Translate this page into:
Non-variceal upper gastrointestinal bleed as first presentation of primary systemic amyloidosis – A case report
Address for correspondence: Dr. Shubham Jain, Department of Gastroenterology, Topiwala National Medical College and BYL Ch. Hospital, Dr. A.L Nair Road, Mumbai- 400 008, Maharashtra, India. Mobile: +91-7710915754. E-mail: dr.shubhamjazz@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Qassim Uninversity and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Gastrointestinal (GI) tract manifestations of amyloid deposition include diarrhea, GI hemorrhage, steatorrhea, or constipation. Here, we report an elderly female presenting with GI hemorrhage due to gastric ulceration and 4–6 polypoidal lesions with intermittent ooze in the duodenum as a first presentation of primary systemic amyloidosis. The bleed was managed with proton-pump inhibitors and hemospray application. She received chemotherapy for multiple myeloma after stabilization. A high index of suspicion is needed to diagnose amyloidosis causing GI hemorrhage.
Keywords
Gastrointestinal amyloidosis
gastrointestinal hemorrhage
hemospray
Introduction
Amyloidosis is a heterogeneous group of diseases, characterized by the misfolding of extracellular proteins, generating insoluble fibrils resulting in disruption of tissue structure and function.[1] Primary amyloidosis is associated with monoclonal light chains in serum and/or urine with 15% of patients having multiple myeloma.[2] Secondary amyloidosis is caused by the deposition of amyloid A (AA) protein, which results from proteolytic cleavage of the circulating acute-phase reactant serum AA.[3] Polarized light microscopy shows apple green birefringence following staining with Congo red dye. On electron microscopy, amyloid fibrils are ~10 nm in diameter.
GI involvement is uncommon in patients with amyloidosis, with a reported risk of only about 3%. The gastrointestinal (GI) tract manifestations include diarrhea, steatorrhea, constipation, or GI bleeding. Peptic ulcer disease, polyps, and Crohn’s disease are included in the differential diagnosis. Primary systemic amyloidosis involving the GI tract can present with bleeding. However, disease diagnosis with GI bleed as the first presentation is unusual. We are reporting a patient with primary systemic amyloidosis who presented with melena.
Case Report
A 54-year-old female presented with right upper quadrant pain for 2 months. It was dull aching, intermittent, non-radiating, and aggravated by meals. She had intermittent episodes of passage of black tarry stools associated with easy fatigability. Bilateral lower limb swelling with abdominal distension was noted for 1 month. She had anorexia and loss of weight. On examination, she had pallor, tachycardia, pedal edema, raised jugular venous pressure, and mild tender hepatomegaly. Investigations revealed microcytic hypochromic anemia, thrombocytopenia, and positive occult blood in the stool. Except for the reversal of albumin/globulin ratio, rest of the liver function tests were normal. Ultrasound abdomen showed hepatomegaly (16.5 cm) with mild ascites. Portal vein and spleen were normal. Ascitic fluid had high protein and high serum ascites albumin gradient (SAAG >1.1) with 300 white cells predominantly lymphocytes. Upper GI endoscopy showed multiple linear ulcers without active bleeding in the gastric body and fundus with thickened gastric folds; the rapid urease test was negative [Figure 1]. Esophageal and duodenal mucosa was normal. There was dense neutrophilic inflammation suggestive of acute gastritis on histology with no evidence of malignancy. Computed tomography of the abdomen revealed mild irregular wall thickening in fundus and body. The right and left atrial enlargement with diastolic dysfunction was noted on the 2D-echocardiogram. The patient was started on diuretics and pantoprazole. She improved and was discharged with a diagnosis of congestive cardiac failure secondary to anemia due to peptic ulcer bleed. She was readmitted after 2 months with a history of generalized weakness and easy fatiguability with melena for 8 days. On admission, hemoglobin was 4.5 g/dl, total leukocyte count and platelets were normal. The patient was transfused two units of blood. Repeat upper GI endoscopy showed partially healed gastric ulcers [Figure 2a] and multiple polypoidal lesions in the duodenum with intermittent ooze [Figure 2b]. Bleeding was controlled with hemospray and injection adrenaline (1:10,000). After stabilization, biopsies were taken 3 days later from duodenal polypoidal lesions and the gastric ulcers. Histology revealed moderate chronic lymphoplasmacytic infiltrate with lamina propria showing glassy pink material separating glands suggestive of amyloidosis [Figure 3a]. Rectal fat pad biopsy and gastric mucosal biopsy showed amyloid deposits with apple-green birefringence on polarized microscopy [Figure 3b]. Serum electrophoresis showed increased IgG lambda light chains – 452 mg/L (N-0.57–2.63 mg/dl). Twenty-four hours urinary protein was normal. Bone marrow aspiration shows monoclonal population of plasma cells with positive amyloid staining with congo red stain. Based on gastrointestinal amyloid deposits with positive congo red staining, presence of IgG lambda-type M protein in serum and clonal plasma cells in bone marrow a diagnosis of primary systemic AL amyloidosis was made. Serum β2 microglobulin levels were 3400 mcg/ml (N-0–3 mcg/ml). Chemotherapy with Bortezomib and low-dose dexamethasone (M-Dex) was started by the hematologist. Follow-up endoscopy at 2 months showed a reduction in the size of the polypoidal lesions. The patient was asymptomatic at 6 months and then lost to follow-up. This was an unusual presentation of systemic amyloidosis in an elderly patient with melena, thickened gastric folds, and duodenal polypoidal lesions.
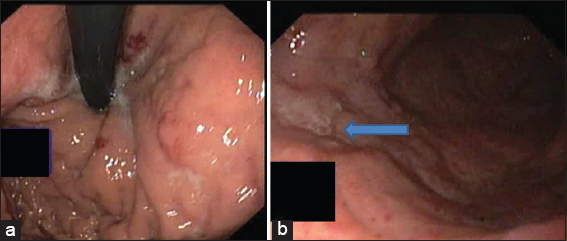
- Upper GI endoscopy showing multiple ulcerations in (a) body and (b) fundus of stomach
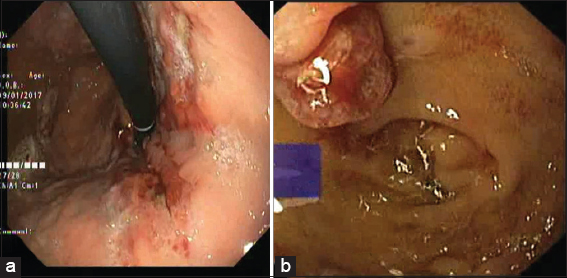
- Upper GI endoscopy on readmission showing (a) healed ulceration in fundus and (b) multiple polypoidal lesions in duodenum (D1)
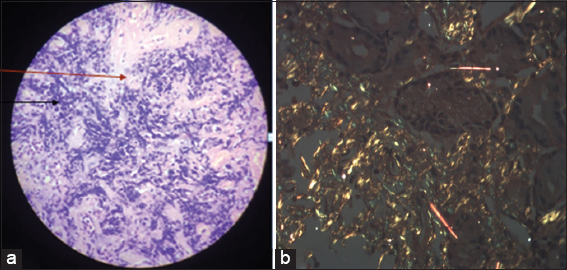
- (a) Histopathology of body of stomach showing moderate chronic lymphoplasmacytic infiltrate (black arrow) with lamina propria showing glassy pink material separating glands (red arrow) suggestive of amyloidosis. (b) Staining with Congo red showed characteristic apple green birefringence under polarized light (×10)
Discussion
Every part of the GI tract can be affected by amyloidosis, but the sites most frequently involved are the small bowel and the stomach, occasionally the colon, and rarely the esophagus.[4] Patients with alimentary tract amyloidosis can have varied presentations depending on the site involved. Various presentations include weight loss, diarrhea, abdominal pain, malabsorption, esophageal reflux, and varying degrees of upper and lower GI bleeding, including fatal hemorrhage.[5] Liver involvement can lead to jaundice, steatorrhea, anorexia, and those related to portal hypertension such as ascites and splenomegaly.[6] GI bleeding in the form of melena was the presentation in our patient. Sridhar et al. reported GI bleed in an already known case of amyloidosis.[7] Similarly, Singh et al. reported a case that presented with melena and hematemesis, but also a known case of amyloidosis.[8] Hence, all previous cases reported with GI bleed were already known to have amyloidosis. Rarely, amyloidosis can present with multiple massive GI bleeding, which can be fatal.[9] Cause for bleeding may be related to small-vessel fragility due to amyloid infiltration and impaired hemostasis caused by factor X deficiency. Menke et al. reported gastric involvement in 8% of patients by biopsy and 12% by autopsy but only 1% is symptomatic. Symptoms can be epigastric pain, nausea, vomiting, hematemesis, and gastric outlet obstruction due to submucosal tumors, polyps, or antral narrowing.[10] Endoscopic appearances of GI amyloidosis as described by Tada et al. can be fine granular mucosa, easy friability, polypoidal protrusions, erosions, and ulcerations. These findings were most marked in the second portion of the duodenum.[11] Similar polypoidal lesions with oozing in the duodenum and multiple ulcers in the stomach were the cause of melena in our patient. Screening endoscopic biopsies of the GI tract are diagnostic in most cases of systemic amyloidosis. The frequency of amyloid deposition in the biopsy specimens was 100% in the duodenum, 95% in the stomach, 91% in the colorectum, and 72% in the esophagus.[11] Diagnosis requires confirmation of the presence of amyloid deposition by histology and characteristic specific staining with Congo red.[3] Treatment is directed at the underlying cause and aimed to arrest amyloid accumulation and formation by reducing the abundance of the fibril precursor protein. At present no endoscopic treatment guidelines exist to manage bleeding from gastric amyloidosis. Moreover, endoscopic therapy has largely been ineffective in this setting. GI complications are managed with symptomatic control.[12] Limitation of the case report is a lack of long-term follow-up of the patient.
Conclusion
Gastroduodenal deposition of amyloid can present as upper GI bleeding due to polypoidal lesions, which can be the first presentation of the disease. Hence a high index of suspicion is necessary for its early diagnosis, especially in the elderly.
Authors’ Contributions
Suhas Udgirkar, Shubham Jain, and Sanjay Chandnani did the drafting of the manuscript; Suhas Udgirkar, Shubham Jain, and Pravin Rathi managed the case. Rima Kamat contributed in histopathological assessment. Suhas Udgirkar and Qais Contractor did the critical revision of the manuscript for important intellectual content and supervised the study.
Authors’ Declaration Statements
The authors’ guarantee that the work is original and does not infringe copyright or other party’s property rights. All authors have read and approved this submission and have given appropriate credit to everyone who participated in this work.
Patient consent
The patient’s consent was taken before reporting the case.
Availability of data and material
The data used in this study are available and will be provided by the corresponding author on a reasonable request.
Competing interest
None to declare.
Funding statement
None to declare
References
- Emerging treatment approaches for the systemic amyloidosis. Kidney Int. 2005;68:1377-90.
- [Google Scholar]
- Primary systemic amyloidosis:Clinical and laboratory features in 474 cases. Semin Hematol. 1995;32:45-59.
- [Google Scholar]
- Gastrointestinal manifestations of amyloidosis. Am J Gastroenterol. 2008;103:776-87.
- [Google Scholar]
- CASE REPORT:Gastrointestinal amyloidosis with ulceration, hemorrhage, small bowel diverticula, and perforation. Digest Dis Sci. 2003;48:2023-6.
- [Google Scholar]
- Gastric amyloidosis causing nonvariceal upper gastrointestinal bleeding. ACG Case Rep J. 2019;6:3-4.
- [Google Scholar]
- Upper gastrointestinal bleeding due to amyloidosis in a patient with multiple myeloma. Clin Gastroenterol Hepatol. 2016;14:A22-3.
- [Google Scholar]
- Gastrointestinal amyloidosis presenting with multiple episodes of gastrointestinal bleeding. Cardiovasc Intervent Radiol. 2009;32:577-80.
- [Google Scholar]
- Symptomatic gastric amyloidosis in patients with primary systemic amyloidosis. Mayo Clin Proc. 1993;68:763-7.
- [Google Scholar]
- Endoscopic and biopsy findings of the upper digestive tract in patients with amyloidosis. Gastrointest Endosc. 1990;36:10-4.
- [Google Scholar]
- Gastrointestinal amyloidosis:Approach to treatment. Curr Treat Options Gastroenterol. 2003;6:17-25.
- [Google Scholar]







