Translate this page into:
Candida species colonization in oral lichen planus: A meta-analysis
Address for correspondence: Alberto Rodriguez-Archilla, Department of Stomatology, Oral Medicine Unit, Faculty of Dentistry, Colegio Maximo, s/n. Campus de Cartuja, 18071-Granada, Spain. Phone: +34 958 244 085. E-mail: alberodr@ugr.es
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Qassim Uninversity and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective:
Oral lichen planus (OLP) is a common chronic inflammatory disease that affects 1%–2% of the population. Candida species superinfection can aggravate the symptoms of OLP, especially of the erosive forms, and promote malignant transformation by producing carcinogenics as nitrosamines or acetaldehyde. On the other hand, antifungal treatment of Candida-infected OLPs improves the clinical symptoms of the disease. The objective of this study is to assess the possible influence of Candida species colonization in oral lichen planus.
Methods:
A search for studies on Candida and oral lichen planus was performed in the following databases: PubMed (MEDLINE, Cochrane Library), Web of Science (WoS), and Scopus. Data analysis: The estimated prevalence of Candida detection in OLP was calculated according to the DerSimonian and Laird random model. For dichotomous outcomes, the estimates of effects of an intervention were expressed as odds ratio (OR) using Mantel-Haenszel (M-H) method with 95% confidence intervals.
Results:
Twenty-four studies were included in this meta-analysis. The estimated prevalence of Candida species detection in oral lichen planus (OLP) lesions was 37.00% (95% CI: 30.52–43.72%). OLP patients were almost 2½ times more likely to be infected by Candida species compared to healthy controls (OR: 2.48, P < 0.001). Likewise, Candida species superinfection was more likely in erosive OLP forms (OR: 2.53, P < 0.001), and by non-albicans Candida species (OR: 2.33, P = 0.02).
Conclusions:
More than one-third of OLP lesions are infected by Candida species, modifying their biological behavior.
Keywords
Candida
candidiasis oral
microbiology
lichen planus oral
Introduction
Oral lichen planus (OLP) is a common chronic inflammatory disease that affects 1–2% of the population, most commonly women in their fifth and sixth decades of life. OLP usually presents with recurrences and periods of clinical exacerbation and remission. OLP etiology remains unknown. An immune response presumably mediated by CD4+ and CD8+ T lymphocytes appears to develop in the oral mucosa, which produces cytokines such as interleukin-2 and tumor necrosis factor and induces a chronic inflammatory response and apoptosis of keratinocytes.[1] OLP lesions are characterized by a bilateral and symmetrical distribution, mainly affecting the buccal mucosa, the tongue, and gingiva. Anderson’s clinical classification distinguishes between six clinical forms: Reticular, atrophic, erosive, papular, plaque, and bullous. A typical manifestation of all OLP clinical forms is the presence of white striae called Wickham striae. Reticular OLP is the most common clinical form. It presents as asymptomatic white striae lesions with a reticular disposition. The OLP atrophic-erosive forms are usually symptomatic and present as erythematous-ulcerated areas with peripheral white striae.[2]
Although the possible relationship between Candida species colonization and OLP is not sufficiently clear, antifungal treatment of Candida-infected OLP lesions improves the clinical symptoms of the disease. In the oral microbiota, Candida albicans is found in approximately 8% of the healthy population. In OLP patients, this percentage is much higher. There is a positive correlation between the presence of Candida species in OLP lesions and interleukin-17 levels.[3] This interleukin plays a fundamental role in many immune regulatory functions through the production of immune signaling molecules that are involved in the pathogenesis of OLP. Both high IL-17 expression in the epithelial cells and markedly elevated serum levels have been observed in these patients compared to subjects without OLP.[4]
Candida superinfection may aggravate the OLP symptoms, especially of the erosive forms. Candida metabolism produces various carcinogenic agents such as nitrosamine or acetaldehyde.[5] OLP lesions exposed to established risk factors for oral malignancy such as smoking and alcohol consumption or Candida species superinfection requires special monitoring due to their increased malignant transformation potential.[6] This study purposed to analyze the repercussion of Candida superinfection on oral lichen planus.
Methods
All research steps, including search, study selection, data extraction, and evaluation, were performed independently by the two authors (ARA and SFT). Later, they agreed on which articles to consider in this study.
A search for studies on the Candida species detection and oral lichen planus up to November 2021 was conducted in the following databases: PubMed (MEDLINE, Cochrane Library), Web of Science (WoS), and Scopus. Search strategies included the combination of Medical Subjects Headings (MeSH) and free-text terms. The search terms were the following: “lichen planus, oral” [MeSH Terms] AND “Candida” [MeSH Terms]; “oral lichen planus” AND “Candida;” TITLE-ABS-KEY (“oral lichen planus” AND “Candida”). Articles without date or language of publication restrictions were initially included. The exclusion criteria were as follows: (a) Articles with relevant risk of bias (<6 points) according to the Newcastle-Ottawa methodological quality assessment scale,[7] (b) articles without full-text availability, (c) studies without clinical data, and (d) studies with non-usable data.
Statistical analysis
The MedCalc Statistical Software version 20.019 (MedCalc Software Ltd. Ostend, Belgium) program was used to calculate the estimated prevalence according to the DerSimonian and Laird random model. In addition, the RevMan 5.4 meta-analysis program (The Cochrane Collaboration, Oxford, UK) was applied for the dichotomous variables using the odds ratio (OR) with the Mantel-Haenszel Chi-square (MH) formula, all with 95% confidence intervals (95% CI). Heterogeneity was determined according to the Higgins statistic (I2) values. In cases of high heterogeneity (I2 > 50%), the random-effects model was performed. The minimum level of significance was set at P < 0.05. Publication bias was estimated using the funnel plot and the Egger test, with P < 0.1 as statistically significant.
Results
After the initial search, 232 articles were recorded (31 in PubMed, 118 in WoS, and 83 in Scopus) from 1974 to 2020, 30 of them duplicates, leaving 202 eligible articles. The exclusion criteria were as follows: (a) Articles with a relevant risk of bias (<6 points) according to the Newcastle-Ottawa methodological quality assessment scale[7] (n = 41), (b) articles without full-text availability (n = 45), (c) studies without clinical data (n = 32), and (d) studies with non-usable data (n = 60). After applying these criteria, 24 studies were included in this meta-analysis [Figure 1].
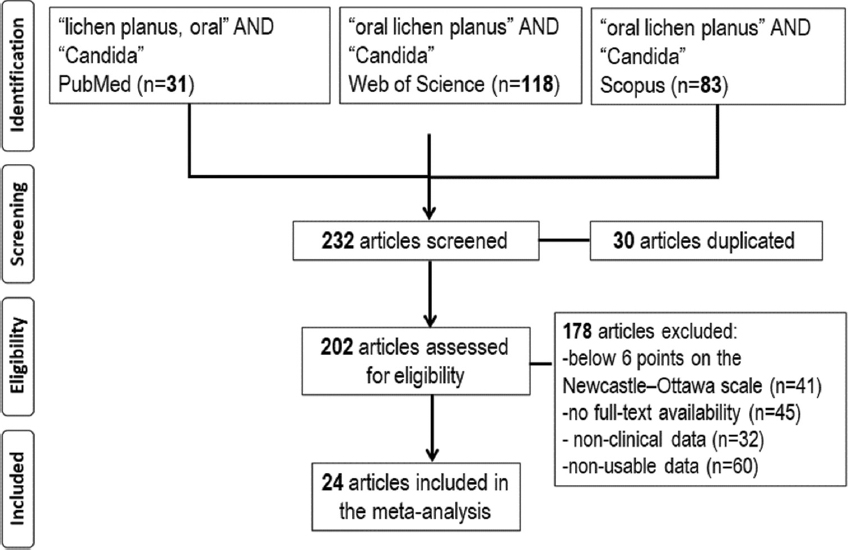
- Flow diagram of study selection
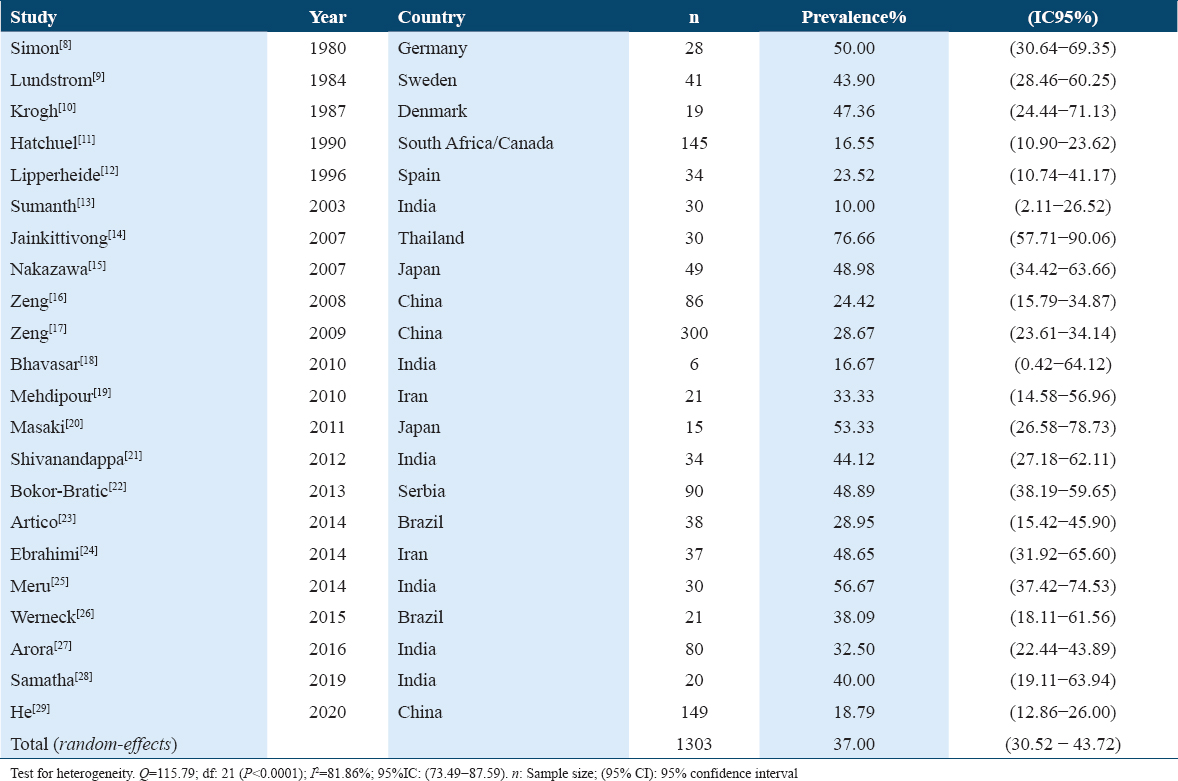
Table 1 presents the prevalence of Candida species detection in 1303 patients with oral lichen planus (OLP) considered in 22 studies[8-29] carried out in 13 different countries. The estimated global prevalence was 37.00% (95% CI: 30.52–43.72%). The variability by studies ranged from the maximum prevalence of 76.66% (95% CI: 57.71–90.06%) found in the study by Jainkittivong (Thailand, 2007)[12] to the minimum of 10.00% (95% CI: 2.11–26.52%) observed in the study by Sumanth (India, 2003).[11]
Fifteen studies[9,12-20,22,23,27-29] examined Candida species detection in oral lichen planus (OLP) patients and controls without the disease [Figure 2]. OLP patients were 2.48 times more likely to be infected by Candida species, with a highly statistically significant relationship (OR = 2.48; 95% CI: 1.94–3.18; P < 0.001).
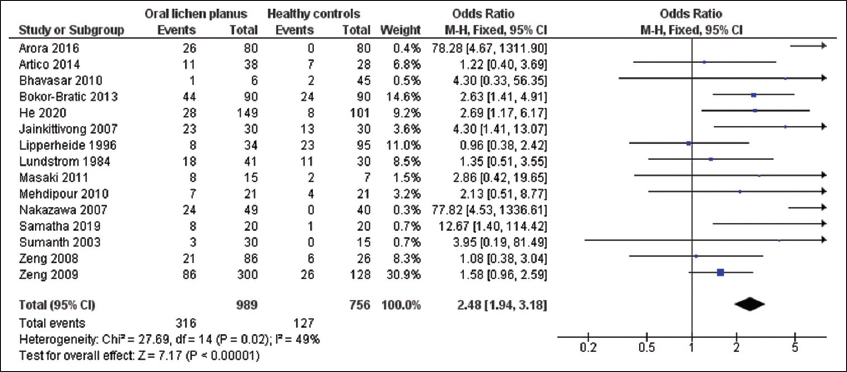
- Study data and forest plot graph on the Candida species detection in patients with oral lichen planus (OLP) and controls without the disease
The funnel plot for Candida species detection showed some asymmetry, suggesting that there may be publication bias, as presented in Figure 3. Egger’s test results (t = 1.54, P = 0.02) indicated evidence of publication bias.
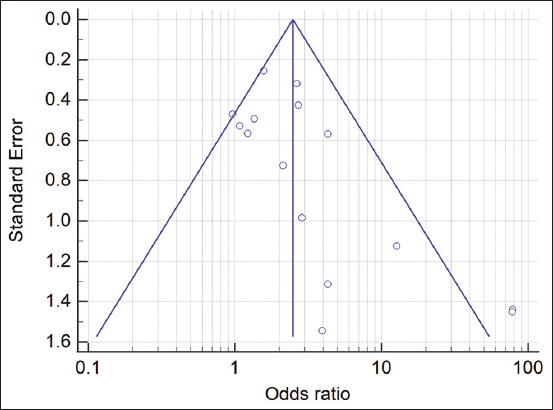
- Funnel plot for publication bias
The odds ratios and 95% confidence intervals for oral lichen planus (OLP) clinical forms (non-erosive/erosive) and different Candida species detection (C. albicans/C. non-albicans) in OLP patients are shown in Table 2.

Ten studies[11,16,17,21,24,25,27,29-31] assessed Candida species superinfection according to the OLP clinical form, observing that erosive OLP patients increased 2.53-fold the probability of Candida superinfection in their lesions. After the statistical analysis, highly significant differences were found between Candida species detection and OLP clinical form (OR = 2.43; 95% CI: 1.92–3.33; P < 0.001).
Five studies[12,14,20,22,23] investigated the different Candida species (C. albicans/C. non-albicans) in OLP patients, finding that Candida non-albicans species were 2.33 times more frequent in OLP lesions. Statistical analysis showed a significant association (OR = 2.33; 95% CI: 1.13–4.81; P = 0.02).
Discussion
Data from 24 studies on Candida species superinfection of lesions from oral lichen planus (OLP) patients have been included in the present meta-analysis.
In this study, in 1303 OLP patients considered in 22 studies,[8-29] the estimated prevalence of Candida species was 37%, with an interval that ranged between 10.0% and 76.6%. An increase in Candida species superinfection has been observed in subjects with other predisposing factors such as immunodeficiencies, diabetes mellitus, corticosteroids or antibiotics treatment, xerostomia, or poor oral hygiene. Oral lichen planus, especially the erosive clinical forms in which there is a loss of mucosal integrity, favors candidal colonization. Candida species may produce endogenous N-nitrosobenzylmethylamine, a carcinogen that could play a role in the potential malignant transformation of oral lichen planus.[27]
In this study, OLP patients were 2.48 times more likely to present Candida species superinfection, with highly statistically significant differences (P < 0.001). Of the 15 studies that analyzed Candida detection in OLP lesions, 14 of them[9,13-20,22,23,26-28] agreed in pointing out this higher detection of Candida species in OLP patients, and only one[12] did not observe it. The possible relationship between Candida species infection and the etiology of OLP has disconcerted many researchers for a long time and is not yet fully clarified. Nevertheless, Candida species infection is common in OLP patients. Candida albicans stimulation could cause leukocyte dysfunction, inhibiting both lymphocyte proliferation and cytokine production, modulating the immune response in oral lichen planus. All these changes modify the biological behavior of OLP lesions.[29] In addition, the use of antifungals together with other drugs in the OLP treatment induces a significant improvement in the lesions and, on some occasions, changes from erosive OLP clinical forms to asymptomatic OLP reticular forms.[27] On the other hand, the use of corticosteroids for the OLP treatment leads to immunosuppression, decreases salivary flow, and creates an ideal microenvironment for the proliferation and growth of Candida species, which makes OLP patients, especially susceptible to this fungal infection.[14]
OLP patients with erosive clinical forms were 2.53 times more likely to have Candida superinfection, with a highly statistically significant association (P < 0.001). The 10 studies[11,16,17,21,24,25,27,29-31] that examined this parameter confirmed this finding of a greater presence of Candida species in erosive lesions.
The different clinical forms of OLP can be classified into two main groups: Non-erosive (predominantly white) and erosive (predominantly red) clinical forms. Non-erosive OLP forms are usually asymptomatic and do not require medical treatment, only regular follow-up of the lesions. However, the erosive OLP forms, which present as superficial ulcerations, often cause significant symptoms. Erosive OLP patients require treatment and frequent follow-up due to the greater potential for malignant transformation of these OLP clinical forms.[2] Candida species detection rates in OLP patients are higher in erosive than in non-erosive forms. Several reasons could justify this finding: (1) Lesions from erosive OLP show superficial ulceration with the breakdown of mucosal integrity, facilitating the invasion and Candida species colonization of oral mucosa and (2) erosive OLP lesions modify the oral microenvironment, allowing some Candida species to better adapt to this situation, influencing the pathogenesis and progression of oral lichen planus.[17]
In the present meta-analysis, Candida non-albicans infection was 2.33 times more frequent in OLP lesions with a statistically significant relationship (P = 0.02). All the studies[12,14,20,22,23] about the different species of the genus Candida that infected the OLP lesions indicated this higher prevalence of the non-albicans Candida species in the OLP cases.
The frequency of Candida species detection is significantly higher in OLP patients compared to healthy controls. Of all Candida species, C. albicans is the most frequently detected species in the normal oral microbiota as a harmless commensal microorganism. In some oral lesions, it becomes an opportunistic pathogenic microorganism, favoring candidal infection. However, in OLP patients, other non-albicans Candida species such as C. glabrata or C. parapsilosis are identified, which are rarely found in the oral microbiota of the healthy population. Specifically, C. glabrata is less susceptible to the antifungal action of beta-defensins, histatins, or salivary mucins. It also has phospholipase activity that induces the destruction of cell membranes, promoting tissue invasion of the microorganism. This action is increased in the case of OLP patients who, moreover, present tissue alterations and disturbances in the immune response.[20]
This study has some limitations. Publication bias requires a careful interpretation of the results about Candida species detection in OLP patients versus healthy controls. The different methods for Candida species detection performed could influence the results. The number of Candida species infected cells in the OLP lesions was not quantified either. Similarly, the extension and severity of the OLP lesions could not be adequately assessed. Some possible confounding factors such as tobacco and/or alcohol consumption could not be considered.
New studies are required to establish the real role of Candida species infection in the etiopathogenesis of oral lichen planus (OLP). This infection seems to affect the biological behavior of these lesions and their potential for malignant transformation.
Conclusions
In this meta-analysis, the estimated prevalence of Candida species detection in oral lichen planus (OLP) lesions was 37.00%. OLP patients were almost 2½ times more likely to be infected by Candida species compared to healthy controls (OR: 2.48). Likewise, Candida species superinfection was more likely in erosive OLP forms (OR: 2.53) and by non-albicans Candida species (OR: 2.33).
Authors’ Declaration Statements
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The data used in this study are available and will be provided by the corresponding author on a reasonable request.
Competing interests
None.
Funding
None.
Authors’ Contributions
The two authors contributed equally to the study design, data collection, analysis, and manuscript preparation. Both approved the final version.
Acknowledgments
None.
ORCID link of the submitting author: https://orcid.org/0000-0003-1023-943X
References
- Diagnostic criteria of oral lichen planus:A narrative review. Acta Clin Croat. 2020;59:513-22.
- [Google Scholar]
- Are fungi responsible for the pathogenesis of oral lichen planus? Med Hypotheses. 2021;156:110689.
- [Google Scholar]
- The microbiology of oral lichen planus:Is microbial infection the cause of oral lichen planus? Mol Oral Microbiol. 2018;33:22-8.
- [Google Scholar]
- 2021. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-analyses. Ottawa, Canada: The Ottawa Hospital; Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- Prevalence rate of Candida in the oral cavity of patients with oral Lichen planus. Arch Dermatol Res. 1980;267:317-8.
- [Google Scholar]
- Yeast species and biotypes associated with oral leukoplakia and lichen planus. Oral Surg Oral Med Oral Pathol. 1987;63:48-54.
- [Google Scholar]
- Candidal infection in oral lichen planus. Oral Surg Oral Med Oral Pathol. 1990;70:172-5.
- [Google Scholar]
- Presence of Candida in oral lichen planus and leukoplakia. J Indian Acad Oral Med Radiol. 2003;15:154-7.
- [Google Scholar]
- Candida in oral lichen planus patients undergoing topical steroid therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:61-6.
- [Google Scholar]
- The cytological findings of oral inflammatory lesions, lichen planus and leukoplakia coexisted with and without Candida:With special reference to clinical, histopathological, immunohistochemical and flow cytometrical analyses. Int J Oral Med Sci. 2007;6:81-90.
- [Google Scholar]
- Genotypic profiles and virulence attributes of Candida albicans isolates from patients with oral lichen planus. APMIS. 2008;116:284-91.
- [Google Scholar]
- Carriage rate and virulence attributes of oral Candida albicans isolates from patients with oral lichen planus:A study in an ethnic Chinese cohort. Mycoses. 2009;52:161-5.
- [Google Scholar]
- Prevalence of Candida species in erosive oral lichen planus. J Dent Res Dent Clin Dent Prospects. 2010;4:14-6.
- [Google Scholar]
- Detection and identification of non-Candida albicans species in human oral lichen planus. Microbiol Immunol. 2011;55:66-70.
- [Google Scholar]
- Unstimulated whole salivary flow rate and anxiolytics intake are independently associated with oral Candida infection in patients with oral lichen planus. Eur J Oral Sci. 2013;121:427-33.
- [Google Scholar]
- Prevalence of Candida spp., xerostomia, and hyposalivation in oral lichen planus--a controlled study. Oral Dis. 2014;20:e36-41.
- [Google Scholar]
- Prevalence of Common Candida species in oral lichen planus patients:A cross-sectional study in South of Iran. Galen Med J. 2014;3:252-5.
- [Google Scholar]
- Isolation and identification of Candida species from various clinical forms of leukoplakia and oral lichen planus. Int J Dent Res Develop. 2014;4:1-6.
- [Google Scholar]
- Phenotypic variability and therapeutic implications of Candida species in patients with oral lichen planus. Biotech Histochem. 2016;91:237-41.
- [Google Scholar]
- Candida and oral lichen planus-a case control study. Indian J Contemp Dent. 2019;7:24-8.
- [Google Scholar]
- A pilot study:A possible implication of Candida as an etiologically endogenous pathogen for oral lichen planus. BMC Oral Health. 2020;20:72.
- [Google Scholar]
- Hypha essential genes in Candida albicans pathogenesis of oral lichen planus:An in-vitro study. BMC Oral Health. 2021;21:614.
- [Google Scholar]
- Factors influencing the presence of Candida dubliniensis and other non-albicans species in patients with oral lichen planus:A retrospective observational study. Clin Oral Investig. 2022;26:333-42.
- [Google Scholar]







