Translate this page into:
Cardiotoxicity in cancer patients treated with chemotherapy: A systematic review
Address for correspondence: Maria Adriely Cunha Lima, Av. Dr. José Tomaz D’Avila Nabuco, 700 - Farolândia, Aracaju - SE, 49030-270. Phone: 7999926-7799. E-mail: mariaadrielycunha@hotmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Qassim Uninversity and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective:
The objective of the study was to assess the incidence of chemotherapy cardiotoxicity.
Methods:
This is a systematic review carried out through the PubMed, VHL and Scientific Electronic Library Online databases, using the descriptors “Cardiotoxicity” and “Chemotherapy” associated with the Boolean operator “AND.” Initially, 15,090 articles were found between 2015 and 2021. After applying the defined inclusion and exclusion criteria, 80 studies remained, of which 27 underwent complete reading, after which all were included in the study.
Results:
In total, 32,009 cancer patients were analyzed, of which 27,270 (85.2%) were female. Breast cancer was the most frequent neoplasm, with 11,145 (34.8%) cases. Regarding the type of chemotherapy, anthracycline was the most prevalent, analyzed in 18 (66.7%) studies, followed by trastuzumab, in 9 (33.3%) studies. Of the studies evaluated, five did not present any case of cardiotoxicity, a total of 2255 (8.3%) cases were recorded, in addition other outcomes mentioned in patients after chemotherapy were arrhythmia (n = 522), acute coronary syndrome (n = 185), diastolic dysfunction (n = 184), cardiomyopathy (n = 161), and arterial hypertension (n = 89).
Conclusion:
Post-chemotherapeutic cardiotoxicity was mentioned in most studies, being present in a relevant percentage of the sample. Furthermore, these patients may develop other cardiovascular events.
Keywords
Cardiotoxicity
chemotherapy
oncology
Introduction
Chemotherapy cardiovascular involvement includes the development or exacerbation of cardiovascular diseases. In addition to cardiotoxicity, these patients are at increased risk of developing other cardiovascular complications, especially arrhythmias, ventricular dysfunction, systemic arterial hypertension, acute coronary syndrome, cardiomyopathy, and thromboembolic diseases.[1-4] Anthracyclines and related compounds are among the chemotherapeutic agents most associated with the development of cardiotoxicity, in addition, to these other related drugs are alkylating agents (cyclophosphamide), monoclonal antibodies (rituximab), protein kinase inhibitors, and HER2-targeted therapies (trastuzumab).[1,4]
Chemotherapeutic cardiotoxicity can be defined as any functional and/or structural alteration of the cardiovascular system induced by cytotoxic antineoplastic drugs, being measured through the reduction of the left ventricular ejection fraction (LVEF), it can manifest in an acute, subacute, or chronic form.[2,5] Cardiotoxicity represents one of the most significant adverse effects of this type of therapy. It is currently the second most common cause of morbidity and mortality in patients with the previous cancer treatment, second only to oncological causes (recurrence or secondary neoplasms).[3,4]
The appearance of chemotherapeutic cardiotoxicity may imply the interruption of chemotherapeutic treatment and, consequently, compromise the cure or adequate control of the neoplasm, in addition to the fact that heart failure may compromise the evolution of the case. It is also noteworthy that cardiotoxicity can be classified as type I, in which myocytes are affected by the toxic effects of drugs (e.g., those caused by anthracyclines) and suffer irreversible damage, and in type II, in which cardiac function is not compromised, is determined to be irreversible (such as that caused by trastuzumab).[1,4,6,7]
This review seeks to assess chemotherapeutic cardiotoxicity, so that it is possible to analyze the epidemiological aspects related to this complication, such as risk factors, the most recurrent alterations and the most commonly associated antineoplastic drugs. Furthermore, it was also possible to assess the incidence of other cardiovascular complications resulting from the use of chemotherapy.
Methods
This is a systematic review that aims to assess the profile of chemotherapy cardiotoxicity cases. For this, searches were carried out in the Scientific Electronic Library Online, virtual health library and Medical Literature Analysis and Retrieval System Online (Medline/PubMed) databases using the descriptors “Cardiotoxicity” and “Chemotherapy,” in addition to of the Boolean operator “AND,” so that it was possible to maximize the number of studies found.
The inclusion criteria defined were original articles published between 2015 and 2021 in any language with descriptors present in the title and/or abstract, while those that did not have content to achieve the proposed objective, in addition to a case report and case series, were excluded. Initially, 15,090 articles were found. Subsequently, the inclusion criteria were applied, excluding 15,010 articles [Figure 1].
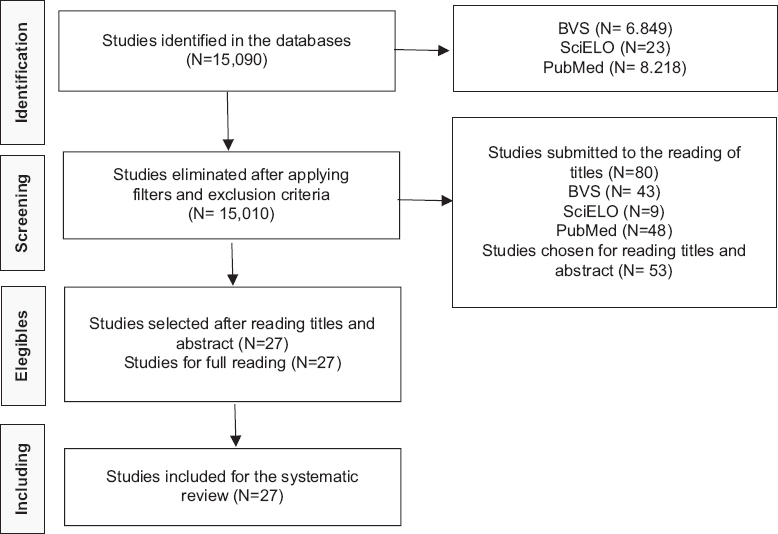
- Study PRISMA flow diagram. Source: survey data, 2021
After that, 80 studies were submitted for reading the title, of these 53 were selected for reading the title and abstract, of which 26 were excluded, leaving 27 studies for full reading. Then, all 27 articles were included to compose the database of this review, as they contained enough data to achieve the proposed objective. For data extraction, the Microsoft Office Excel®8 program was used to store and tabulate the data obtained.
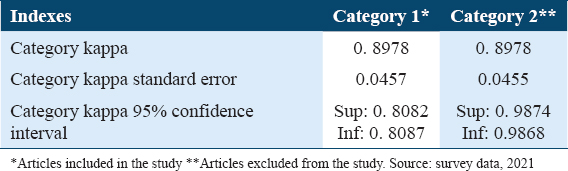
The Kappa index was used to assess the agreement of the evaluators in the selection of articles. The value 0.8978 was obtained, which, according to Landis and Koch9, suggests excellent reliability, since the closer the value to 1, the greater the agreement of the reviewers. Therefore, it was possible to proceed with the systematic review [Table 1].
Results
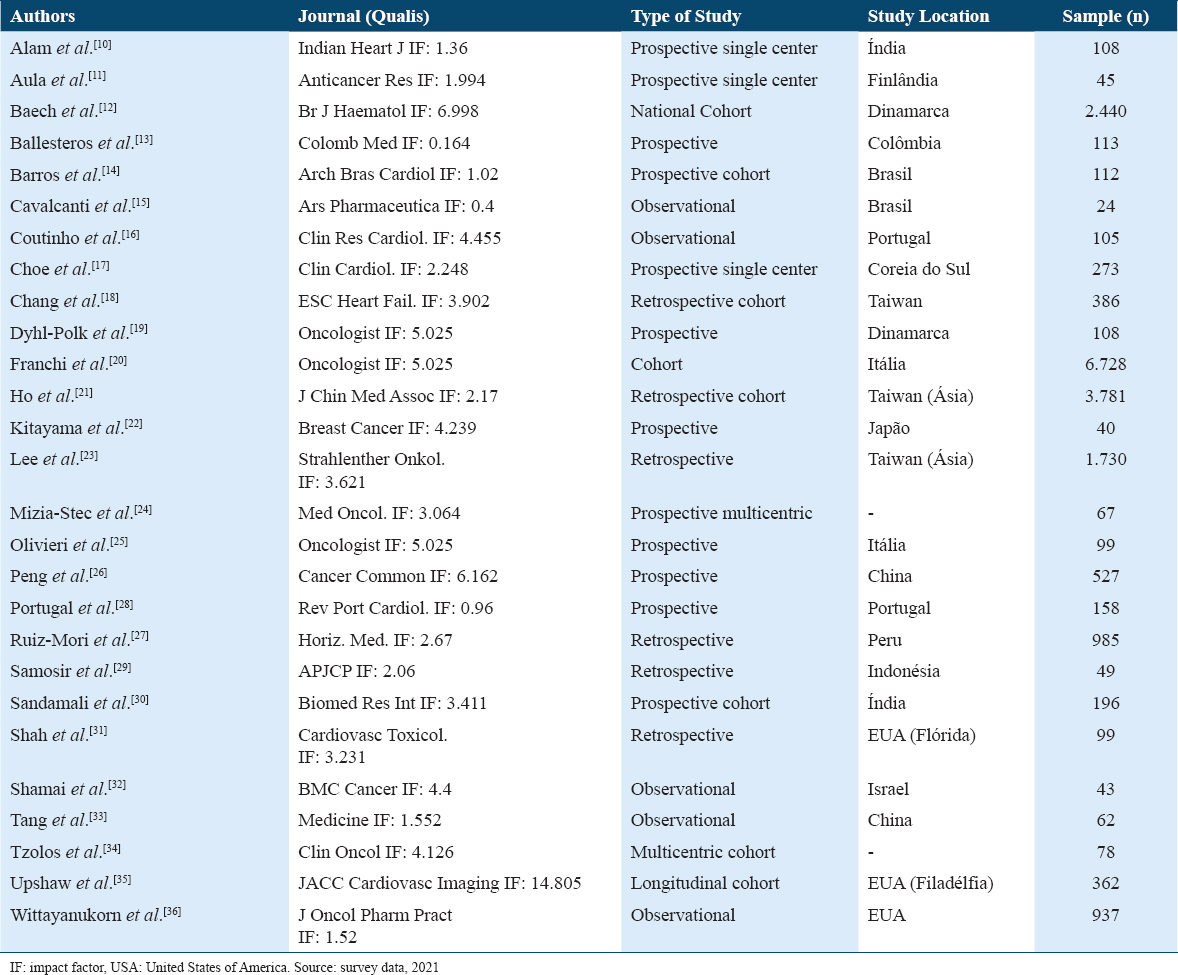
In Table 2, it is possible to analyze that only 2 (7.4%) studies had an impact factor lower than 1.02. With regard to the study location, a heterogeneous distribution is noted, with the United States being the country with the most number of studies, totaling 3 (11.1%). Furthermore, regarding the number of cancer patients analyzed, there were a total of 32,009 cases.
From Table 3, it is observed that most of the patients analyzed were female, in which there were 27,270 (85.2%) cases, while there were 4533 (14.2%) of males, with 12 studies not included males in the sample. Breast cancer was the most frequent neoplasm, with 11,145 (34.8%) cases, followed by colorectal cancer, with 3925 (12.3%). Other reported neoplasms were lymphoma, leukemia, osteosarcoma, and sarcoma.
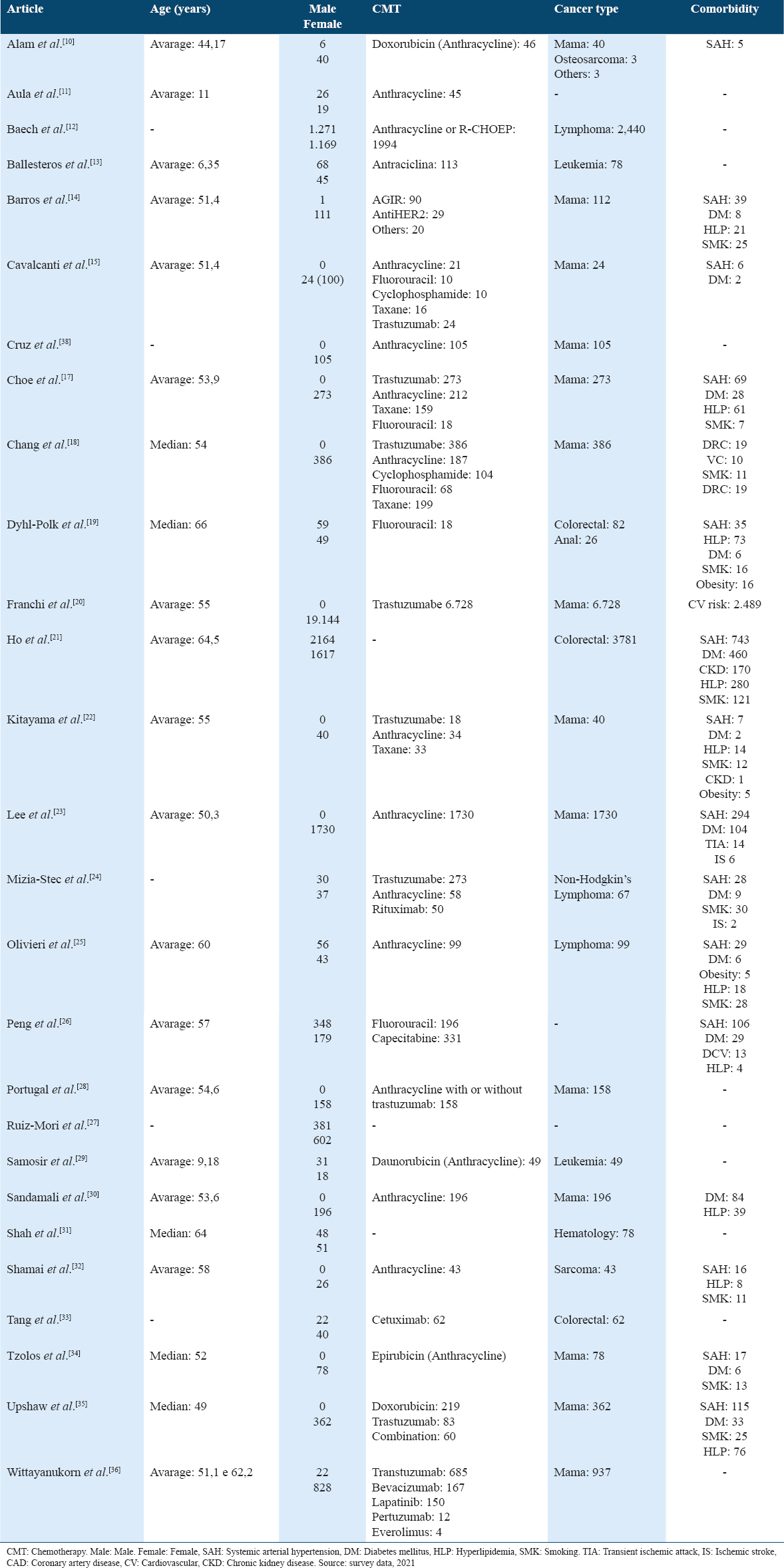
Some studies did not mention the presence of comorbidities in the sample, when analyzing this variable, cardiovascular risk was reported in 2489 (9.1%) patients, arterial hypertension in 1509 (5.5%), and diabetes mellitus in 777 (2.8%). Other comorbidities mentioned in the studies were current smoking, hyperlipidemia, chronic kidney disease, and obesity. Regarding the type of chemotherapy, anthracycline was the most prevalent, analyzed in 18 (66.7%) studies, followed by trastuzumab, in 9 (33.3%) studies.
When analyzing the presence of cardiotoxicity in the analyzed patients, five studies did not present any case. In total, 2,255 (8.3%) cases of cardiotoxicity were recorded, and the study by Wittayanukorn et al.[36] had only cases of cardiotoxicity in its sample, and the causes were later evaluated. In addition to cardiotoxicity, other outcomes were evaluated, such as the development of arrhythmia (n = 522), acute coronary syndrome (n = 185), diastolic dysfunction (n = 184), cardiomyopathy (n = 161), and arterial hypertension (n = 89) after chemotherapy [Table 4].
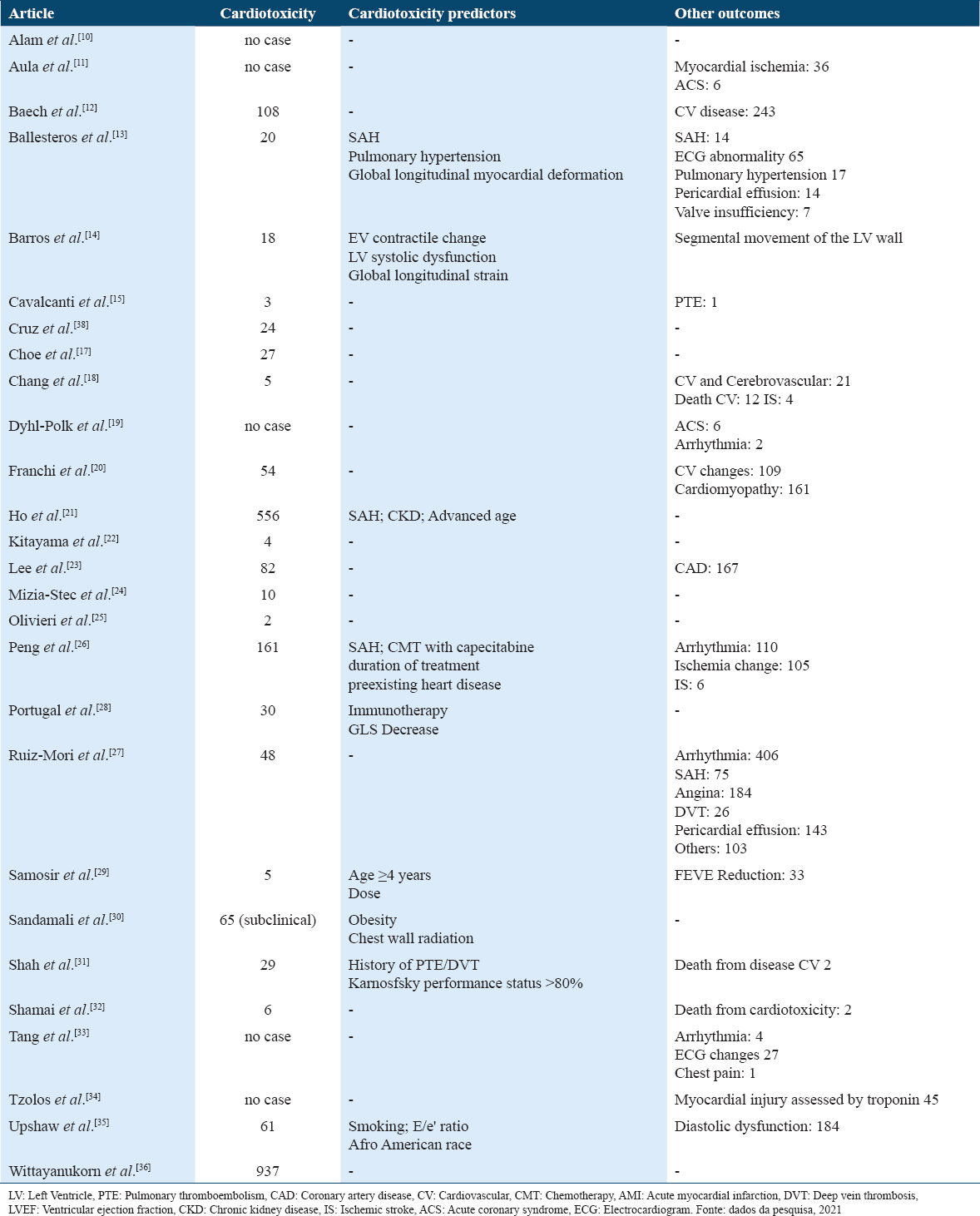
Regarding the risk factors for the development of cardiotoxicity, only nine studies evaluated this, and the presence of arterial hypertension was mentioned by three studies, being the most cited factor. From Table 4, it can be observed that there were 16 deaths from cardiovascular causes, two of these cases were due to cardiotoxicity.
Discussion
This review showed a higher prevalence of female patients in the analyzed studies, with breast cancer and colorectal cancer being, respectively, the most prevalent in the sample. Likewise, chemotherapy with anthracycline combined or not with trastuzumab was the most frequent. In fact, breast cancer is currently the most commonly diagnosed malignancy in the world, followed by lung cancer and colorectal cancer, respectively.[37]
Anthracyclines are antineoplastic agents commonly used in adjuvant or neoadjuvant therapy, as well as in the treatment of metastases. This class of drugs, represented by doxorubicin, epirubicin and others, forms a complex with DNA, inhibiting the synthesis of nucleic acids and proteins, through cleavage by topoisomerase II, which results in its cytotoxic activity. The mechanisms of cardiotoxicity described for this class involve lipid peroxidation, oxidative stress, apoptosis and necrosis of cardiac cells, in addition to the impairment of proteins and transcription factors relevant to the heart, resulting in a negative balance of sarcomeric proteins and an imbalance in cardiac function.[1,3,38]
In the study, it was possible to observe the presence of cardiovascular risk factors in cancer patients, such as systemic arterial hypertension, diabetes mellitus, smoking, hyperlipidemia, obesity, and chronic kidney disease. In addition to these, it is known that other factors such as inflammatory diseases, chronic obstructive pulmonary disease, and the presence of cancer are associated with high cardiovascular risk.[39] Therefore, care with the identification and control of cardiovascular risk factors should be redoubled in patients undergoing chemotherapy with potential for cardiotoxicity.[40,41]
Cardiotoxicity was recorded in 8.3% of cancer patients who underwent chemotherapy, similar to the study by Volkova and Russell[42] observed that anthracycline can induce heart failure in up to 8.7% of cases, while Curigliano et al.[43] reported that in patients treated with trastuzumab the cardiotoxicity is up to 3.8% and with cyclophosphamide it varies between 7 and 28%. The increased risk for the development of cardiotoxicity associated with the use of anthracyclines is related to younger patients, the female sex, the rapid rate of drug infusion, the high cumulative dose, and the presence of hypocalcemia, hypomagnesemia, and previous cardiovascular diseases, such as coronary heart disease and hypertension.[40] Consistent with these facts, this study found risk factors associated with chemotherapy cardiotoxicity, such as the presence of systemic arterial hypertension.
The study by Wittayanukorn et al.[36] evaluated reports of chemotherapy-related cardiotoxicity in breast cancer patients in the United States, identifying 937 cases. The most prevalent drug was trastuzumab in combination with doxorubicin or cyclophosphamide, with 25% of the total reports being associated with death or disability. Such findings are in agreement with the study by Chen et al.,[44] which presents evidence demonstrating that the use of anthracyclines with trastuzumab increases the risk of heart failure, while the study by Seidman et al.[45] demonstrates an association of the use of anthracyclines with cyclophosphamide and trastuzumab with the incidence of dysfunction heart. In addition to these outcomes, this review also demonstrates the occurrence of arrhythmias, acute coronary syndrome and cardiomyopathy in patients undergoing chemotherapy, evidencing the existing cardiovascular risk.
In view of all these factors discussed, it is important to reaffirm the need to know the cardiotoxic effects of chemotherapy, as well as the cardiovascular risk that the patient presents, so that the likelihood of poor treatment outcomes is combated. In addition, the cardiovascular risks and cardiotoxicity demonstrated in this work may help the surveillance of adverse events in cancer patients undergoing chemotherapy, as well as guide future studies.
Conclusions
Cardiotoxicity was highlighted as relevant in most studies, presenting a percentage of review cases in this review, in addition to cardiovascular manifestations such as coronary syndrome and systemic arterial hypertension from chemotherapy. Finally, regarding the types of chemotherapy, the most prevalent was anthracycline followed by trastuzumab.
Conflict of Interest
Nothing to declare.
Funding Source
None.
References
- I Brazilian Cardio-Oncology Guideline of the Brazilian Society of Cardiology. Arch Brazil Cardiol. 2011;96:2011.
- [Google Scholar]
- Cardiotoxicity associated with cancer therapy:Pathophysiological mechanisms and prevention strategies. Rev Port Card. 2013;32:395-409.
- [Google Scholar]
- 2021. Cardiotoxicity of Cancer Chemotherapy Agents Other than Anthracyclines, HER2-Targeting Agents, and Fluoropyrimidines. Waltham: UpToDate; Available from: https://www.uptodate.com/contents/cardiotoxicity-of-cancer-chemotherapy-agents-other-than-anthracyclines-her2-targeted-agents-and-fluoropyrimidines?search=quimioterapia%20cardiotoxicidade&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1
- Clinical Manifestations, Monitoring and Diagnosis of Anthracycline-induced Cardiotoxicity. Waltham: UpToDate; Available from: https://www.uptodate.com/contents/clinical-manifestations-monitoring-and-diagnosis-of-anthracycline-induced-cardiotoxicity?search=quimioterapia%20cardiotoxicidade&source=search_result&selectedTitle=2~150&usage_type=default&display_rank=2
- Prevention and monitoring of cardiac dysfunction in adult cancer survivors:Clinical practice guideline from the American Society of clinical oncology. J Clin Oncol. 2017;35:893-911.
- [Google Scholar]
- MICROSOFT Project for Windows 95. In: Versão 4.1. [S. l.]. Redmond: Microsoft Corporation; 1995.
- [Google Scholar]
- The measurement of observer agreement for categorical data. Biometrics. 1997;33:159-74.
- [Google Scholar]
- To study the usefulness and comparison of myocardial strain imaging by 2D and 3D echocardiography for early detection of cardiotoxicity in patients undergoing cardiotoxic chemotherapy. Indian Heart J. 2019;71:468-75.
- [Google Scholar]
- Adjuvant breast cancer treatments induce changes in homoarginine level- A prospective observational study. Anticancer Res. 2017;37:6815-24.
- [Google Scholar]
- Cumulative anthracycline exposure and risk of cardiotoxicity;a Danish nationwide cohort study of 2440 lymphoma patients treated with or without anthracyclines. Br J Haematol. 2018;183:717-26.
- [Google Scholar]
- Early-onset cardiotoxicity assessment related to anthracycline in children with leukemia. A prospective study. Colomb Med (Cali). 2021;52:e2034542.
- [Google Scholar]
- Left ventricular regional wall motion abnormality is a strong predictor of cardiotoxicity in breast cancer patients undergoing chemotherapy. Arq Bras Cardiol. 2019;112:50-6.
- [Google Scholar]
- Adverse reactions for the use of the monoclonal trastuzumab antibody in the treatment of patients with HER2 positive breast câncer. Ars Pharm. 2017;58:171-4.
- [Google Scholar]
- Three-dimensional speckle-tracking echocardiography for the global and regional assessments of left ventricle myocardial deformation in breast cancer patients treated with anthracyclines. Clin Res Cardiol. 2020;109:673-84.
- [Google Scholar]
- Prolonged electromechanical delay as an early predictor of trastuzumab-induced cardiotoxicity in patients undergoing treatment for breast cancer. Clin Cardiol. 2018;41:1308-14.
- [Google Scholar]
- Risks of trastuzumab-related cardiotoxicity in breast cancer patients in Taiwan. ESC Heart Fail. 2021;8:5149-58.
- [Google Scholar]
- Myocardial ischemia induced by 5-fluorouracil:A prospective electrocardiographic and cardiac biomarker study. Oncologist. 2020;26:e403-13.
- [Google Scholar]
- Cardiovascular risk after adjuvant trastuzumab in early breast cancer:An Italian population-based cohort study. Oncologist. 2020;25:e1492-e9.
- [Google Scholar]
- Pre-existing chronic kidney disease and hypertension increased the risk of cardiotoxicity among colorectal cancer patients treated with anticancer drugs. J Chin Med Assoc. 2021;84:877-84.
- [Google Scholar]
- High-sensitive troponin T assay can predict anthracycline- and trastuzumab-induced cardiotoxicity in breast cancer patients. Breast Cancer. 2017;24:774-82.
- [Google Scholar]
- Risk of cardiotoxicity induced by adjuvant anthracycline-based chemotherapy and radiotherapy in young and old Asian women with breast cancer. Strahlenther Onkol. 2019;195:629-39.
- [Google Scholar]
- Chemotherapy and echocardiographic indices in patients with non-hodgkin lymphoma:The ONCO-ECHO study. Med Oncol. 2017;35:14.
- [Google Scholar]
- Modern management of anthracycline-induced cardiotoxicity in lymphoma patients:Low occurrence of cardiotoxicity with comprehensive assessment and tailored substitution by nonpegylated liposomal doxorubicin. Oncologist. 2017;22:422-31.
- [Google Scholar]
- Cardiotoxicity of 5-fluorouracil and capecitabine in Chinese patients:A prospective study. Cancer Commun (Lond). 2018;38:22.
- [Google Scholar]
- Chemotherapy-induced cardiotoxicity at the Instituto Nacional de enfermedades neoplasicas 2012-2016. Horiz Med. 2017;17:24-8.
- [Google Scholar]
- Global and regional patterns of longitudinal strain in screening for chemotherapy-induced cardiotoxicity. Rev Port Cardiol. 2017;36:9-15.
- [Google Scholar]
- Risk factors of daunorubicine induced early cardiotoxicity in childhood acute lymphoblastic leukemia:A retrospective study. Asian Pac J Cancer Prev. 2021;22:1407-12.
- [Google Scholar]
- Anthracycline-induced cardiotoxicity in breast cancer patients from Southern Sri Lanka:An echocardiographic analysis. Biomed Res Int. 2020;2020:1847159.
- [Google Scholar]
- Unanticipated cardiotoxicity associated with targeted anticancer therapy in patients with hematologic malignancies patients:Natural history and risk factors. Cardiovasc Toxicol. 2018;18:184-91.
- [Google Scholar]
- Cardio-toxicity among patients with sarcoma:A cardio-oncology registry. BMC Cancer. 2020;20:609.
- [Google Scholar]
- The cardiotoxicity of cetuximab as single therapy in Chinese chemotherapy-refractory metastatic colorectal cancer patients. Medicine (Baltimore). 2017;96:e5946.
- [Google Scholar]
- Dynamic changes in high-sensitivity cardiac troponin I in response to anthracycline-based chemotherapy. Clin Oncol (R Coll Radiol). 2020;32:292-7.
- [Google Scholar]
- Comprehensive assessment of changes in left ventricular diastolic function with contemporary breast cancer therapy. JACC Cardiovasc Imaging. 2020;13:198-210.
- [Google Scholar]
- Cardiotoxicity in targeted therapy for breast cancer:A study of the FDA adverse event reporting system (FAERS) J Oncol Pharm Pract. 2017;23:93-102.
- [Google Scholar]
- Global cancer statistics 2020:GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-49.
- [Google Scholar]
- Cardiotoxicity in anthracycline therapy:Prevention strategies. Rev Port Cardiol. 2016;35:359-71.
- [Google Scholar]
- 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice:Developed by the task force for cardiovascular disease prevention in clinical practice with representatives of the European Society of cardiology and 12 medical societies with the special contribution of the European Association of preventive cardiology (EAPC) Eur Heart J. 2017;42:3227-337.
- [Google Scholar]
- Update of the cardiovascular prevention guideline of the Brazilian Society of cardiology-2019. Arq Bras Cardiol. 2019;113:787-891.
- [Google Scholar]
- Concomitant management of cancer therapy and cardiac therapy. Biochim Biophys Acta. 2015;1848:2727.
- [Google Scholar]
- Cardiotoxicity of anthracyclines:Prevalence, pathogenesis and treatment. Curr Cardiol Rev. 2011;7:214-20.
- [Google Scholar]
- Cardiotoxicity of anticancer treatments:Epidemiology, detection and management. CA Cancer J Clin. 2016;66:309-25.
- [Google Scholar]
- Risk of cardiac dysfunction with trastuzumab in breast cancer patients:A meta-analysis. Cancer Treat Rev. 2011;37:312-20.
- [Google Scholar]
- Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20:1215-21.
- [Google Scholar]







