Translate this page into:
Antimicrobial screening to molecular docking of newly synthesized ferrocenyl-substituted pyrazole
Address for correspondence: Dr. Manoj Kumar, Department of Chemistry, Maharishi Markandeshwar University, Sadopur, Ambala, India. E-mail: manojraju27@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Qassim Uninversity and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective:
Microbial diseases are snowballing at an alarming proportion. Therefore, the intent of this study was to inspect the antimicrobial action of ferrocenyl-substituted pyrazole against various human pathogenic Gram-positive, Gram-negative, and fungal microbial strains. Pyrazoles have been recognized for over a century as a significant and bioactive class of heterocyclic compounds. The association of pyrazoles with a ferrocene moiety may give new class of compounds. The present study was designed to synthesize biological active ferrocenyl-substituted pyrazole through a novel route.
Methods:
The anhydride of ferrocenyl-substituted pyrazole, namely, (S)-(3-(3-(carboxyamino)-3H-pyrazol-4-yl)cyclopenta-1,3-dien-1-yl)(cyclopenta-1,3-dien-1-yl)iron was synthesized using expansion cyclocondensation. FTIR, NMR, and GC-MS were performed to analyze the structure of the synthesized ferrocenyl-substituted pyrazole. Antimicrobial, DNA photo-cleaving, and anti-angiogenic activities of ferrocenyl-substituted compounds were studied.
Results:
Anhydride of (S)-(3-(3-(carboxyamino)-3H-pyrazol-4-yl)cyclopenta-1,3-dien-1-yl)(cyclopenta-1,3-dien-1-yl)iron obtained with yield of 87%. Spectral analysis confirmed the formation of anhydride. The synthesized compound was found to be biological active in the range of 85–95 μg/ml.
Conclusion:
This study described the novel method for the synthesis of biologically active anhydride of ferrocenyl-substituted pyrazole. The study demonstrations that synthesized ferrocenyl-substituted pyrazole in today’s situation is the encouraging antimicrobial mediator against the human pathogens. In addition, it may open new doors to initiate research against drug resistance bacteria with possible biomedical applications.
Keywords
Biological screening
expansion cyclocondensation
ferrocenyl-substituted pyrazoles
molecular docking
spectroscopic investigations
Introduction
Presently, ferrocenyl-substituted pyrazole compounds are being discovered exponentially due to their remarkable use in biomedical therapeutics.[1] Ferrocene own great antimicrobial applicability with lower toxicity rate to human cells in numerous studies.[2] These days microbial infections are emerging with antibiotics resistance and signify a serious problematic to the human health. According to a report, a large quantity of deaths are triggered due to drug-resistant microbial infection.[3] The chemistry of heterocyclic compounds is a fascinating area of research having wider horizon. Synthesis of heterocyclic compounds is interesting and challenging due to its theoretical implications and diversity of its synthetic procedures. These compounds have great industrial and biological significance.[4,5] Nowadays, therapeutic scientific experts are utilizing ferrocene into their medication plan system due to its uniqueness toward biological systems. Ferrocene is known to have excellent therapeutic potential due to its controllable toxicity, great redox properties, and steadiness.[6-8] Moreover, many ferrocenyl compounds possessing antitumor, antimalarial, and DNA cleavage properties have been reported.[9-14] Synthesis of potent tranquilizer[12-14] and anti-malignant agents[15-18] using ferrocene has also been reported. However, pyrazole having ferrocenyl substituent has been found to have enhanced anti-cancer properties.[19]
The literature also revealed some ferrocenyl-substituted pyrazoles with antimicrobial[20] and DNA cleavage[21] properties. The current study discloses synthesis of a novel anhydride of ferrocenyl-substituted pyrazole and its biological potential supported with molecular docking interactions. In the present study, a broad spectrum of bioactivities including antimicrobial, DNA photo-cleavage, and anti-angiogenesis of synthesized compounds was evaluated. Antimicrobial and DNA cleavage studies of ferrocenyl-substituted pyrazoles revealed their promising role to control infectious diseases. On the other hand, angiogenesis is an essential process to supply nutrient and oxygen for the proliferation of tumor. Therefore, inhibition of angiogenesis can be considered a potential drug target in cancer therapy.[22-26]
In the present study, synthesis, characterization, and antimicrobial applications of ferrocenyl-substituted pyrazoles were carried out to open new avenue for scientific community against pathogenic microbial species. In addition, tested compounds may provide an alternative research to investigate their effect against drug resistant pathogenic microbial strains.
Methods
1H NMR spectra were recorded on BrukerAvance II NMR Spectrometer at 500MHz utilizing TMS as interior standard. IR spectra were made using Perkin Elmer – Spectrum RX-IFTIR instrument tests were set up as KBr pellets. Mass spectrum (m/z) was made using Gas Chromatography–Mass Spectrometry through SAIF LAB Chandigarh. Each and every engineered mixes used of analytical evaluation secured from LOBA, MERK, and OTTO. Solvents were refined before use. All solvents were of A.R. grade and utilized without any more purification. Products were purified by preparative thin-layer chromatography on silica gel (Merck, Kieselgel60HF254) using the mixtures CH2Cl2/EtOAc or CH2Cl2/MeOH. Melting points were determined in open capillaries.
Experimental
Synthesis of anhydride of (S)-(3-(3-(carboxyamino)-3H-pyrazol-4-yl)cyclopenta-1,3-dien-1-yl)(cyclopenta-1,3-dien-1-yl)iron
For the synthesis of anhydride of (S)-(3-(3-(carboxyamino)-3H-pyrazol-4-yl)cyclopenta-1,3-dien-1-yl)(cyclopenta-1,3-dien-1-yl)iron, Friedel Craft acylation-like conditions were applied. A mixture of acetic acid (0.16 mmol) and hydrochloric acid (0.13 mmol) was treated with (0.005 mol) ferrocene along with Lewis acid AlCl3 refluxing [Scheme 1] with continuous mixing at 100°C for 1 h utilizing magnetic stirrer with hot plate yielding acetoferrocene.
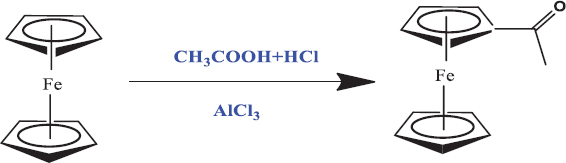
- Synthesis of acetoferrocene
Dicyandiamide was included little parcels to acetoferrocene followed by refluxing with blending utilizing magnetic stirrer with hot plate at 100°C for 120 min. The yellow product, a dimer of (S)-(3-(3-(carboxyamino)-3H-pyrazol-4-yl)cyclopenta-1,3-dien-1-yl)(cyclopenta-1,3-dien-1-yl)iron, obtained after treatment of mixture with iodine crystal (0.001 mol and 0.01 mol) and sodium bicarbonate through refluxing at 100°C for 3 h followed by quenching the reaction mixture in ice. Anhydride of (S)-(3-(3-(carboxyamino)-3H-pyrazol-4-yl)cyclopenta-1,3-dien-1-yl)(cyclopenta-1,3-dien-1-yl)iron was acquired as mustard yellow colored solid [Scheme 2] recrystallized using ethanol in small lots for 2 times. The purity of the compound was checked by thin-layer chromatography using chloroform and petroleum ether in the ratio 9:1.
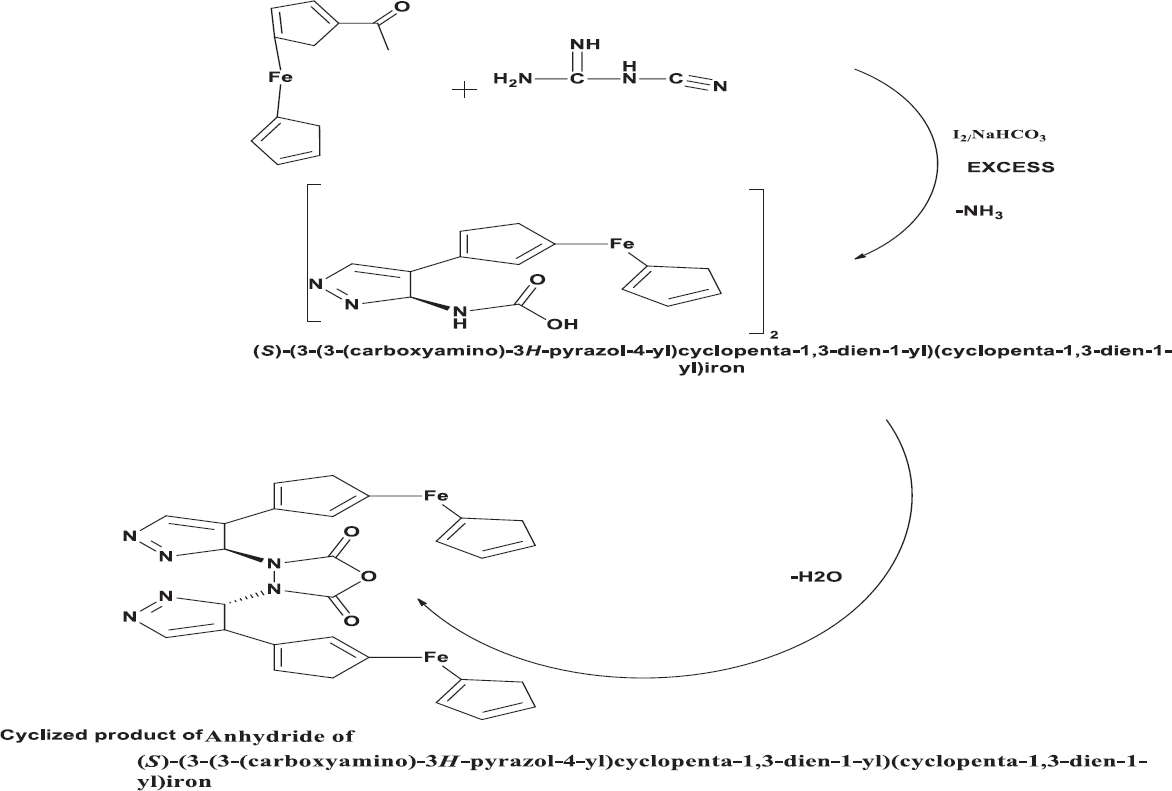
- Synthesis of anhydride of (S)-(3-(3-(carboxyamino)-3H-pyrazol-4-yl) cyclopenta-1,3-dien-1-yl)(cyclopenta-1,3-dien-1-yl)iron
Biological activities
Antimicrobial activity assay
In vitro antimicrobial assay has been evaluated for ferrocenyl-substituted pyrazole against pathogenic lines of bacteria (Staphylococcus aureus and Klebsiella pneumoniae) and fungi (Aspergillus niger and Trichophyton rubrum) using disk plate diffusion assay. Stock solution was prepared in ethanol. Nutrient broth and potato dextrose were used as culture medium for the growth of bacteria and fungi, respectively. Agar-Agar was used as solidifying agent. The various concentrations of ferrocenyl-substituted pyrazole (100, 150, 200, and 250 ppm) were loaded on 5 mm sterilized clear out paper discs and positioned on agar plates accompanied with the aid of incubation at 30°C for 24 h and 72 h to assess the effect of the compound on bacterial and fungal growth, respectively. Neomycin and Fluconazole have been used as trendy antimicrobial agents for bacterial and fungal study, respectively.
DNA photo-cleavage assay
DNA cleavage activity of the synthesized ferrocenyl-substituted pyrazoles had been studied on the supercoiled pUC19 plasmid DNA using agarose gel electrophoresis. The total extent of reaction mixture turned into 10 μl containing 0.5 μg of plasmid DNA in TE (Tris 10 mM, EDTA 0.01 mM, pH 8.0) buffer with different concentrations of synthesized ferrocenyl-substituted pyrazole. The centrifuge tube carrying the reaction aggregate has been placed on the floor of a trans-illuminator (8000 mW/cm) without delay, at 360 nm for 30 min. After irradiation, samples had been similarly incubated at 37°C for 1 h. Irradiated samples have been mixed with a 6X loading dye containing 0.25% bromophenol blue and 30% glycerol. The samples were then analyzed through electrophoresis on a 0.8% agarose horizontal slab gel in Tris-Acetate EDTA buffer (40 mMTris, 20 mM acetic acid, and 1 mMEDTA, pH: 8.0) with assessment to untreated plasmid DNA as a control. Gel turned into stained with ethidium bromide (1 mg/ml) and photographed below UV light.
Chorioallantoic membrane (CAM) assay
Anti-angiogenic activity has been evaluated using ex vivo CAM assay.[23] The fertilized chicken eggs were collected, cleaned with 70% ethanol to avoid any infection, and kept in a humidified (70%) chamber at 37°C. After 48 h, 1 ml of albumin was taken out with a syringe from the lower side of the eggs and the pierced holes were sealed with a sterilized laboratory tape. After 72h of incubation, a small window was opened by removing the eggshell at the blunt end. On confirmation of normal and viable development of the embryo, various concentrations of synthesized ligand (0, 1, and 10 μg) were loaded on 5 mm sterilized filter disks and placed over the surface of extra embryonic membrane, that is, CAM. The windows were sealed with sterilized laboratory tape to prevent external environmental contact and eggs were incubate for 48h. After the treatment, anti-angiogenic effect of ligand was manually counted in terms of branch points over CAM and calculated the percent inhibition as follows:
% Inhibition = Data of control–Data of treated × 100/Data of control
Molecular docking study
Ligand structure preparation
The 2D chemical structures of the ferrocenyl-substituted pyrazole were drawn using ChemDraw 18.0. The geometric and electronic structures of the ligands were optimized using Gaussian 03 software with Hartree-Fock (HF) theory at the B3LYP/3-21G level by employing ab-initio quantum mechanical calculations based on Density Functional Theory (DFT). Viewing of the ferrocenyl-substituted pyrazole was done using Chem3D 18.0 and saved in Mol2 file format. Mol2 file was then converted to PDBQT (Protein Data Bank, Partial Charge (Q), and Atom Type (T)) file format using AutoDock Tools version 1.5.6 by adding Gasteiger charges, merging non-polar hydrogens, detecting aromatic carbons, and rotatable bonds and setting the TORSDOF values.[27]
Protein structure preparation
Bacterial DNA Gyrase enzyme (PDB ID: 6QX2) was obtained in pdb format from protein data bank (PDB; https://www.rcsb.org/structure/6QX2). Protein structure was viewed in BIOVIA Discovery Studio Visualizer, and hetero-atoms, water molecules, ligand groups, and nucleic acid groups were removed. Polar hydrogens were added and non-polar hydrogens were merged using AutoDock Tools version 1.5.6. Missing atoms were checked and repaired before applying Kollman charges.[27,28] The macromolecule was saved in PDBQT file format merged using AutoDock Tools version 1.5.6 for the further application.[27]
Molecular docking
Molecular docking study of the ligands against Bacterial DNA Gyrase enzyme (PDB ID: 6QX2) was performed using AutoDock Vina suite.[29] The binding site coordinates for active Site AD5 (chain s and t): center_x = −68.31; center_y = 96.49; and center_z = −80.28, were assigned using Discovery Studio Visualizer. The grid box employed for specifying the search space was set at 80 × 80 × 80 Å using previously determined coordinates with a default grid point spacing of 0.375 Å. The most convenient conformations were identified based on lowest docked energy (kcal/mol). The further analysis was performed using Discovery Studio Visualizer.[30-32]
Results
Spectral
IR (KBr, in cm-1): 472.20 (Cp-Fe-Cp Str.), 786.44 (C-H Str.), 1105.08 (C-N Str.), 1694.50 (C=N Str.), 1775.53 (C=O Str.) for acid anhydride), 1408.26 (-N-N- Str).
1H NMR: (400MHz, DMSO, in ppm): δ:6.50 (m, 3H, Cp), δ:2.90(s, 1H, cp), δ: 4.30 (s, 1H, CH-N), δ: 5.25 (s, 1H, Py-H).
GC-MS (m/z): 602(M+), 394, 292, 278 (Base peak). Elemental analysis Calcd. (found) % for C28H2256Fe2N6O3: C 55.84 (55.85); H 3.68 (3.69); Fe 18.55 (18.53); N 13.96 (13.97); O 7.97 (7.96). Calculated molecular mass of anhydride of (S)-(3-(3-(carboxyamino)-3H-pyrazol-4-yl) cyclopenta-1,3-dien-1-yl) (cyclopenta-1,3-dien-1-yl) iron is 602 and molecular ion peak(M+) observed at m/z= 601.24 in mass spectra. Fragmentation peaks for different fragments were observed. The base peak has been found at m/z=278 produced by removal of C14H10FeN3O3 (m/z=324) [Figure 1]. The peak at m/z=292 has been labeled for the next fragment which, in turn, produced by removal of C14H12FeN3O2- ion having m/z=310. Similarly, another peak observed at m/z=394 attributed to fragment produced by removal of Cp2Fe2+C2H (m/z=208). These observations are in line with previously published reports.[30-34]
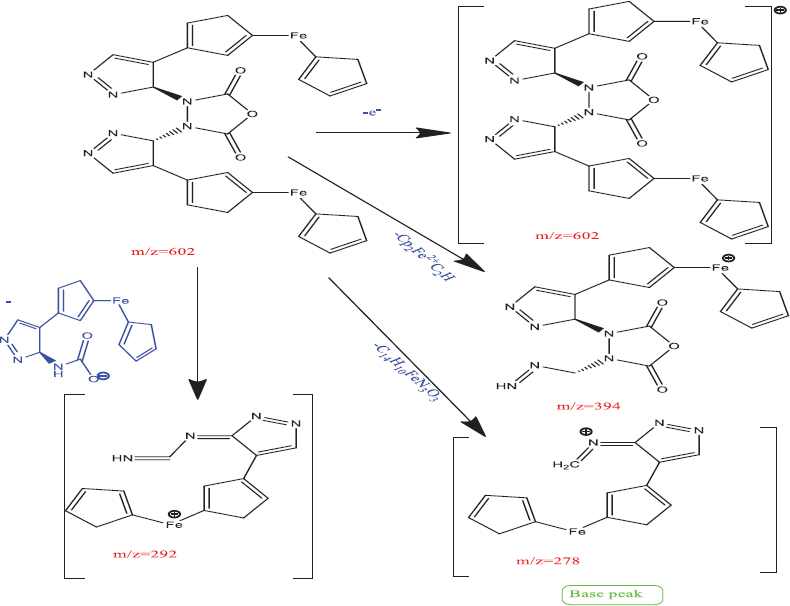
- Proposed fragmentation pattern
Proposed mechanism of synthesis of anhydride of (S)-(3-(3-(carboxyamino)-3H-pyrazol-4-yl) cyclopenta-1, 3-dien-1-yl) (cyclopenta-1, 3-dien-1-yl) iron
Mechanism for the reaction of synthesis of anhydride of (S)-(3-(3-(carboxyamino)-3H-pyrazol-4-yl) cyclopenta-1, 3-dien-1-yl) (cyclopenta-1, 3-dien-1-yl) iron as stated in Scheme 2 follows three steps mechanism.
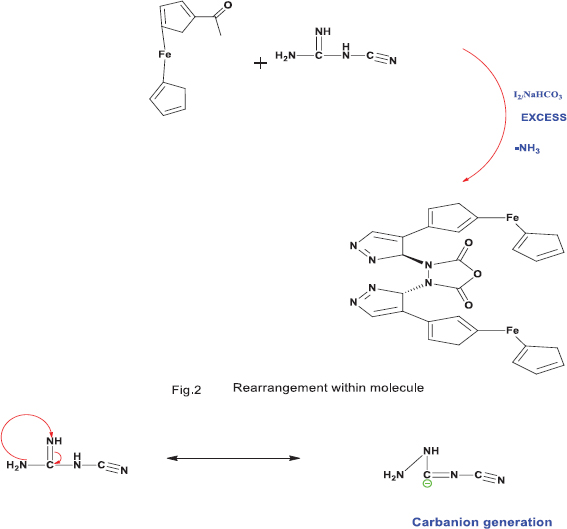
Step 1: Rearrangement
In first step, rearrangement [Figure 2] occurred within molecule of dicyanodiamide which resulted in the formation of a carbanion as active site for the reaction to attack.
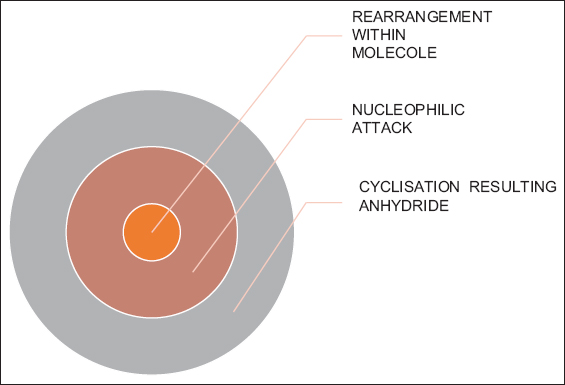
- Proposed sequence of chemical reactions
Step 2: Nucleophile attack
In second step [Figure 3], generated nucleophile attacked on acetoferrocene which resulted in the formation of (4-(5-cyanamido-3H-pyrazol-4-yl) cyclopenta-1, 3-dien-1-yl) (cyclopenta-1, 3-dien-1-yl) iron by the removal of water molecule and rearrangement of hydrogen. After that, Iodine in presence of sodium bicarbonate oxidized the (4-(5-cyanamido-3H-pyrazol-4-yl)cyclopenta-1,3-dien-1-yl)(cyclopenta-1,3-dien-1-yl)iron which resulted in the formation of dimer of (S)-(3-(3-(carboxyamino)-3H-pyrazol-4-yl)cyclopenta-1,3-dien-1-yl)(cyclopenta-1,3-dien-1-yl)iron.[33-39]
- Nucleophilic attack
Step 3: Cyclization of anhydride product
The ability of aromatic carboxylic acids to form different dimers and multimers is due to presence of hydrogen-bonding donor and acceptor cites. Due to it, they are able to form cyclized anhydrides by escaping a single molecule of water. The tendency to form cyclic rings either five membered or six membered depends on the structure of carboxylic acids as completely known in the formation of lactones and cryptands. Acids in which there are two carboxyl groups separated by a chain of more than five carbon atoms (n > 5) for the most part have unexceptional properties, and the carboxyl groups behave more or less independently of one another. However, when the carboxyl groups come closer the possibilities for interaction increases. The dicarboxylic acid goes to self-condensation at room temperature easily with acquiring thermal heat only. Thus, cyclic dicarboxylic anhydride product was the final product [Figure 4] obtained.[40]
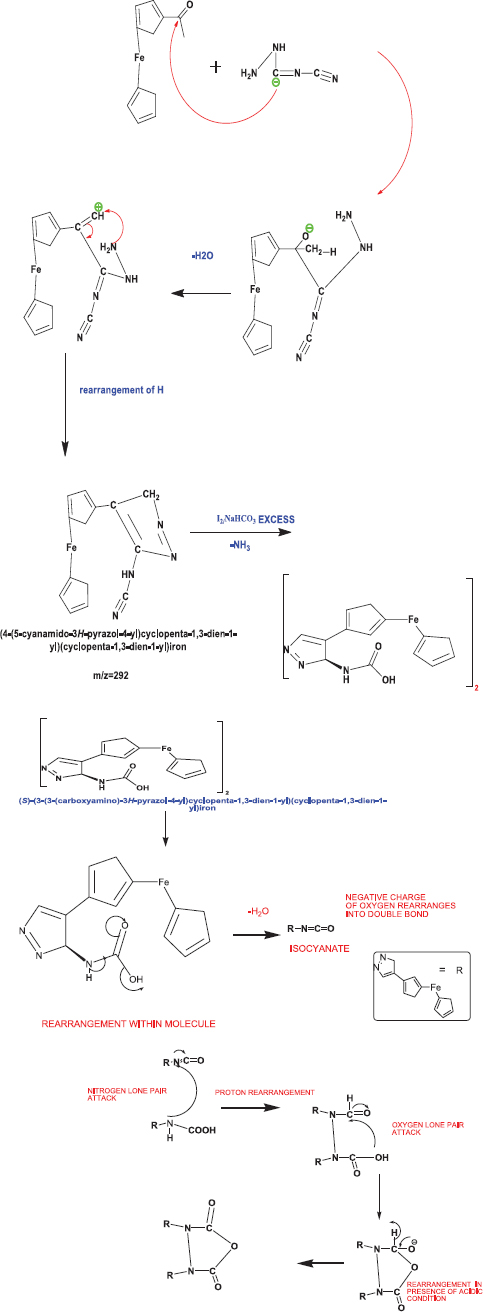
- Cyclization of anhydride product
Antimicrobial activity
The newly designed compound was elevated for their antimicrobial potential against S. aureus and K. pneumoniae bacterial stains, and A. niger and Trichophyton rubrum fungal strains, taking fluconazole and neomycin as standard positive control, respectively. The compound was found potent against the tested microbial strains. The inhibitory action of the compound was measured in terms of zone of inhibition [Tables 1 and 2, Figures 5-7] and MIC values were found in the range of 85–95 mg/ml.
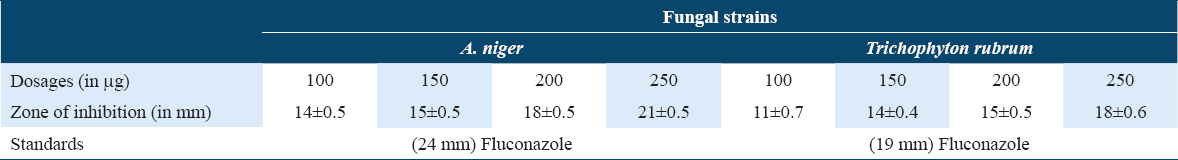
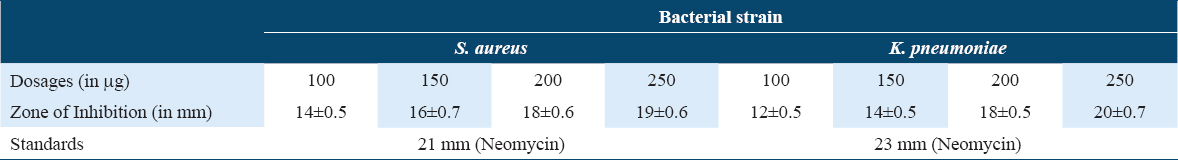
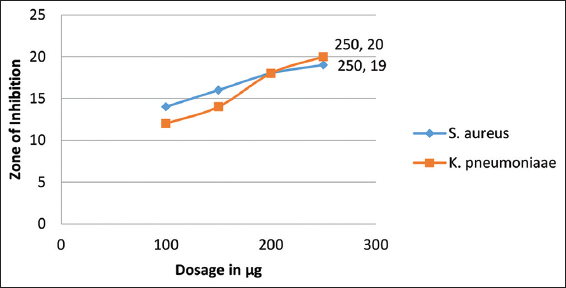
- Graphical representation of Antifungal effects of cyclopenta-2,4-dien-1-yl(2-(1-phenyl-1H-pyrazol-4-yl)cyclopenta-2,4-dien-1-yl)iron(III)
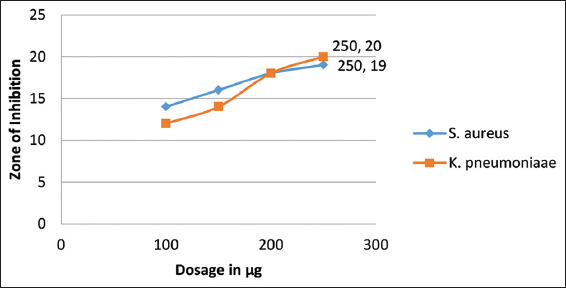
- Graphical representation of antibacterial effects of anhydride of cyclopenta-2,4-dien-1-yl(2-(1-phenyl-1H-pyrazol-4-yl)cyclopenta-2,4-dien-1-yl)iron(III)
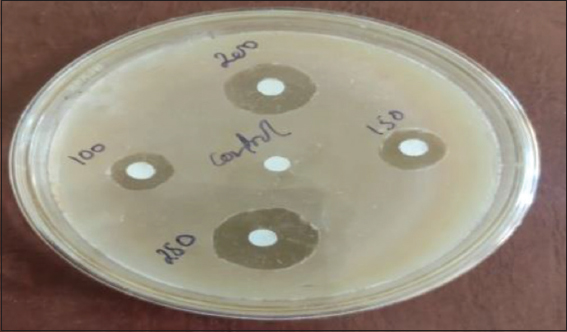
- Antimicrobial assay
DNA photo-cleavage activity
The DNA biding potential of the synthesized compound was carried out using super coiled plasma DNA by gel electrophoresis. The activity results revealed that the tested compound has potential of cleaving DNA helix.
Anti-angiogenic activity
The anti-angiogenic potential of the synthesized compound was carried out at various concentrations (0, 20, 40, 60, and 80 μg/ml). A dose dependent anti-angiogenic effect was observed, maximum inhibition (94%) of blood vessels was observed at a concentration of 80 μg/ml. The anti-angiogenic results of the compound are displayed in Figure 8 in the form of bar diagram.
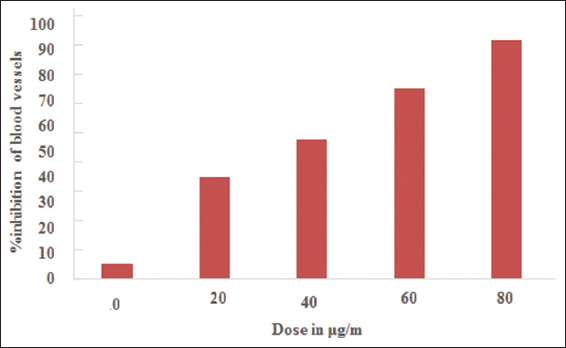
- Anti-angiogenic activity
Molecular docking study
The docking studies were carried on bacterial DNA gyrase enzyme (PDB ID: 6QX2) using Autodock vina tools. The docking analysis showed that the synthesized ferrocenyl-substituted pyrazole interacts with 3 amino acid residues that are located in the active site of DNA gyrase enzyme by −9.6 kcal/mol binding energy. Results also revealed that one conventional H bond and pi-alkyl interactions are formed with alanine amino acid located at 588 positions [Figure 9a and b]. Besides that, one carbon hydrogen and pi sigma bonds were also observed with asparagine and leucine amino acids located at 109 and 298 positions, respectively.
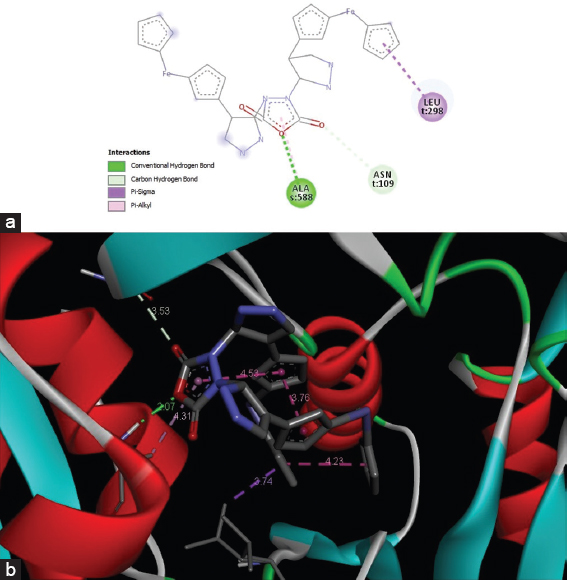
- (a and b) 2D and 3D interactions of the Synthesized Ferrocenyl-substituted pyrazole with DNA gyrase protein (PDB ID 6QX2)
Discussion
Investigations of anti-infectious agents are the need of an hour. Emergence rate of infectious diseases are found to be increasing in recent years. According to the WHO reports, novel antibiotics that are targeting pathogenic microbes may not only help to reduce deaths due to infections but also lower down the threat of drug resistance microbes.
IR: The Eclipsed IR spectra of Ferrocene shows signal in region (400–500 cm-1) for ten localized Fe-C bonds found at 472.20 cm-1. Aromatic C-H stretching out of plane bending lies in between (900–690 cm-1) observed at 786.44 cm-1, C-N stretching usually observed in region (1350-1000 cm-1) came at 1105.08 cm-1, C=N stretching lies in region (1690–1640) found at 1694.50 cm-1,[41,42] and band found at 1775.53cm-1 attributed to C=O stretching of carboxylic acid anhydride. C-O stretch found at 813.6 cm-1–999.69 cm-1.[43,44] The band observed in region 1408.28 cm-1 attributed to (-N-N-) stretching.[45] 1H NMR: The 1H NMR spectral study of prepared compound reveals the presence of cyclopentadienyl protons, these were observed as a multiplet at 6.50 ppm and singlet at 2.90 ppm, and these values are in agreement with previous findings. The chemical shift value of >CH-N lies at 4.30ppm due to the deshielding of the proton by a nitrogen atom. Pyrazole proton was found as a singlet at 5.25 and 5.40 ppm region.[46-48] The antimicrobial effect of synthesized ferrocenyl-substituted pyrazole toward harmful human pathogenic microorganisms has been studied. Neomycin and Fluconazole were used as standard positive control. The compounds were found very potent against tested bacteria and fungi. The antimicrobial action of ferrocenyl-substituted pyrazoles was measured in term of zone of inhibition [Tables 1 and 2, Figures 5-7] and MIC values were found to be in the range of 85–95 mg/ml. Previous evidences have supported that pyrazole based derivatives possesses broad spectrum of antimicrobial effects. Bekhit and Abdel-Aziem investigated the antimicrobial effect of novel pyrazole[49] derivatives against Gram-positive and Gram-negative bacterial as well as pathogenic fungi. Recently, Mandal et al. and Munyaneza et al. have also reported the promising antimicrobial activities of metal complexes of pyrazole.[50,51] Previously proposed mechanisms from the results of fluorescence and transmission electron microscopy (TEM) have suggested that pyrazole-derived compounds (such as pyrazole-arginine) possesses anti-bacterial effect possibly through disruption of the cell membrane and leakage of cytosolic content that finally leads to cell lysis.[52] In addition, pyrazole analogs acted as inhibitors of multidrug efflux pump which is known to be an important mechanism for microbial resistance toward antibiotics.[53]
In recent years, heterocyclic compounds have been widely studied for their DNA cleavage properties.[54] The photo-induced DNA cleavage has been carried out using super coiled plasmid DNA by gel electrophoresis. The result of DNA photo-cleavage investigations indicated that synthesized compound has good potential of cleaving DNA helix. Previously it has been reported that super-coiled plasmid DNA migrate relatively faster than nicked DNA during electrophoresis.[55] Super coiled form is relaxed to produce an open circular form which is slower moving if one strand of Super Coiled form is cleaved. If both strands of super coiled form are cleaved, a nicked form is generated which migrate in between the super coiled and open circular form.[56,57] The conversion of form I to form II was observed with the treatment of synthesized ferrocenyl-substituted pyrazole in comparison to untreated plasmid DNA which indicates its DNA-cleavage potential. Previously, Han et al. have reported the role of pyrazole-based ligands as promising DNA cleaving action[58] through hydrolytic mechanisms. More recently, Feng et al. have investigated DNA binding capabilities of pyrazole derivatives and suggested their promising role in designing the chemotherapeutic drugs.[50] Furthermore, anti-angiogenic activity of synthesized ferrocenyl-substituted pyrazole ligand was investigated at various concentrations (0, 20, 40, 60, and 80 μg/ml) [Figure 8]. Dose dependent anti-angiogenic effect of synthesized ligand was observed. The maximum of 94% blood vessel inhibition effect was seen at concentration of 80 μg/ml. It has been found that developing blood vessels are essential for the growth of tumor.[59-61] The previous studies have suggested the promising role of anti-angiogenic agents to prevent cancer cell growth.[62-64] Therefore, the results of present study could be considered in near future to design novel anti-cancer drugs. Molecular docking studies were performed against bacterial DNA gyrase enzyme, an ATP dependent, unique type II topoisomerase.[65] Due to the crucial roles of DNA gyrase enzyme in DNA synthesis, it is considered as the most important target for pyrazole derivatives and its inhibitors significantly show the killing effect on the microorganisms by blocking DNA synthesis.[66] The molecular docking studies put forward a better insight for understanding the binding mechanisms and molecular interactions between drug candidates and target proteins.[67] In accordance with the anti-microbial activity studies, the docking studies showed that the synthesized ferrocenyl-substituted pyrazole interacts with 3 amino acid residues that are located in the active site of DNA gyrase enzyme by −9.6 kcal/mol binding energy. Results revealed that one conventional H bond and pi-alkyl interactions are formed with alanine amino acid located at 588 positions [Figure 9]. Besides that, one carbon hydrogen and pi sigma bonds were also observed with asparagine and leucineaminoacids located at 109 and 298 positions, respectively. As per literature records, it has been found that binding energy of −9.6 kcal/mol is a very significant value.
Conclusion
In the present examination, ferrocenyl subbed pyrazole has been planned and integrated by methods for novel course and portrayed. The IR, 1H NMR, and mass information affirmed the proposed structure and geometry. It is evident from the affirmations that such holding is conceivable. The antimicrobial examination indicated that both ferrocenyl subbed pyrazoles are solid antimicrobial specialists, and further, DNA photo-cleavage examination affirmed that the two mixes have great DNA cleavage potential. The delayed consequences of the test examination of ferrocenyl subbed pyrazoles give new technique toward new assistant topic and further new research. The results of molecular docking study are in agreement with biological activities of synthesized pyrazole.
Authors’ Declaration Statements
Ethics approval and consent to participate
Not applicable.
Availability of data and material
The data used in this study are available and will be provided by the corresponding author on a reasonable request.
Competing interests
There is no conflicts of interest many of the authors concerning the publishing this manuscript.
Funding statement
None.
Authors’ Contributions
All authors contributed equally.
Acknowledgment
We would like to thank Department of Chemistry and authorities of Maharishi Markendeshwar University, Sadopur-Ambala for providing all necessary support to pursue the research. We are also thankful to SAIF, Punjab University, Chandigarh for providing necessary instrumental support.
ORCID link of the submitting author: https://orcid.org/0000-0002-2062-9602
References
- Novel ferrocenyl pyrazoles inhibit breast cancer cell viability via induction of apoptosis and inhibition of PI3K/Akt and ERK1/2 signaling. Chemicoboil Interact. 2017;263:28-35.
- [Google Scholar]
- Design and synthesis of ferrocene?tethered pyrazolines and pyrazoles:Photophysical studies, protein?binding behavior with bovine serum albumin, and antiproliferative activity against MDA?MB?231 triple negative breast cancer cells. Appl Organ Chem. 2021;35:e6248.
- [Google Scholar]
- Novel ferrocenyl linked pyrazoline analogs as potent antiamoebic agents. J Heterocycl Chem. 2016;53:473-8.
- [Google Scholar]
- The Chemistry of Heterocyclic Compounds:Pyrazoles, Pyrazolines, Pyrazolidines, Indazoles and Condensed Rings. New York: Interscience, John Wiley &Sons; 1967.
- The Chemistry of the Heterocyclic Compounds. New York: Academic Press; 1961.
- Replacement of aromatic or heteroaromatic groups in nonsteroidal antiinflammatory agents with the ferrocene group. J Med Chem. 1983;26:226-9.
- [Google Scholar]
- Thirteen-week, repeated inhalation exposure of F344/N rats and B6C3F1 mice to ferrocene. Toxicol Sci. 1993;21:127-39.
- [Google Scholar]
- Hemilability of hybrid ligands and the coordination chemistry of oxazoline?based systems. Angew Chem Int Ed Engl. 2001;40:680-99.
- [Google Scholar]
- The Bipyridines, Advances in Heterocyclic Chemistry. Vol 35. Amsterdam: Elsevier; 1984. p. :281-375.
- Imidazole and benzimidazole derivatives as chemotherapeutic agents. Mini Rev Med Chem. 2005;5:409-24.
- [Google Scholar]
- Facile synthesis of novel tetrasubstituted 1-pyrazolines from Baylis-Hillman adducts and acyl diazomethanes. Tetrahedron Lett. 2013;54:3846-50.
- [Google Scholar]
- Recent advances on the synthesis and reactivity of isoxazoles. Curr Org Chem. 2005;9:925-58.
- [Google Scholar]
- Synthesis and biological activity of prostaglandin lactones. J Med Chem. 1983;26:226-9.
- [Google Scholar]
- Monosubstituted derivatives of ferrocene. Ferrocene-containing penicillins and cephalosporins. J Organ Chem. 1991;401:81-5.
- [Google Scholar]
- Ferrocene compounds XXII. Synthesis and reactions of some ferrocenylthiaaliphatic acids. J Organ Chem. 1996;507:215-20.
- [Google Scholar]
- Synthesis and structure-activity relationships of novel 1-arylmethyl-3-aryl-1H-pyrazole-5-carbohydrazide derivatives as potential agents against A549 lung cancer cells. Bioorg Med Chem. 2007;15:6893-9.
- [Google Scholar]
- Synthesis and antimicrobial screening of some novel ferrocenyl derivates of pyrazole analogues. Int J Res Pharm Chem. 2014;4:473-8.
- [Google Scholar]
- Palladium-catalyzed asymmetric allylic amination using ferrocenyl pyrazole ligands:Steric control of h3-allyl configuration and site-selective nucleophilic attack. J Amm Chem Soc. 1996;118:1031-7.
- [Google Scholar]
- The structure-based design, synthesis and biological evaluation of DNA-binding bisintercalating bisanthrapyrazole anticancer compounds. Bioorg Med Chem. 2008;16:3959-68.
- [Google Scholar]
- Synthesis, DNA binding and cytotoxicity of new pyrazole emodin derivatives. European J Med Chem. 2006;41:1041-7.
- [Google Scholar]
- Deguelin targets multiple oncogenic signaling pathways to combat human malignancies. Pharmacol Res. 2021;166:105487.
- [Google Scholar]
- Emodin:A metabolite that exhibits anti-neoplastic activities by modulating multiple oncogenic targets. Toxicol In Vitro. 2021;73:105142.
- [Google Scholar]
- Molecular targets of celastrol in cancer:Recent trends and advancements. Crit Rev Oncol Hematol. 2018;128:70-81.
- [Google Scholar]
- The chick embryo chorioallantoic membrane as a model to investigate the angiogenic properties of human endometrium. Gynecol Obstet Investig. 1999;48:108-12.
- [Google Scholar]
- Molecular docking studies of curcumin natural derivatives with DNA topoisomerase I and II-DNA complexes. Interdiscip Sci Comput Life Sci. 2014;6:285-91.
- [Google Scholar]
- Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem. 1998;19:1639-62.
- [Google Scholar]
- AutoDock Vina:Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31:455-61.
- [Google Scholar]
- High-resolution mass spectrometry Part VI. A hydrogen rearrangement in the mass spectra of pyrazole oximes. J Chem Soc C Organ. 1969;19:2497-9.
- [Google Scholar]
- Diaryl dihydropyrazole-3-carboxamides with significant in vivo antiobesity activity related to CB1 receptor antagonism:Synthesis, biological evaluation, and molecular modeling in the homology model. J Med Chem. 2007;50:5951-66.
- [Google Scholar]
- Simple and efficient one-pot synthesis, spectroscopic characterization and crystal structure of methyl 5-(4-chlorobenzoyloxy)-1-phenyl-1H-pyrazole-3-carboxylate. Crystals. 2012;2:967-73.
- [Google Scholar]
- Imidazole-pyrazole hybrids:Synthesis, characterization and in-vitro bioevaluation against α-glucosidase enzyme with molecular docking studies. Bioorg Chem. 2019;82:267.
- [Google Scholar]
- Ferrocenium promoted oxidation of benzyl amines to imines using water as the solvent and air as the oxidant. ACS Sustain Chem Eng. 2018;7:479-86.
- [Google Scholar]
- C70 as a photocatalyst for oxidation of secondary benzylamines to imines. Org Lett. 2016;18:184-7.
- [Google Scholar]
- Comparison of porous iron trimesates basolite F300 and MIL-100 (Fe) as heterogeneous catalysts for lewis acid and oxidation reactions:Roles of structural defects and stability. Acs Catalys. 2012;2:2060-5.
- [Google Scholar]
- Togni A, Hayashi T, eds. Ferrocenes. Homogeneous Catalysis. Organic Synthesis. Materials Science. United States: VCH Verlag Sgesellschaft, Weinheim, Wiley Online Library; 1995. p. :448-9.
- Synthesis, molecular docking and in vitro screening of some newly synthesized triazolopyridine, pyridotriazine and pyridine-pyrazole hybrid derivatives. Molecules. 2018;23:2548.
- [Google Scholar]
- The reaction of 1,2,3-thiadiazoles with base. I. A new route to 1-alkynyl thioethers. J Chem Soc. 1996;446:1-7.
- [Google Scholar]
- Molecular iodine-catalyzed facile procedure for N-Boc protection of amines. J Org Chem. 2006;71:8283-6.
- [Google Scholar]
- Parallel synthesis of 4, 5-dihydro-1, 2, 4-oxadiazoles using soluble polymer support. Tetrahedron Lett. 2003;44:4113-5.
- [Google Scholar]
- Microwave-assisted efficient, one-pot, three-component synthesis of 3, 5-disubstituted 1, 2, 4-oxadiazoles under solvent-free conditions. Tetrahedron Lett. 2006;47:2965-7.
- [Google Scholar]
- p-(3, 3-Dimethyl-1-triazeno) benzonitrile. Acta Crystallogr Sect C Crystal Struct Commun. 1988;44:1651-3.
- [Google Scholar]
- One-and two-photon spectroscopy of donor-acceptor-donor distyrylbenzene derivatives:Effect of cyano substitution and distortion from planarity. J Phys Chem A. 2002;106:11470-80.
- [Google Scholar]
- Highly regioselective synthesis of 1-Aryl-3,4,5-substituted pyrazoles. Syn Lett. 2006;19:3267-70.
- [Google Scholar]
- 1, 3-Diketones from acid chlorides and ketones:A rapid and general one-pot synthesis of pyrazoles. Org Lett. 2006;8:2675-8.
- [Google Scholar]
- Design, synthesis and biological evaluation of some pyrazole derivatives as anti-inflammatory-antimicrobial agents. Bioorg Med Chem. 2004;12:1935-45.
- [Google Scholar]
- Synthesis and pharmacological activities of pyrazole derivatives:A review. Molecules. 2018;23:134.
- [Google Scholar]
- Synthesis, characterization and in vitro biological activities of pyrazolylpalladium(II) complexes towards selected strains. Synthesis Catalys. 2018;3:100020.
- [Google Scholar]
- Pyrazole derived ultra-short antimicrobial peptidomimetics with potent anti-biofilm activity. Eur J Med Chem. 2017;125:551-64.
- [Google Scholar]
- Pyrazolo [4, 3-c][1, 2] benzothiazines 5, 5-dioxide:A promising new class of Staphylococcus aureus NorA efflux pump inhibitors. J Med Chem. 2012;55:3568-72.
- [Google Scholar]
- Synthesis, DNA-binding and photocleavage studies of Ru (II) complexes of phenyl-(4, 5, 9, 14-tetraaza-benzo [b] triphenylen-1, 1-yl)-methanone. J Chem Sci. 2009;121:397-405.
- [Google Scholar]
- Synthesis, characterization and biological studies of novel schiff base viz. Bis-1,1'- (pyridine-2,6-diyldieth-1-yl-1-ylidene) biguanidine and their transition metal complexes. Asian J Chem. 2019;31:799-804.
- [Google Scholar]
- In vitro bacteriostatic and DNA interaction studies of drug-based mixed-ligand complexes of cobalt (II) Med Chem Res. 2011;20:220-30.
- [Google Scholar]
- Spectral, viscometric and electrochemical studies on mixed ligand cobalt (III) complexes of certain diimine ligands bound to calf thymus DNA. Inorg Chim Acta. 2002;337:420-8.
- [Google Scholar]
- Pyrazole bearing molecules as bioactive scaffolds:A review. Chin Chem Lett. 2015;26:534.
- [Google Scholar]
- Fisetin and quercetin:Promising flavonoids with chemopreventive potential. Biomolecules. 2019;9:174.
- [Google Scholar]
- Molecular mechanisms of action of genistein in cancer:Recent advances. Front Pharmacol. 2019;10:1336.
- [Google Scholar]
- Garcinol exhibits anti-neoplastic effects by targeting diverse oncogenic factors in tumor cells. Biomedicines. 2020;8:103.
- [Google Scholar]
- Mechanistic insight into carnosol-mediated pharmacological effects:Recent trends and advancements. Life Sci. 2017;169:27-36.
- [Google Scholar]
- Molecular aspects of melatonin (MLT)-mediated therapeutic effects. Life Sci. 2015;135:147-57.
- [Google Scholar]
- Ursolic acid (UA):A metabolite with promising therapeutic potential. Life Sci. 2016;146:201-13.
- [Google Scholar]
- Synthesis, characterization, theoretical, anti-bacterial and molecular docking studies of quinoline based chalcones as a DNA gyrase inhibitor. Bioorg Chem. 2014;54:31-7.
- [Google Scholar]
- Synthetic methods and antimicrobial perspective of pyrazole derivatives:An insight. Anti Infect Agents. 2020;18:207-23.
- [Google Scholar]
- Molecular docking studies of gyrase inhibitors:Weighing earlier screening bedrock. In Silico Pharmacol 20219. ;1:1-10.
- [Google Scholar]







