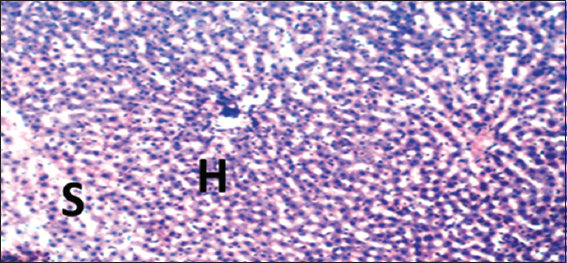
- Photomicrograph of liver of rat of experimental Group 10, which received only 10 mg/kg bodyweight Annona muricata (3 weeks), respectively. Hematoxylin and Eosin × 100. H = Hepatocytes, S = Blood sinusoids and CV = Central Vein. Histopathological evaluations showed normal histoarchitecture of the liver components
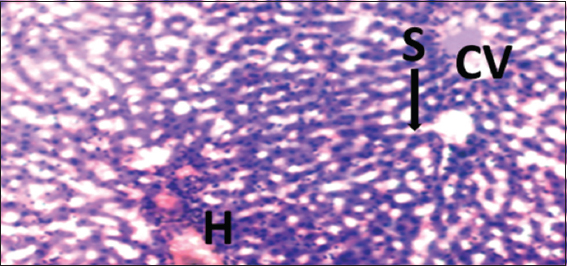
- Photomicrograph of liver of rat of experimental Group 11, which received co-administration of 100 mg/kg bodyweight lead acetate + 15 mg/kg bodyweight Morinda lucida (5 weeks). Hematoxylin and Eosin × 100. H = Hepatocytes, S = Blood sinusoids and CV = Central Vein. Histopathological evaluations showed normal histoarchitecture of the liver components
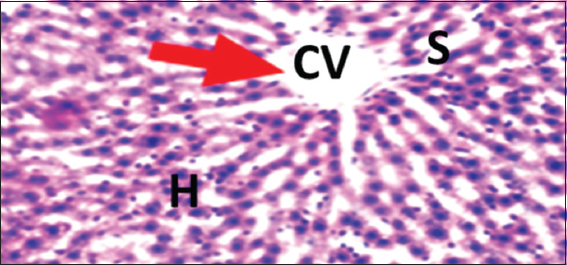
- Photomicrograph of liver of rat of experimental Group 12, which received co-administration of 100 mg/kg bodyweight lead acetate + 10 mg/kg bodyweight Annona muricata (5 weeks). Hematoxylin and Eosin × 100. H = Hepatocytes, S = Blood sinusoids and CV = Central Vein. Histopathological evaluations showed normal histoarchitecture of the liver components
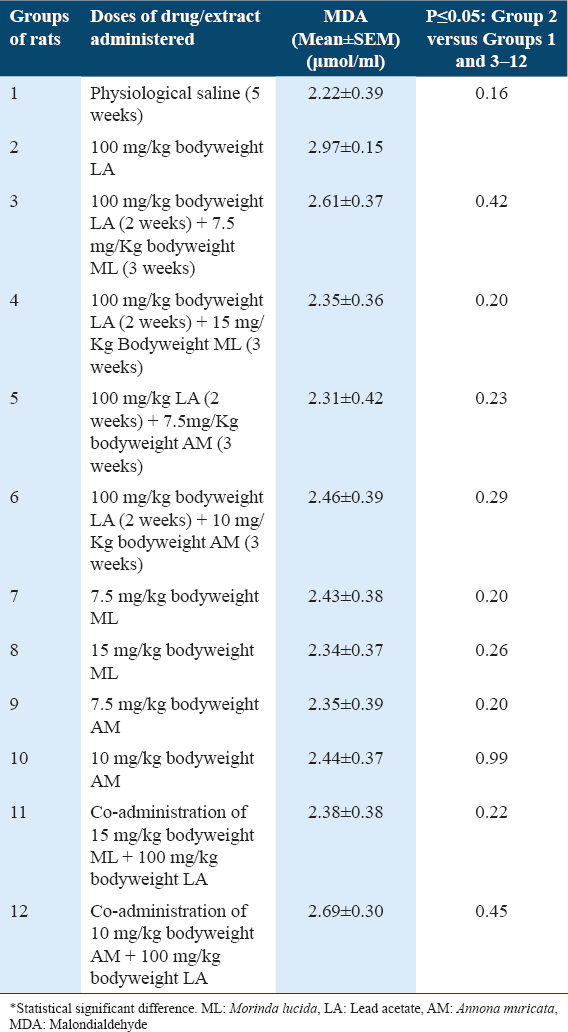
Malondialdehyde (MDA) concentrations in liver tissues of rats
Results showed statistically non-significant higher (P ≥ 0.05) MDA levels in rats of Group 2 when compared with Group 1 [Table 1]. In addition, results showed statistically non-significant lower (P ≥ 0.05) MDA levels in rats of Groups 3–12, when compared with Group 2 [Table 1].
Sera melatonin concentrations in rats
Results showed statistically significant lower (P ≤ 0.05) levels of melatonin in rats of Group 2 when compared with Group 1 [Table 2]. In addition, results showed statistically significant higher (P ≤ 0.05) levels of melatonin in rats of Groups 5–12, when compared with Group 2 [Table 2]. However, there were statistically non-significant higher (P ≥ 0.05) levels of melatonin in rats of Group 4, when compared with Group 2 [Table 2].

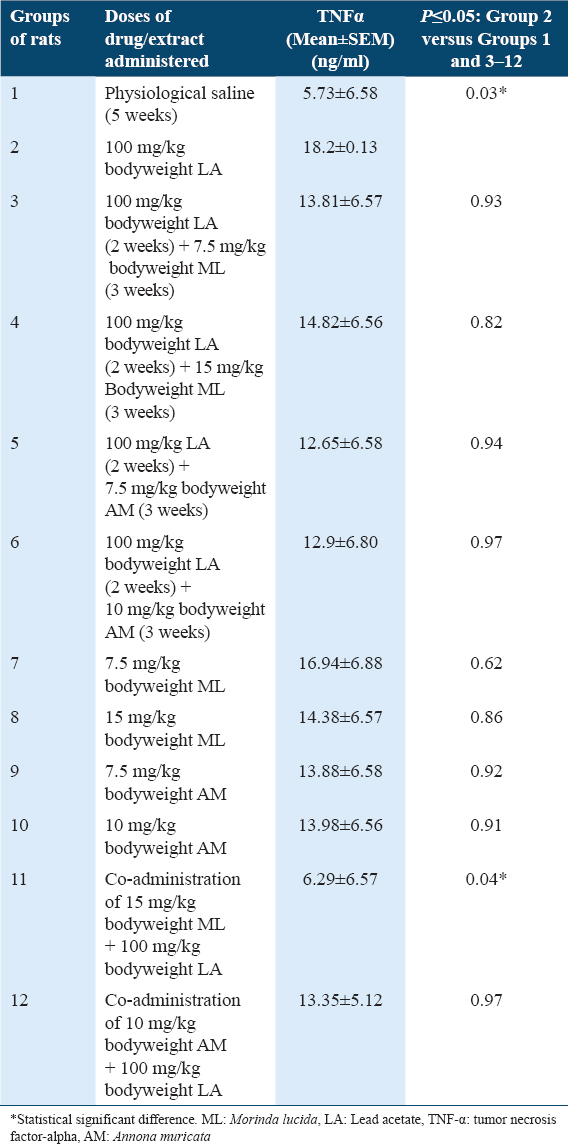
Sera TNF-α concentrations in rats
Results showed statistically significant higher (P ≤ 0.05) levels of TNF-α in rats of Group 2 when compared with Group 1 [Table 3]. In addition, results showed statistically significant lower (P ≤ 0.05) levels of TNF-α in rats of Group 11, when compared with Group 2 [Table 3]. However, there were statistically non-significant lower (P ≥ 0.05) levels of TNF-α in rats of Groups 3–10 and 12, when compared with Group 2 [Table 3].
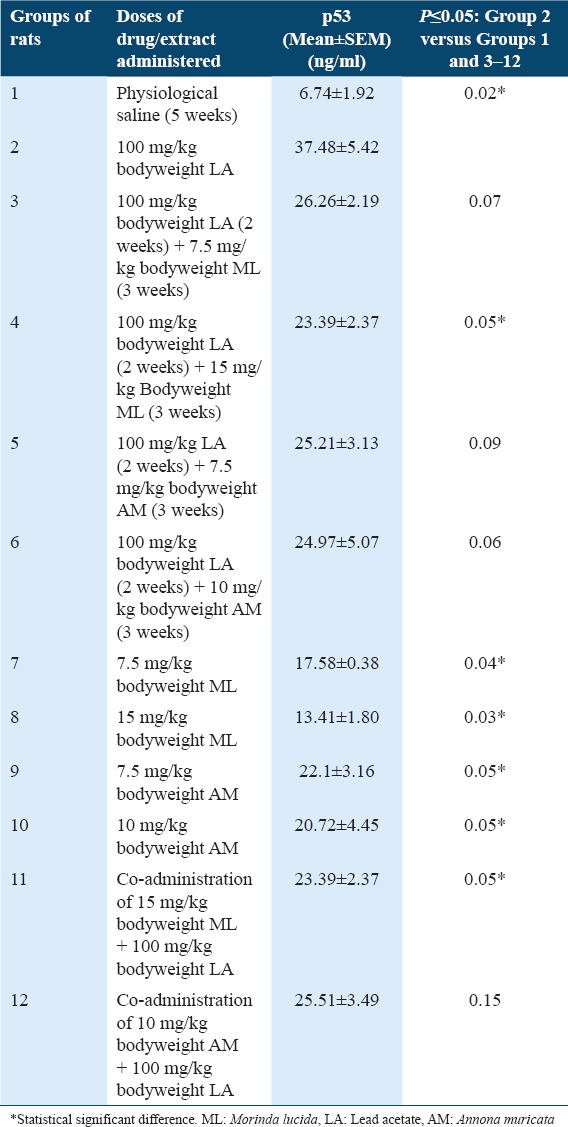
p53 concentrations in Liver tissues of rats
Results showed statistically significant higher (P ≤ 0.05) levels of p53 in rats of Group 2 when compared with Group 1 [Table 4]. In addition, results showed statistically significant lower (P ≤ 0.05) levels of p53 in rats of Groups 4 and 7–11, when compared with Group 2 [Table 4]. However, there were statistically non-significant lower (P ≥ 0.05) levels of p53 in rats of Groups 3, 5, 6, and 12, when compared with Group 2 [Table 4].
Discussion
Histopathological evaluations showed normal histoarchitectures of the liver in rats of Groups 1 – 12 [Figures 1-8]. There were normal cellular density and staining characteristics of hepatocytes, hepatic sinusoids, and central veins. The nuclei of hepatocytes were well characterized with no apparent large vacuolations around them. This implied that administrations of 100 mg/kg bodyweight of Lead acetate (LA), 7.5 and 15 mg/kg bodyweight of MLF1 (extracted from Morinda lucida leaves) and 7.5 and 10 mg/kg bodyweight of AMF1 (extracted from Annona muricata leaves) to rats did not result in evident histopathological change of the liver after 5 weeks of exposure at the light microscopic level. This could be due to the fact that the cyto-toxicity of adverse chemical agents is exposure-dependent and drug-induced toxicity is usually first elicited on molecular markers, while further exposure will result in evident histo-pathology at tissue level.
Lipid peroxidation results in increased oxidative stress, compromised cell membrane, cellular damage and production of mutagenic aldehydes such as Malondialdehyde.[7] In addition, there is increased lipid peroxidation in inflammatory and cancer conditions.[7] Hence, increased Malondialdehyde levels implies increased oxidative stress. The treatment of rats of Group 2 with 100 mg/kg bodyweight of LA resulted in non-significant increased MDA levels when compared with control Group 1 [Table 1], indicating LA-induction of oxidative stress. This observation is in agreement with those of Abdelhamid et al.,[6] who reported that the administration of 100 mg/kg bodyweight of LA resulted in marked increased MDA levels and prominent reduction in the antioxidant system in rats.
Do MLF1 and AMF1 have cyto-protective and antioxidant potentials against LA-induced oxidative stress? Post-treatments of LA-induced oxidative stress with 7.5 and 15 mg/kg bodyweight of MLF1, 7.5 and 10 mg/kg bodyweight of AMF1 resulted in decreased MDA levels, when compared with Group 2 [Table 1]. Hence, MLF1 and AMF1 possess cytoprotective and antioxidant potentials.
Do MLF1 and AMF1 have chemopreventive and antioxidant potentials against LA-induced oxidative stress? The co-administrations of 100 mg/kg bodyweight of LA with 15 mg/kg bodyweight of MLF1 and 10 mg/kg bodyweight of AMF1 resulted in decreased MDA levels, when compared with Group 2 [Table 1]. Therefore, MLF1 and AMF1 possess chemopreventive antioxidant potentials.
Melatonin is a mitochondria-targeted antioxidant,[26] and its increased level is associated with reduced DNA and cellular damage, decreased oxidative stress, inflammation, and proliferation.[8-10,26] The treatment of rats of Group 2 with 100 mg/kg bodyweight of LA resulted in significant decreased Melatonin levels when compared with control Group 1 [Table 2], indicating LA-induction of prominent reduction in the antioxidant system. This observation is in agreement with those of Abdelhamid et al.,[6] who reported that the administrations of 100 mg/kg bodyweight of LA resulted in marked increased MDA levels and prominent reduction in the antioxidant system in rats.
Do MLF1 and AMF1 have cytoprotective and antioxidant potentials against LA-induced prominent reduction in the antioxidant system? Post-treatments of LA-induced prominent reduction in the antioxidant system with 15 mg/kg bodyweight of MLF1, 7.5 and 10 mg/kg bodyweight of AMF1 resulted in increased melatonin levels, when compared with Group 2. Hence, MLF1 and AMF1 possess pro-Melatonin, cytoprotective, and prominent promotion of the antioxidant system potentials.
Do MLF1 and AMF1 have chemopreventive and antioxidant potentials against LA-induced prominent reduction in the antioxidant system? The co-administrations of 100 mg/kg bodyweight of LA with 15 mg/kg bodyweight of MLF1 and 10 mg/kg bodyweight of AMF1 resulted in increased melatonin levels, when compared with Group 2 [Table 2]. Therefore, MLF1 and AMF1 possess pro-Melatonin, chemopreventive, and prominent promotion of the antioxidant system potentials.
When cells are exposed to chemical or physical injury, anoxia or starvation, there is activation of cellular immune response, inflammation, and induction of release of cytokines. Inflammation results in cell damage through necrosis which is characterized by leakage of cell contents into the adjacent tissues and capillary transmigration of granulocytes to the injured tissue.[11-13] The release of cellular contents into the extracellular space results in the induction of apoptosis of adjacent cells. Hence, in chemicals-induced mutagenesis, the upregulation of TNF-α is a characteristic immune response mechanism for the resolution of cancer-associated chronic inflammation, resulting in induction of necrosis and consequent apoptosis. TNFa is a pro-inflammatory cytokine,[11-13] hence, in chemicals-induced mutagenesis, the upregulation of TNFa is a characteristic immune response mechanism for the resolution of cancer-associated chronic inflammation, resulting in induction of necrosis and consequent apoptosis. This makes TNFa a biomarker or relevant risk factor for tumorigenesis, tumor progression, invasion and metastasis.[11-13]
The treatment of rats of Group 2 with 100 mg/kg bodyweight of LA resulted in significant increased TNF-α levels when compared with control Group 1 [Table 3], indicating LA-induction of cancer-associated inflammation. This observation is in agreement with a previous study which reported that the administrations of 100 mg/kg bodyweight of LA resulted in significant upregulation of inflammatory markers in rats.[7]
Do MLF1 and AMF1 have cytoprotective and anti-inflammatory potentials against LA-induced cancer-associated inflammation? Post-treatments of LA-induced cancer-associated inflammation with 7.5 and 15 mg/kg bodyweight of MLF1, 7.5 and 10 mg/kg bodyweight of AMF1 resulted in the downregulation of TNF-α level, when compared with Group 2 [Table 3]. Hence, MLF1 and AMF1 possess cytoprotective, anti-inflammatory, and anticancer potentials.
Can MLF1 and AMF1 protect the organism and prevent LA-induced cancer-associated inflammation? The co-administrations of 100 mg/kg bodyweight of LA with 15 mg/kg bodyweight of MLF1 and 10 mg/kg bodyweight of AMF1 resulted in decreased TNF-α levels, when compared with Group 2 [Table 3], and thus protected the rats against LA-induced cancer-associated inflammation. This suggests that MLF1 and AMF1 possess chemopreventive anti-inflammatory potentials.
The tumor suppressor gene p53 is a pro-apoptotic gene which functions to induce cell cycle arrest in cells with damaged DNA and chromosomal aberrations, preventing the transfer of damaged DNA to daughter calls. The expression of the functionally latent form of p53 is usually low in normal conditions, however, p53 is up-regulated in response to abnormal conditions such as inflammation and DNA damage.[14-16] Previous study reported that administration of 10-100mg/kg bodyweight of Lead acetate resulted in increased expressions of p53 and Bax with accompanied imbalance of Bax/Bcl-2 ratio.[27]
The treatment of rats of Group 2 with 100 mg/kg bodyweight of LA resulted in significant increased p53 levels when compared with control Group 1 [Table 4], indicating LA-induction of mutagenesis. This result is in agreement with previously reported LA-induced increased p53 expression.[27] It must be noted that with increased lipid peroxidation, reduced melatonin levels, cancer-associated inflammation, and response upregulation of MDA and TNF-α levels in only the rats of Group 2 [Tables 1-3], the characteristic expected immune response and DNA/cell-damaged repair mechanism is induction of apoptosis and upregulation of p53 pro-apoptotic gene. p53 upregulation is, however, as long as it takes for p53 activated apoptotic mechanism to complete the repair of damaged DNA/cells. Thereafter, p53 expression returns to normal status as the cells re-enter the normal cell cycle.[13-15] In the absence of cancer treatment, the sustained upregulation of p53 in rats of Experimental Group 2 at the end of the 5-week experimental procedure implied rapid and sustained LA-induced mutagenesis. This will gradually inhibit and prolong the DNA repair mechanism of p53 until its actions are spent out in-order to allow for un-inhibited mutagenesis. Consequently, cancer cells survival and tumorigenesis will prevail.
Do MLF1 and AMF1 have cyto-protective and anticancer potentials against LA-induced mutgenesis? Post-treatments of LA-induced mutagenesis resulted in significant [Group 4] and non-significant downregulation [Groups 3, 5 and 6] of p53 levels in comparison with Group 2 [Table 4] implied that MLF1 and AMF1 possess pro-apoptotic, tumor suppression and anticancer potentials which will bring p53 to normal levels after quick resolution of mutagenesis.
Can MLF1 and AMF1 protect the organism and prevent LA-induced mutagenesis? The co-administrations of 100 mg/kg bodyweight of LA with 15 mg/kg bodyweight of MLF1 and 10 mg/kg bodyweight of AMF1 resulted in decreased p53 levels, when compared with Group 2 [Table 4], and thus protected the rats against LA-induced mutagenesis. This suggests that MLF1 and AMF1 possess chemopreventive, proapoptotic, and anticancer potentials. The observed anticancer potentials of MLF1 and AMF1 in this study are in agreement with previously reported anti-proliferation and tumor inhibitory potentials of ML leaves[28] and AM leaves[29] against HL-60 leukemia cells and breast cancer MCF-7 cells, respectively.
Conclusion
Post-treatments of Lead acetate-induced toxicity with doses of MLF1 (extracted from Morinda lucida leaves) and AMF1 (extracted from Annona muricata leaves) resulted in decreased levels of sera TNF-alpha, liver Malondialdehyde and liver p53 in rats. In addition, post-treatments of Lead acetate-induced toxicity with doses of MLF1 and AMF1 resulted in increased sera Melatonin levels in rats. These observations implied that MLF1 and AMF1 conferred some degrees of antioxidant, pro-Melatonin, anti-inflammatory, anti-proliferation, anticancer and hepato-protective potentials against Lead acetate-induced toxicity.
Recommendations
The observations of this study implied that MLF1 (extracted from ML leaves) and AMF1 (extracted from AM leaves) possess antioxidant, pro-Melatonin, anti-inflammatory and anticancer potentials. This study recommends that the active components responsible for the anticancer potentials of MLF1 and AMF1 be further evaluated as potential drug candidates in in vitro and in vivo cancer models.
Limitations of the study
The present study is limited to evaluation of the anticancer potentials of isolated and fractionated compounds (MLF1: Extracted from ML leaves and AMF1: Extracted from AM leaves).
Authors’ Declaration Statements
Ethics approval and consent to participate
All experimental protocols were approved and performed in accordance with the guiding principles of the UERC.
Availability of data and materials
The datasets computed and/or analyzed during this study are available from the corresponding author on reasonable request.
Authors’ Contributions
AAA, MOA, AOO, and REK designed, performed the project work, analyzed, and interpreted the data. AAA was the main contributor in writing the manuscript. AAA, MOA, AOO, REK, OA, ST, OO, RB, SA, TA, and MA contributed to analyzing, interpretation of the data and writing of the manuscript. All authors read and approved the final manuscript.
Acknowledgments
The authors acknowledge the technical support of Laboratory staff members of the Departments of Anatomy and Chemistry of the University of Ilorin, Nigeria.
References
- Prevalence and Determinants of childhood lead poisoning in Zamfara state, Nigeria. J Health Pollut. 2014;6:1-9.
- [Google Scholar]
- Effect of lead acetate toxicity on experimental male albino rat. Asian Pac J Trop Biomed. 2012;2:41-6.
- [Google Scholar]
- Lead organ and tissue toxicity:Roles of mitigating agents (Part 2) Br J Pharmacol Toxicol. 2013;5:1-15.
- [Google Scholar]
- Hepatotoxic effects of lead acetate in rats:Histopathological and cytotoxic studies. J Cytol Histol. 2014;5:256.
- [Google Scholar]
- Indigofera oblongifolia prevents lead acetate-induced hepatotoxicity, oxidative stress, fibrosis and apoptosis in rats. PLoS One. 2016;11:e0158965.
- [Google Scholar]
- Ameliorative effect of curcumin against lead acetate-induced hemato-biochemical alterations, hepatotoxicity, and testicular oxidative damage in rats. Environ Sci Pollut Res. 2020;27:10950-65.
- [Google Scholar]
- Lipid peroxidation in Nigerians affected with heamatological malignancies. Afr J Med Med Sci. 2012;41:145-8.
- [Google Scholar]
- New developments of the effect of melatonin on reproduction. World J Obstet Gynecol. 2013;2:8-15.
- [Google Scholar]
- Signaling pathways of melatonin in prevention of liver disorders via suppressing of oxidative stress in cellular level. Biomed Pharmacol J. 2016;9:972.
- [Google Scholar]
- TNF-induced apoptosis of macrophages following inhibition of NF-B:A central role for disruption of mitochondria. J Immunol. 2004;172:1907-15.
- [Google Scholar]
- Measurement of serum, liver, and brain cytokine induction, thiamine levels, and hepatopathology in rats exposed to a 4-day alcohol binge protocol. Alcohol Clin Exp Res. 2010;34:1858-70.
- [Google Scholar]
- Production of p53 gene knockout rats by homologous recombination in embryonic stem cells. Nature. 2010;467:211-3.
- [Google Scholar]
- Clinicopathological and prognostic significance of Ki-67, caspase-3 and p53 expression in gastric carcinomas. Oncol Lett. 2013;6:1277-84.
- [Google Scholar]
- Anti-peroxidative, protective and ameliorative properties of methanol extract of all parts of Morinda lucida Benth in CCl4-induced liver injury. Natl Prod Chem Res. 2014;1:3.
- [Google Scholar]
- Traditional and medicinal uses of Morinda lucida. J Med Plants Stud. 2018;6:249-54.
- [Google Scholar]
- Antioxidant potential of Morinda lucida and Psidium guajava extracts and actions against paracetamol-induced kidney and liver injuries in rats. Am Int J Biol Life Sci. 2020;2:6-17.
- [Google Scholar]
- Proximate composition, phytochemical analysis, and in vitro antioxidant potentials of extracts of Annona muricata (Soursop) Food Sci Nutr. 2017;5:1029-36.
- [Google Scholar]
- Annona muricata (Annonaceae):A review of its traditional uses, isolated acetogenins and biological activities. Int J Mol Sci. 2015;16:15625-58.
- [Google Scholar]
- Annona muricata Linn leaf as a source of antioxidant compounds with in vitro andtidiabetic and inhibitory potential against a-amylase, a-glucosidase, lipase, non-enzymatic glycation and lipid peroxidation. Biomed Pharmacother. 2018;100:83-92.
- [Google Scholar]
- Histological effects of 25mg/kg/bodyweight of artemether on the trapezoid nuclei of wistar rats. Acta Histochem. 2010;112:193-8.
- [Google Scholar]
- The effects of Moringa oleifera on lipid profile status, heart histology and liver histochemistry in adult wistar rats. CHRISMED J Health Res. 2017;4:104-9.
- [Google Scholar]
- Melatonin as an antioxidant:Under promises but over delivers. J Pineal Res. 2016;61:253-78.
- [Google Scholar]
- Lead induces oxidative stress, DNA damage and alteration of p53, Bax and Bcl-2 expressions in mice. Food Chem Toxicol. 2008;46:1488-94.
- [Google Scholar]
- Antiproliferative, antioxidant activities and apoptosis induction by Morinda lucida and Taraxacum officinale in human HL-60 leukemia cells. J Glob Biosci. 2016;5:4281-91.
- [Google Scholar]
- Potential benefits of Annona muricata in combating cancer:A review. Malays J Med Sci. 2018;25:5-15.
- [Google Scholar]







