Translate this page into:
Pneumoperitoneum secondary to non-specific intestinal cystic pneumatosis: A case report
Address for correspondence: Ivan Lozada Martínez, Medical-Surgical Research Center, University of Cartagena, Cartagena, Colombia. Phone: +57-315-7799823. E-mail: ivandavidlo-ma@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Qassim Uninversity and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Intestinal cystic pneumatosis is a pathological entity of strange presentation, characterized by the presence of extraluminal gas located at the level of the submucosa and/or subserosa of the intestinal walls, forming cystic lesions that generally vary from 0.5 to 2.0 cm presenting an incidence around 0.03% in the general population. We present the case of a patient who presented to the emergency department for sudden abdominal pain, diffuse signs of peritoneal irritation, and a history of previous laparotomy for perforated gastric ulcer as the only relevant history. An X-ray was performed showing pneumoperitoneum, with subsequent histopathological diagnosis of cystic intestinal pneumatosis.
Keywords
Colon
jejunum
medical history taking
pneumatosis cystoides intestinalis
pneumoperitoneum
Introduction
Intestinal cystic pneumatosis is a pathological entity of strange presentation, characterized by the presence of extraluminal gas located at the level of the submucosa and/or subserosa of the intestinal walls, forming cystic lesions that generally vary from 0.5 to 2.0.[1] It has been described that its incidence is around 0.03% in the general population, occurring in ages from 2 to 80 years without distinction of gender or other variable in particular, being its course of approximately 6 months.[2] Its origin is multifactorial, classified as primary or idiopathic in 15% and secondary in 85%, the latter is due to endoscopic processes, immunological alterations, disruption of the intestinal mucosa and multiple intra-abdominal pathologies.[3] This entity is generally asymptomatic, although if it is symptomatic, it manifests itself through an unspecific clinic of abdominal pain and bloating, vomiting, weight loss, and diarrhea with or without blood, depending on its location.[4] Radiologically, when performing an X-ray or tomography of the abdomen, pneumoperitoneum can be evidenced by rupture of cysts at the level of the intestinal wall.[5]
We present the case of a patient who presented to the emergency department for sudden abdominal pain, diffuse signs of peritoneal irritation, and a history of previous laparotomy for perforated gastric ulcer as the only relevant history. An X-ray was performed showing pneumoperitoneum, with subsequent histopathological diagnosis of cystic intestinal pneumatosis. For the publication of this manuscript, the informed consent of the patient was obtained, as well as the approval of the institutional ethics committee.
Case Report
A 45-year-old male patient with a history of previous laparotomy for perforated gastric ulcer that required a Graham patch a year ago, who consulted for clinical signs of 3 days of evolution of abdominal pain in the epigastrium (colic-type pain), intensity: 7/10, associated with vomiting and weight loss, there is no fever or diarrhea. Physical examination shows a slightly distended, soft and depressible abdomen, with generalized pain on palpation and signs of peritoneal irritation. X-ray is the abdomen and chest is requested, in which pneumoperitoneum is evident [Figure 1]. Based on the findings, a diagnostic impression of hollow viscera perforation is performed, requiring urgent surgical management.
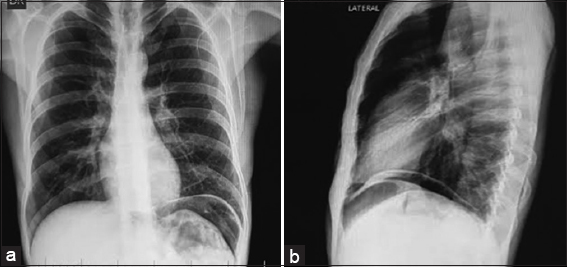
- (a) X-ray of the abdomen in a posteroanterior view showing the left pneumoperitoneum and low insertion of the left diaphragmatic abutment, (b) X-ray of the abdomen with a lateral view showing pneumoperitoneum
The patient is taken to emergency surgery, undergoing an exploratory laparotomy where multiple cystic lesions with gas content are found at the subserosa level, involving the small intestine, transverse colon and greater omentum [Figure 2], so it was decided to perform resection and latero-lateral anastomosis with mechanical suture at the jejunum level, which had a transition zone and a greater cystic compromise. The rest of the injuries were left intact for later medical management. The surgical pieces were sent to histopathology for evaluation [Figure 3].
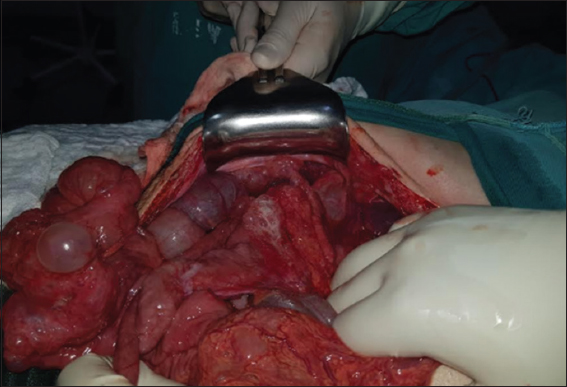
- Cystic lesions in the small intestine, mainly in the jejunum with a stenotic area 110 cm from the Treitz ligament, another cystic zone 160 cm from the same ligament without light compromise, cystic lesions in the transverse colon subserosa without light compromise and greater omentum with multiple cystic lesions without any repercussion
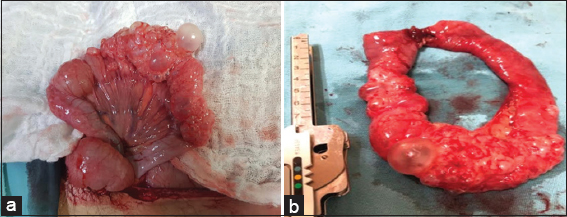
- (a) Small intestine (jejunum) 110 cm from Treitz ligament, 8 cm stricture area composed of multiple cystic lesions and local thickening, (b) 15 cm surgical jejunal piece that compromises the stenotic area due to cysts with closed edges by mechanical suturing
After surgery, the patient is taken to the general hospitalization room, where he received medical management with antibiotic and oxygen. During the post-operative ileus that resolved with potassium replacement, mobilization out of bed, respiratory incentive, and chewing gum. He was finally discharged with warning signs.
Fifteen days after discharge, he attended to the outpatient basis where a healthy wound is observed, without abdominal pain or any other added symptoms. It carries the histopathological result, which reports intestinal cystic pneumatosis and chronic granulomatous inflammation secondary to an undetermined foreign body.
Discussion
Although the pathogenesis of intestinal cystic pneumatosis is unknown, several hypotheses have been put forward that could explain its presentation. These include local inflammatory processes, physical damage to the intestinal mucosa, nutritional imbalance and dysbacteriosis, gastrointestinal dysmotility, and immune dysfunction.[4,6] This can occur in any location at the level of the gastrointestinal tract, from the esophagus to the rectum, with the large intestine being the places of greatest incidence (46%), followed by the small (27%), mixed involvement (7%), and stomach (5%). Furthermore, it has been found in some cases, the presence of gas in the portal vein simultaneously, which considerably increases the complication and mortality rate.[7,8]
Although it has been described in the literature that generalized abdominal pain and diarrhea are the most frequent (53%),[9] it is evident that its clinical presentation is variable, being able to present it diffusely, as in this clinical case, in which the cardinal symptom was the epigastric pain associated with vomiting, which could simulate some localized condition at the stomach level. Approximately 20% of cases present complications such as intestinal obstruction, intussusception, volvulus, hemorrhage, and perforation, thus leading to necrosis, ad considerably worsening the overall prognosis.[2,9]
Secondary cystic pneumatosis of the intestine can be caused by different pathologies of the digestive system, such as peptic ulcer, pyloric stenosis, Crohn’s disease, appendicitis, or necrotizing enterocolitis; Although cases have also been described related to lung diseases (chronic obstructive pulmonary disease or cystic fibrosis), autoimmune (dermatomyositis or scleroderma), infectious (Clostridium difficile, HIV), trauma, or as an adverse effect of pharmacotherapy with α-glucosidase inhibitors.[8,10] The interesting thing about this case is that the only relevant data that could explain the pathogenesis of cystic intestinal pneumatosis is laparotomy for gastric ulcer perforation carried out a previous year in addition to the time of evolution of the disease (>6 months), becoming a new factor to keep in mind in the evaluation of acute abdomen of this type of patients. Although it is documented in the literature that endoscopic processes could generate this pathology, the patient did not have these antecedents, adding that he did not have underlying pathologies either.
X-ray of the abdomen and chest showing pneumoperitoneum, and observation of circumferential (cystic) collections of air adjacent to the lumen of the intestine running in parallel with the wall of the intestine, or linear collections without air contrast or air levels fluids during CAT scan, they perform the diagnosis.[10-12]
Those asymptomatic patients benefit more from conservative treatment, while symptomatic patients due to intestinal decompression, parenteral nutrition, and hydroelectrolytic replacement, with surgery being limited to those complicated cases in which intestinal obstruction, visceral perforation, or the presence of a neoplasm are found.[8,9,11,12] A positive response has been reported with metronidazole and hyperbaric therapy; however, the quality of this evidence is unfortunately low.[13]
Authors’ Declaration Statements
Ethics approval and consent to participate
Institutional approval was obtained for the publication of this case.
Consent for publication
Informed consent was obtained for the publication of this case.
Availability of data and material
The data used in this study are available and will be provided by the corresponding author on a reasonable request.
Competing Interests
None declared.
Funding Statement
None.
Authors’ Contributions
All authors contributed equally to this manuscript.
ORCID link of the submitting author: https://orcid.org/0000-0002-1960-7334
Conclusion
Intestinal cystic pneumatosis is a rare presentation entity, adjacent complications can occur that worsen the prognosis of the disease and increase the probability of dying, so it is essential to diagnose it and monitor it. The clinical history is of vital importance, since it allows identifying factors that contributed to the pathogenesis of the disease, as well as in this case, in which a gastric ulcer laparotomy could predispose its presentation.
References
- Asymptomatic pneumoperitoneum in pneumatosis coli:A misleading operative indication. Int J Surg Case Rep. 2020;69:92-5.
- [Google Scholar]
- Pneumatosis cystoides intestinalis:A case report and literature review. BMC Gastroenterol. 2019;19:176.
- [Google Scholar]
- Pneumatosis cystoides intestinalis:Case report and review of literature. Clin J Gastroenterol. 2020;13:31-6.
- [Google Scholar]
- Pneumatosis cystoides intestinalis:Six case reports and a review of the literature. BMC Gastroenterol. 2018;18:100.
- [Google Scholar]
- Intestinal pneumatosis. An uncommon cause of acute abdomen. Gastroenterol Hepatol. 2019;42:557-8.
- [Google Scholar]
- Pneumatosis cystoides intestinalis:Not uncommon cause of free air in acute abdomen. J Visc Surg. 2019;156:177-8.
- [Google Scholar]
- A systematic analysis of pneumatosis cystoids intestinalis. World J Gastroenterol. 2013;19:4973-8.
- [Google Scholar]
- Pneumomediastinum and pneumoperitoneum secondary to cystic intestinal pneumatosis after percutaneous endoscopic gastrostomy placement. Cir Esp. 2017;95:474-84.
- [Google Scholar]
- Segmental pneumatosis cystoides coli:Computed tomography-facilitated diagnosis. Rev Esp Enferm Dig. 2016;108:510-3.
- [Google Scholar]
- Pneumatosis cystoides intestinalis:An unusual cause of intestinal ischemia and pneumoperitoneum. Int Surg. 2015;100:221-4.
- [Google Scholar]
- Characteristics of pneumoperitoneum due to intestinal cystic pneumatosis. Gastroenterol Hepatol. 2015;38:283-3.
- [Google Scholar]







