Translate this page into:
Mycochemistry, antioxidant content, and antioxidant potentiality of the ethanolic extract of Pleurotus florida and its anti-cancerous effect on HeLa cancer cell line, and antitumor effect on HeLa-implanted mice
Address for correspondence: Swapan Kumar Ghosh, Molecular Mycopathology Lab, Cancer Research Unit, PG Department of Botany, Ramakrishna Mission Vivekananda Centenary College (Autonomous), Rahara - 700 118, Kolkata, India. E-mail: gswapan582@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Qassim Uninversity and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objectives:
Cervical cancer is increasing worldwide and is becoming resistant to the existing drugs in clinical practice. Here, ethanolic extract of fruit body of Pleurotus florida was evaluated as antioxidant, anticancer agent against HeLa cell lines and anti-tumor against cervical cancer in mice model.
Methods:
Fruit bodies of P. florida in 90% ethanol, and the P. florida ethanolic extract (PFEE) was subsequently investigated for its antioxidant content and activity, anticancer properties against the cervical cancer cell line, HeLa, and antitumor activity against HeLa implanted mice.
Results:
The antioxidant activity bioassay showed that the IC50 of PFEE was 41.17 ± 1.42a μg/ml. The cytotoxicity assay revealed that PFEE caused inhibition of cell proliferation. At the highest dose (1,250 μg/ml) after 24 h, 48 h, or 72 h of treatment, the percentages of cell growth inhibition were 75.22%, 77.77%, and 84.65%, respectively. It revealed that PFEE-treated cells became rounded and the nuclei became fragmented. PFEE induced intracellular generation of reactive oxygen species and reduced the mitochondrial membrane potential. PFEE also led to an up regulation of the apoptotic genes for caspases-3, -9, and Bax, whereas Bcl-2 gene was down regulated, and it also promoted the expression of p53. Cell cycle analysis revealed that cell cycle was arrested at the G0/G1 checkpoint. PFEE suppressed metastasis and colonization. At a dosage of PFEE of 50 mg/kg of body weight, a 66.72% reduction in the size of tumors and an 87.44% reduction in the tumor weight were observed in mice.
Conclusions:
It has demonstrated that PFEE is a highly potent anti-cervical cancer agent in vitro and in vivo.
Keywords
Apoptosis
colonization
Cytotoxicity
metastasis
migration
Introduction
More than two decades have passed since the concept of “functional foods” was first introduced in food science.[1] At present, consumers are interested in bioactive food products that are beneficial to health and reduce the risk of disease. Some epidemiological studies revealed that ideal diet containing vegetables, whole grains, fruits, seeds, and herbs, may decrease the risk of the progression of many ailments such as diabetes, cancer, and cardiovascular disease.[2] Alhumaydhi[3] reported that honey has profound protective effect against toxicity caused by application of chemotherapeutant in BALB/C mice. Mushrooms have been recognized as one of the important functional foods for humans. They have been collected and cultivated for hundreds of years in Asian countries, such as China and Japan[4] and they have a long history of use in view of their health promotion benefits.[5] Numerous reports have been published in recent years on mushroom biochemistry, and on their nutritional and functional properties.[6,7] The number of different mushroom species on earth is estimated at 140,000, of which perhaps only ~10% are known to science. Furthermore, of those ~14,000 species currently recorded, ~50% are considered to possess varying degrees of edibility.[8] At present, mushrooms, or the extracts derived thereof, are used globally in the form of dietary supplements for cancer management.[9,10] Oyster mushrooms (of the Pleurotus genus) are edible and are widespread throughout the hardwood forests of the world.[11] Now cultivation and popularity of the Pleurotus species has increased markable throughout the world as it is easy to cultivate and it has high nutritional and medicinal values. It is the 3rd largest cultivated mushroom in the world.[12] P. florida belongs to the family Pleurotaceae and order Agaricales and is one of the widely cultivated mushroom species, although the number of studies that have been conducted on its anticancer activity in the cervical cancer cell line, HeLa, and other cancer cell lines, are very few.[11] Other species of Pleurotus, however, have drawn the attention of scientists in terms of investigating their anticancer properties. Pleurotus ostreatus is an edible mushroom that has been widely investigated for a variety of properties, including its antitumor effects.[13] Ren[14] reported that water-soluble polysaccharide extracts of Pleurotus eryngii, Pleurotus austral and 14 other species of mushroom exhibited direct antitumor activity against the C32 and WM-266-4 cell lines. Since a large number of the compounds have been shown to act synergistically, it is worth testing the anti-proliferative effects of the whole mushroom extract, rather than simply its individual components. This principle (synergy) is compatible with similar natural biological products, such as the essential oils, which are more effective when used as whole products, as potential unwanted side-effects are quenched, or nullified, by the presence of individual components. Previously, numerous researchers have worked with various solvent extracts of mushrooms (ethanolic, methanolic, water, hexane, etc.) on different human cancer cell lines, although the consensus opinion is that ethanolic extracts derived from mushrooms are most effective for this purpose.[15,16] The use of the ethanol for extraction has some advantages over other polar solvents as it is able to dissolve both polar and non-polar substances and rather non-toxic in comparison to methanol while the use of methanol is limited due to its toxic effects. Water use for extracting requires care for evaporating.[17,18] Rather ethanolic solvent is more effective for extracting maximum antioxidant content.[19] On the other hand, among women in India, approximately one-third of the total number of newly diagnosed cases of cancer are of cervical cancer, resulting from infection with certain types of human papilloma virus. This disease is also called as “hidden or silent” cancer as the patient is generally asymptomatic. More than 50% of international cases occur within the Asia-Pacific region, and India has the top predicted figure of cases of this cancer.[20] Every year in India, 122,844 women are diagnosed with cervical cancer, and 67,477 die from the disease.[21,22] Cancers of the cervix and the uterus are a major problem for women in West Bengal.[23] Therefore, in the present study, P. florida (fruit body) ethanolic extract (PFEE) was evaluated for its antioxidant content, activity, anticancer properties, including anti-proliferative effects and other properties, such as induction of apoptosis, mitochondrial membrane potential (MMP) reduction, generation of reactive oxygen species (ROS), and anti-colonization and anti-migration effects. To this end, the antitumor effects of PFEE were investigated on the HeLa cervical cancer cell line, and on HeLa cell-implanted Swiss albino mice.
Materials and Methods
Mushroom collection and identification
The cultivated basidiocarps were collected from Baruipur market, District-24-Parganas(S), Kolkata, India and carried to the laboratory in a biodegradable polythene bag. All morphological characteristics, such as color, shape, and so on, were recorded, a spore print was taken, and anatomical characters were noted, identifying them by consulting the published keys.[24-26] The genomic DNA was isolated[27] and purified from fresh mushroom samples, and ITS1-5.8S-ITS2 marker zone of rRNA was amplified by PCR protocol, modified from Gardes and Bruns[28] with primers ITS-1F (5′-CTTGGTCATTTAGAGGAAGTAA-3′) and ITS4 (5′ TCCTCCGCT TATTGATATGC3′) The PCR products were sent to the SCI GENOME laboratory in Kerala for sequencing. The obtained sequence was subjected to BLAST and submitted to GenBank, NCBI, Baltimore, USA.
Solvent extraction
The collected fruit bodies of the mushroom were thoroughly washed with tap-water, and subsequently air-dried in an oven at 50°C for 48 h. The dried fruit bodies were chopped into pieces, and then grinded into powder using a mixer grinder.
Dip method
700 g of dry mushroom powder was weighed and then it was dipped in ethannol perpetuating proper ratio (1:10), stored at incubator 45°C for 7 days. After 7 days, the mixture was filtered through Whatman No. 4, followed by Whatman No.1 filter papers (GE Healthcare Life Sciences, Little Chalfont, UK). The ethanol was removed from the extract using a rotary vacuum evaporator at 40°C, and the remaining solvent was removed using a freeze-drier. The resultant powder is recognized as PFEE (Pleurotus florida Ethanolic Extract) was kept in an airtight condition in a refrigerator at 4°C. Extract to be used in the in vitro assays was dissolved in plain Dulbecco’s Modified Eagle medium (DMEM) (Himedia Laboratory), and sterilized using a 0.22 mm Millipore filter. The prepared extract was further diluted with DMEM to the desired concentrations prior to use.
Soxhlet extraction method
Dried mushroom powder (600 g) was packed into a Soxhlet apparatus and decoction with 500 mL of ethanol at 45°C for 6 h. The elicitation was strained through Whatman filter paper No. 1, and the filtrate was kept and the ethanol was removed from the extract using a rotary vacuum evaporator at 40°C, and the remaining solvent was removed using a freeze-drier. The further steps were as earlier method.
Mycochemical screening of PFEE
Mycochemical screening was performed to identify the myco-compound/secondary metabolites in the PFEE sample in this present observation by way of color checks.
Alkaloid test
A quantity of 0.2 g PFEE was weighed and warmed in 2% sulfuric acid in test tube for 2 min, and a few drops of Dragendroff’s reagent were added and the presence of orange red precipitation indicated the presence of alkaloid.[29]
Test for steroids
A few drops of acetic acid anhydride were added to 0.2 g of PFEE in a test tube. Concentrated H2SO4 was carefully delivered drop by drop to the examination tube via the inner wall. The presence of a brown ring on the surrounding floor, which indicated the presence of steroids.[30]
Test for terpenoids
Approximately 0.2 g of PFEE was confiscated and solvated in 2 ml of chloroform in test tube; after filtration, filtrate was added by 3 ml of concentrated sulfuric acid in a test tube and in the end perceived reddish brown shade which indicated for the presence of terpenoid.[31]
Test for flavonoid
Around 0.2 g of PFEE was confiscated and solvated with 4 ml of distilled water in a test tube then appended with diluted sodium hydroxide and diluted hydrochloride solution delivered and described a yellow coloration which implied for the presence of flavonoid. It became colorless while on addition of a few drops of dilute acid which changed into yellow color indicating the presence of flavonoid.[32]
Test for saponins
About 2 ml of the PFEE were mixed with 10 mL of distilled water in a test tube and that was shaken vigorously for about 5 min, and then it turned into stored for 30 min and found for honey comb forth, which turned into indicating the presence of saponin.[29]
Test for tannin
A quantity of 0.2 g of PFEE turned into mingled with 4 ml of distilled water in a test tube and heated on water bath. The aggregate was blended and ferric chloride turned into appended to the filtrate and descried for dark inexperienced answer that indicated the presence of tannin.[33]
Test for phenol
Approximately 5 ml of sample PFEE mixed with 5% ferric chloride in a test tube and kept for the genesis of deep blue color which stipulated the presence of phenol.[34]
Test for phlobatannins
Almost 2 ml of PFEE solution have been mixed with dilute HCl in a test tube and located for pink precipitation that indicated the presence of phlobatannins.[29]
Divergent biochemical assay
Total phenol content assessment
Folin-reagent Ciocalteu’s method was used to determine the total phenol content of various solvent extracts.[35] The mixture of 0.1 ml Folin-reagent Ciocalteu’s (0.5 N) reagent and 0.5 ml PFEE solution was incubated for 15 min at room temperature. Then 2.5 ml of saturated sodium carbonate solution was added, and the mixture was incubated at room temperature for 30 min. At 760 nm, the absorbance was measured and compared to a Gallic acid calibration curve. In the end, the result was expressed as mg of Gallic acid equivalent (GAEs) per gram of dry mushroom weight and it was presented as the mean ± SD of triplicate.
Total flavonoid content assessment
The TFC of the PFEE was determined using a standard method proposed by Meda et al.[36] Briefly in a test tube, equal amount of 2% AlCl3 (made in absolute methanol) and extract (1 mg/ml) were incubated for 10 min at room temperature. The mixture was then incubated for 10 min and the absorbance was measured at 415 nm. Different concentrations of quercetin (0–10 μg/ml) were used as a standard. The result was expressed as mg of Quercetin equivalent (QEs) per g of mushroom dry weight, and it was presented as the mean presented as the mean ± SD of triplicate.
Total ascorbic acid content assessment
Total ascorbic acid content was determined following the Folin–Ciocalteu reagent method by Jagota and Dani[37] with slight modifications. The PFEE (0.5 ml) were combined with 0.8 ml of 10% trichloroacetic acid and rapidly shaken, then refrigerated for 5 min before centrifugation at 3000 rpm for 5 min. The extract (0.2 ml) was then dilute to 2 ml with distilled water. Folin-Ciocalteu 2.0 M was diluted 10 times in distilled water. The combination was then given 0.2 ml of the diluted reagent. The mixture was then incubated for 10 min at room temperature. The absorbance was measured at 760 nm. Different concentrations of ascorbic acid (0–10 mg/ml) were used to make a standard curve. Finally, the results were reported as mg of ascorbic acid equivalent (AAEs) present per g of mushroom extract, and presented as the mean ± SD of triplicate
Antioxidant activity assay
1,1-diphenyl-2-picrylhydrazyl (DPPH) assay
The radical scavenging activity (RSA) of PFEE was assessed using the DPPH method. DPPH, a stable free radical with an at 515 nm, was used to study the radical scavenging effect of decoction. As antioxidants deliver protons to these radicals, their absorption reduces. The drop in absorbance is regarded as a measure of the magnitude of radical-scavenging. The free-radical scavenging capacities of the tracts were calculated using the DPPH test. The RSA of DPPH was evaluated using a modified Bondet et al. technique.[38] Different concentrations (50–150 μg/ml) of PFEE were made, and here, 0.9 ml of DPPH solution (0.1 mM) was added to a test tube with 100 μl of each concentration of PFEE powder separately. Control was prepared with ethanol. The reaction mixture was incubated for 2 h at RT, and the absorbance was measured at 515 nm with a spectrophotometer. The scavenge potentiality of PFEE was ultimately measured. The synthetic antioxidant BHA (butylated hydroxyanisole) was used as positive control. The percent reduction in DPPH was estimated using the following formula:
% DPPH= [(Ac–As)/Ac] ×100
As is the absorbance of sample and Ac is the absorbance of control. Then the IC50 value (50% inhibition) was calculated.
Anti-cancerous activity
Cell culture
The human cervical cell line, HeLa, at passage number 30 (purchased from The National Centre for Cell Science, Savitribai Phule Pune University, Pune, India] was cultured in DMEM supplemented with L-glutamine, 10% (v/v) fetal bovine serum, 100 μg/ml streptomycin, and 250 IU/ml penicillin (all purchased from HiMedia Laboratories Pvt. Ltd., Mumbai, India) in 75-mm tissue culture flasks at 37°C in a humidified atmosphere of 5% CO2 until the cells reached 70-80% confluence.[39] For maintenance, cultures were passaged weekly, and the culture medium was changed twice a week.
Cytotoxicity/cell proliferation assay
The effect of PFEE on cell proliferation of the HeLa cell line was assessed using a methyl thiazolyl tetrazolium (MTT) assay (reagents provided by HiMedia Laboratories Pvt Ltd).[40] Briefly, 10 × 104 cells/well of a 96-well culture plates were seeded with fresh DMEM medium containing 10% FBS and antibiotics overnight until the cells reached 80% confluence. Subsequently, the culture was washed with 10% phosphate-buffered saline (PBS) and treated with PFEE dissolved in DMEM at various concentrations (250, 500, 1000, and 1250 μg/ml), and the cells were then incubated at 37°C in an atmosphere of 5% CO2 and 95% air, where DMEM used as vehicle control. After 24 h, 48 h, and 72 h of treatment, cells were washed with phosphate-buffered saline, and 100 μl of 500 μg/ml MTT solution (dissolved in phenol red-free DMEM) was added to each well. The cells were subsequently further incubated for 3 h, and after having discarded the media, 100 μl dimethyl sulfoxide (DMSO) was added to dissolve the crystals. The plate was read using a microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA) at an absorbance of 570 nm. The HEK 293 cell line was taken and subjected to MTT assay at the highest tested concentration of PFEE (1250 μg/ml) to check the cytotoxicity of this extract toward a normal human cell line. The IC50 value (i.e., the concentration that led to a 50% killing of the cells) was calculated by plotting a dose-response graph of the cytotoxicity values obtained using the following formula:
% Cell cytotoxicity=100 - [AControl - Atest /AControl] x 100 ×100] The data points represent the mean ± standard deviation or each experiment, and the experiments were repeated at least 3 times.
HeLa cell morphology examined under a phase contrast microscope
To investigate the cell morphology, 2 ×105 HeLa cells/well were seeded into6-well plates, and at 80–90% confluence the cells were treated with various concentrations of PFEE (500, 750, and 1000 μg/ml) where DMEM used as vehicle control. After 24 h, images of the cells were captured using an Olympus phase contrast microscope (at a magnification of ×20; Olympus Corporation, Tokyo, Japan).[41]
HeLa nuclear morphology examined by Hoechst-33342 staining under a fluorescence microscope
To determine the morphological changes (chromatin condensation) of the cell nuclei by PFEE, Hoechst-33342 (Thermo Fisher Scientific, Inc.) staining was performed, according to the method described by Allen et al.[42] In brief, 2 × 105 HeLa cells/well were seeded onto a 6-well plate, and after having reached 95% confluence, the cells were treated with two concentrations of PFEE (500 and 1000 μg/ml) supplemented with DMEM, where DMEM used as a vehicle control. After 48 h incubation, the cells were washed with PBS, followed by fixing of the cells with 3.7% formaldehyde for 25 min. After washing twice with PBS, cells were permeabilized with 0.1% Triton X-100 for 15 min. Subsequently, the cells were stained with 5 μg/ml Hoechst 33342 dyes for 10 min at room temperature in the dark. After washing twice with PBS, stained nuclei were observed under an inverted Olympus fluorescence microscope (Olympus Corporation) at a magnification of ×20.
Cell cycle analysis
It was based on flow cytometric DNA analysis of cells that distributed at different stages in the cell cycle as described by Pozarowski and Darzynkiewicz.[43] In brief, HeLa cells (7.5 × 105) were seeded in 100 mm dishes, and cultured in DMEM containing 10% FBS for 24 h, followed by incubation with PFEE (500, 750, and 1000 μg/ml) at 37°C for 24 h, where DMEM used as vehicle control. After the period of incubation, the cells were harvested, washed with Dulbecco’s PBS containing 1% FBS, and re-suspended in 50 μg/ml propidium iodide. Samples were analyzed on a fluorescence-activated cell sorting (FACS Calibur BD Bioscience, USA). The fractions of cells in the various phases of the cell cycle (G0/G1, S, and G2/M) are shown as a percentage of the total cells analyzed.
Cell migration assay using the scratch method
The migration assay was performed following the method described by Lee et al.[44] but with certain modifications. Briefly, 10 × 103 HeLa cells/well were cultured in a 24-well plate. After having reached 90% confluence, the center of the culture dishes was scratched with a 200 μl pipette tip. The cells were subsequently washed 2 times with PBS, prior to incubation with PFEE at concentrations of 500 and 1000 μg/ml. After 24 h incubation, images of the cells were captured at a magnification of ×10. The relative migration rate was estimated by measuring the distance the cells had migrated for 24 h, with the data being normalized against cells cultured in DMEM alone as the control. The relative migration rate was calculated as compared with the control group.
Clonogenic assay
This assay was performed following the method described by Crowley et al.[45] In brief, harvested HeLa cells were seeded in 6-well plates at a concentration of 500 cells/well. Subsequently, culture medium containing PFEE at various concentrations (50, 75, 100, and 125 μg/ml) was added to each well soon after the cells had attached themselves to the wells. One negative control (DMEM vehicle without PFEE) set of experiments was also performed. Colony formation was assessed after incubation for 14 day(s) in culture medium, which was replaced every 4 days. Colonies were fixed and stained with 3.7% formaldehyde and 0.5% crystal violet; subsequently, the colonies were counted manually under a microscope, and images of the cells were captured. Colonies consisting of >50 cells were counted using a colony counter, and the results are reported as a percentage of colonies formed using the following equation:
% Colonies formed= (colonies formed in treated sample/colonies formed in untreated sample) ×100.
Microscopic determination of ROS
ROS was determined by staining using 2,7-dichlorodihydrofluorescein di-acetate(H2DCFDA) and 4’,6-diamidino-2-phenylindole (DAPI; both purchased from Thermo Fisher Scientific, Inc.). In brief, 2 × 105 cells/well were seeded onto a 6-well plate, and after having reached 90% confluence, cells were treated with PFEE at various concentrations (500, 750, and 1250 μg/ml) before incubation at 37°C for 2h, where DMEM used as vehicle control. After the 2 h incubation, the existing medium was discarded. The cells were subsequently re-suspended in serum-free DMEM media, 1μl H2DCFDA and DAPI were added, and the cells were further incubated for 30 min. Finally, the cells were washed 3 times with PBS, serum-free media were added, and the cells were observed under an inverted fluorescence microscope. Ascorbic acid and hydrogen peroxide (H2O2) were used as positive and negative controls, respectively.[46]
Qualitative determination of DNA fragmentation using agarose gel electrophoresis or DNA laddering assay
DNA fragmentation a distinctive feature used to determine the extent of apoptosis. This is an early event, which occurs before any shifts in plasma membrane permeability. The DNA fragmentation assay is used to visualize the endonuclease cleavage products of apoptosis on an agarose gel electrophoresis. For this assay, 4 × 105 cells were seeded on to a 35 mm plate at 37°C. After 24 h, cells were treated with different concentrations of PFEE (500, 825, and 1000 μg/ml), and subsequently incubated for 24 h, 48 h, or 72 h. After each respective time of incubation, cells were washed with PBS buffer, trypsinized and finally the total genomic DNA of the cells was extracted using radio-immunoprecipitation (RIPA) assay buffer (Abcam, Cambridge, UK). The extracted DNA was resolved on a 1.5% agarose gel containing ethidium bromide (HiMedia Laboratories Pvt. Ltd.) in 40 mM Tris-acetate buffer (pH 7.5), electrophoresis at 50V for 4 h. Images of the DNA fragments were captured using a gel documentation system (Bio-Rad Laboratories, Inc.).[47]
MMP analysis
Cells (2 × 105/well) were seeded on to a 24-well plate in DMEM. After a 24 h incubation at 37°C in an atmosphere of 5% CO2, cells were treated with (500, 750, and 1000 μg/ml) PFEE, followed by incubation for 12 h, where DMEM used as vehicle control. At the end of the incubation period, cells were washed with 1× PBS, and subsequently incubated with 10 μl 200 μM JC-1 at 37°C in 5% CO2 and 95% air for 30 min. Finally, the cells were examined under an Olympus fluorescence microscope with a red and green filter (Olympus Corporation).[48]
Protein isolation and western immunoblotting
Cells (4 × 105 cells/well) were seeded on to a 6-well plate, followed by an overnight incubation (37°C). After discarding the media, cells were washed with 1× PBS and treated with 500, 750, and 1000 μg/ml PFEE for 24 h, where DMEM used as vehicle control. After 24 h incubation, each well was washed with 1× PBS (1 ml), and subsequently the cells were trypsinized (1X) and incubated at 37°C in a cell culture incubator for 4 min. Following trypsinization, 2 ml fresh DMEM media were added to each well, and the cells were collected in a 1.5 ml centrifuge tube. Each centrifuge tube was centrifuged at 5000 rpm, 4°C for 5 min, and after discarding the supernatant, the cell pellets were dissolved in 1 ml 1× PBS, followed by a further centrifugation at 5000 rpm for 5 min. Total protein isolation was performed in 1X RIPA buffer (Abcam) as per the manufacturer’s protocol, and the protein concentration was measured using the Puregene® bicinchoninic acid BCA) method. Protein samples (100 μg) were subjected to SDS-PAGE: Protein separation was accomplished on a 10% TGX stain-Free™ Fast Cast Acrylamide gel (Bio-Rad Laboratories, Inc.) at 200V for 45 min. Following protein separation, proteins were transferred to a nitrocellulose membrane (Bio-Rad Laboratories, Inc.) using a Trans-Blot® Turbo™ transfer system (Bio-Rad Laboratories, Inc.). Subsequently, the membranes were washed with TBS buffer for 5 min, and blocked with 5% bovine serum albumin (BSA) for 1 h. The effect of PFEE treatment on the expression of certain genes as proteins, like p53, and on anti-apoptotic and apoptotic proteins, such as Bcl-2, Bax, pro-caspase-3, pro- caspase-9, cleaved-caspase-3, and cleaved-caspase-9 (Santacruze Biotechnology, USA), were determined. Each membrane was then incubated with the respective primary antibody at a 1:500 dilutions (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) in 1× antibody dilution buffer (5% BSA and 0.01% thiomershal) at 4°C with agitation. After an overnight incubation, membranes were washed 3 times (10 min each wash) in 1X TBS/Tween 20 (TBST) and incubated for 1h with the respective secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Again, membranes were washed 3 times (10 min each wash) with 1X TBST, and finally the protein expression of each membrane was imaged on photographic film (Carestream) using enhanced chemi-luminescence buffer (Bio-Rad Laboratories, Inc.).[49,50]
Antitumor effects of PFEE in HeLa cell-implanted mice
Swiss albino mice (8–10 weeks old) weighing ~22–26 g were purchased. Animal experiments were conducted on experimental mice. All mice were housed under hygienic/pathogen-free air conditions in a room maintained at 24°C with 50% relative humidity and a 12 h/12 h light-dark cycle. All animal experiments were approved and performed according to the regulations of the Institutional Animal Ethics Committee, Government of India (approval no: 12/P/S/IAEC/2018). The mice were divided into six groups: i.e., control mice supplemented with pellet (control + vehicle; n = 6); ii) controls supplemented with PFEE (500 mg.kg-1) (control + PFEE; n = 6); iii) HeLa cell-implanted mice supplemented with pellet (HeLa + vehicle; n = 6); iv) HeLa cell-implanted mice supplemented with PFEE (10 mg.kg-1 body weight) (HeLa + PFEE 10; n = 6); v) HeLa cell-implanted mice supplemented with PFEE (25 mg/kg) (HeLa + PFEE 25, n = 6); and vi) HeLa cell-implanted mice supplemented with PFEE (50 mg/kg) (HeLa + PFEE 50, n = 6).
For tumor generation, a suspension of 2 × 106 HeLa cells in 0.2 mL DMEM was subcutaneously injected on the dorsal surface of the right-hand legs of the Swiss albino mice, whereas the control (DMEM vehicle) groups were injected with DMEM. The tumors were measured with Vernier calipers every 3–4 days using the formula a2 × bx 0.52 (where b is the shortest diameter and bis the longest diameter).[51] When the tumor volume was measured to be 8.0–10.0 mm3, the mice were randomized. Subsequently, the mice were supplemented daily with vehicle or PFEE at the doses of 10, 25, and 50 mg/kg of body weight for 28 days. After 28 days, all the mice in groups 3-6 were sacrificed, and the tumors were excised from their legs and then weighed. Visceral organs (i.e., the kidney, liver, spleen, and lungs) were morphologically observed for possible side effects of PFEE in the mice.
Statistical analysis
All data were analyzed using Graph Pad Prism 5 software (GraphPad Software, Inc., La Jolla, CA, USA), and one-way analysis of variance was performed to compare differences between the groups. All results are presented as the mean ± standard error from three independent experiments performed in a parallel manner, unless otherwise indicated. Significant level of all data in the presented figures was obtained after Tukey’s test statistical analysis (P < 0.05) from three independent experiments. Statistically significant differences (P < 0.05) among means of experiment results were analyzed by ANOVA and means compared by Duncan’s Multiple Range Tests.
Results
Identification of mushroom sample
The Phenotypically mushroom sample was identified as P. florida by consulting the published keys.[24-26] ITS1-5.8S-ITS2 based identification showed that this mushroom was Pleurotus sp. and published in GenBank, NCBI, Baltimore, USA with Accession no MF045429.1.
Yield of ethanolic extracts
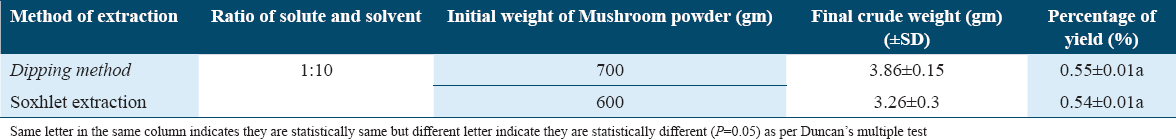
The solvents were evaporated using a rotary evaporator once the extraction was completed, yielding semi-solid bulk crude extracts. The extraction yields of ethanol solvent for P. florida were obtained and presented in the Table 1, it showed that the yields in dip method and Soxhlet method were 0.55 ± 0.01 and 0.54 ± 0.01%, respectively, and they are same statistically (P = 0.05).
Mycochemical screening study (qualitative)
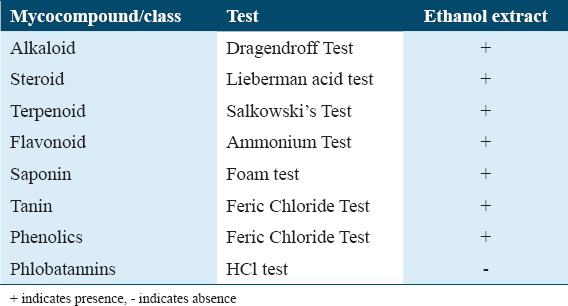
The result presented in Table 2 exhibited that the chemical compounds present in the ethanolic extract were alkaloid, steroid, terpenoid, flavonoid, saponin, tannin, and phenolics but this extract did not contain any phlobatannins.
Total Phenol, flavonoid, and Ascorbic acid content assay
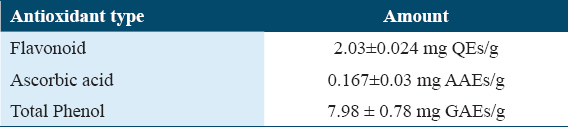
Total phenolic content of ethanolic extract was 7.98 ± 0.78 mg GAEs/g, total flavonoid content of the ethanolic extract was 2.031 ± 0.024 mg quercetin equivalent/g. Ascorbic acid content in the ethanolic extract was 0.167 ± 0.03 mg AAEs/g [Table 3].
Antioxidant activity by DPPH method
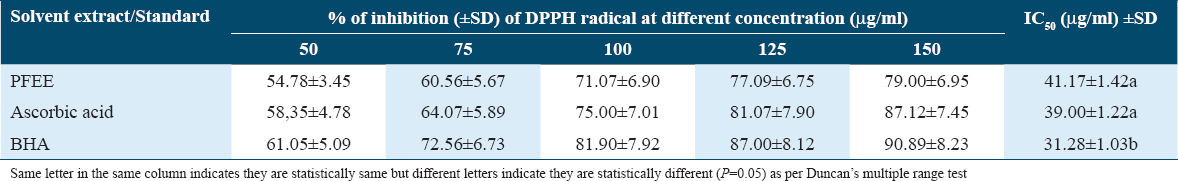
The data presented in Table 4 showed percentage (%) of inhibition (±SD) of DPPH radical at different concentration (μg/ml) of PFEE, ascorbic acid, and BHA. Furthermore, the IC50 (50 percent inhibition) of natural antioxidant of PFEE was 41.17 ± 1.42a μg/ml, while the IC50 of synthetic antioxidant BHA and ascorbic acid (positive control) were 31.28 ± 1.03b and 39.00 ± 1.22a μg/ml, respectively. It clearly indicated that PFEE and ascorbic acids are statistically (P = 0.005) same as per IC50 activity but BHA is different from other two.
Anticancer activity
Effect of PFEE on HeLa cell morphology as measured using phase contrast microscopy
HeLa cell line, treated with various concentrations of PFEE and after 24 h incubation, was examined under a phase contrast microscope to monitor their morphology. The untreated cells (DMEM vehicle control) exhibited the normal spindle shape and reached 90% confluence after 24 h culture [Figure 1a]. Cells treated with 500 and 750 μg/ml PFEE were revealed to be smaller in size; they also lost their spindle shape, and the cell confluence was reduced [Figure 1b and c]. More rounded, shrunken cells were observed for the experiment group treated with 1000 μg/ml PFEE; furthermore, a lot of cell debris was observed, and the cell confluence was markedly lower [Figure 1d].
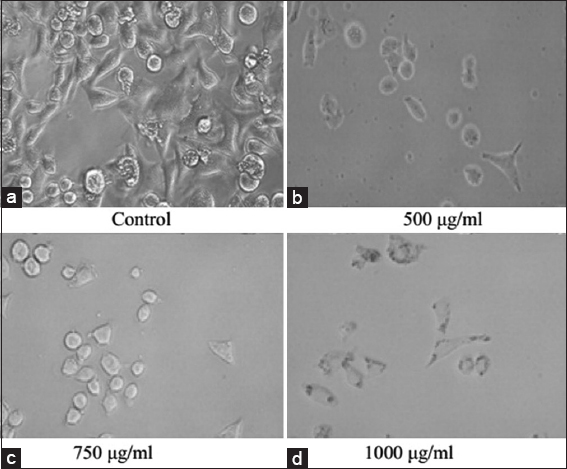
- Effect of PFEE on HeLa cell morphology under phase contrast microscope(20×). (a) Control cells showed normal spindle shaped morphology but (b-d) treated cell showed mostly round shaped morphology after 24 h treatment of PFEE
Effect of PFEE on the nuclei of HeLa cells as observed under an inverted fluorescence microscope
Hoechst-33342 is a fluorescence stain that yields a blue color under a fluorescence microscope. When cells were stained with Hoechst-33342, live cells took up a light blue coloration uniformly, whereas apoptotic cells were stained a deeper shade of blue due to chromatin condensation. Figure 2a revealed that the nuclei of the untreated HeLa cells appeared normal, assuming a light-blue color, and were round and homogeneous, whereas the nuclei of cells treated with PFEE were observed to take up more of the deep-blue stain, and were condensed [Figure 2b], also being irregular in shape and fragmented [Figure 2c]. These results demonstrated that PFEE was effective in inducing apoptosis of the HeLa cells.
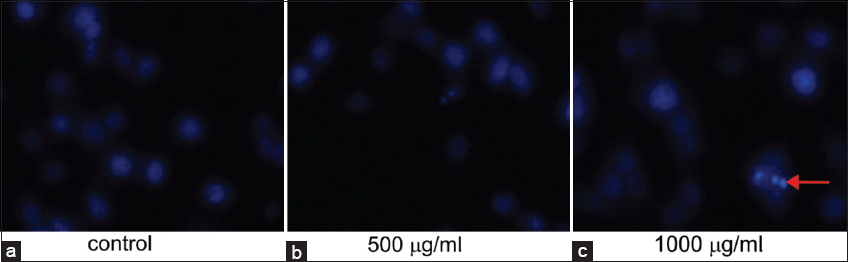
- Effect of PFEE on HeLa nuclear morphology under Inverted Fluorescence microscopy. (a) Control showed Hoechst-33342 stained round nucleus of HeLa cell. (b and c) showed Hoechst-33342 stained nucleus of PFEE (500 and 1000 μg/ml) treated HeLa cell and they were condensed and fragmented. “Arrow” indicates nuclear fragmentations
Cytotoxicity/anti-proliferation effects of PFEE against the HeLa cell line as determined by the MTT assay
Whether PFEE affected HeLa cell proliferation or not was subsequently investigated. The cell proliferation bar diagram [Figure 3] revealed that the negative control (DMEM vehicle) cells (untreated) proliferated during the course of the observed time periods (24 h, 48 h and 72 h). By contrast, cells treated with PFEE at all tested concentrations exhibited a reduced rate of proliferation in a concentration- and time-dependent manner. Cells treated with the highest concentration (1,250 μg/ml) of PFEE tested proliferated the least. The reduction in cell proliferation was observed starting from 24 h after treatment and became more pronounced as the duration of the treatment was extended. Control cells without PFEE treatment exhibited no indication of any decrease in terms of their growth and proliferation. Cells treated with 1250 μg/ml revealed 75.22%, 77.77%, and 84.65% growth inhibition at the 24 h, 48 h, and 72 h time points, respectively [Figure 3a-c]. The highest dose tested of PFEE (1,250 μg/ml) did not reveal any indications of cytotoxicity in the normal HEK 239 cell line at 24 h, 48 h, or 72 h (data not shown).
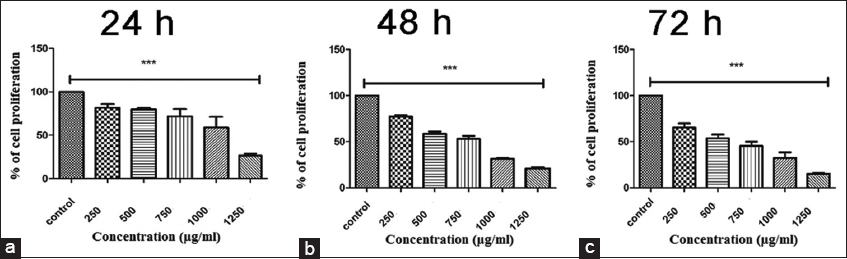
- Cytotoxic effect of PFEE on HeLa cell at (a) 24 h, (b) 48 h, (c) 72 h. in vitro cytotoxic effect of PFEE (0–1250 μg/ml was measured by MTT assay on HeLa cell line. Bar graphs shows % of viable cells (Y axis) against PFEE concentration (X axis). (a-c) demonstrates decreasing percentage of viable cells with increasing concentration of PFEE as compared to untreated cell. Data are representative of three independent experiment and bar graph showed mean ±SEM (**P < 0.05, ***P < 0.001, ns not significant)
Cell cycle arrest by PFEE
The cell cycle distribution of HeLa cells was subsequently investigated using flow cytometry with the PI staining method [Figure 4]. The data presented in Table 5 revealed that, for the negative control (DMEM vehicle), the percentage of cells distributed in the G0/G1 phase of the cycle was 63.1 ± 5.23%, although on treatment with PFEE at concentrations of 500, 750, and 1000 μg/ml, the percentages of cells distributed in the G0/G1 phase increased gradually, to 70.6 ± 6.67%, 72.7 ± 6.79%, and 77.9 ± 7.01%, respectively. Hence, the arrest of cell cycle was happened at G0/G1 by this PFEE.
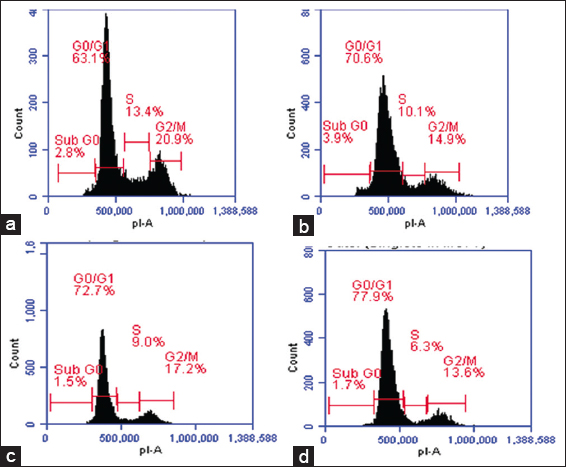
- Flow cytometric detection of the effect of PFEE on HeLa cell cycle phase distribution. (a) Control. (b) 1000 μg/ml. (c) 1250 μg/ml (d) 1500 μg/ml shows interfere with cell cycle population by inducing G0/G1 arrest of HeLa cell in vitro, in 24 h
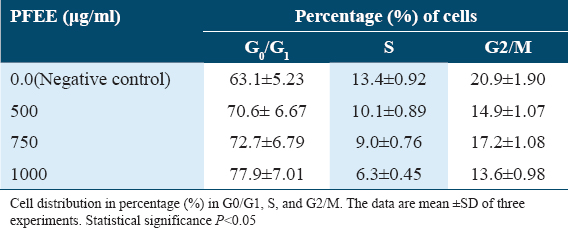
Effect of PFEE on induction of apoptosis as measured by protein expression with a western blotting assay
To understand how PFEE induced apoptosis of cancer cells and inhibited cell proliferation, the gene expressions of pro- and anti- apoptosis genes such as, P53, Pro-caspase 3, Pro-caspase 9, Cleaved caspase 3, Cleaved caspase 9, Bax and Bcl-2, were detected by bands in western blotting. The density of bands between the target protein and actin (control) was calculated. In control, band densities of P53, Bcl-2, Pro-caspase 3, Pro-caspase 9, Cleaved caspase 3, Cleaved caspase 9, and Bax, were 1.86 ± 0.45, 0.95 ± 0.22, 1.79 ± 0.49, 1.00 ± 0.49, 0.74 ± 0.05, 0.64 ± 0.02, and 0.55 ± 0.04%, respectively. In highest dose, band densities of P53, Bcl-2, Pro-caspase 3, Pro-caspase 9, Cleaved caspase 3, Cleaved caspase 9, and Bax were 4.15 ± 0.65, 0.39 ± 0.04, 0.42 ± 0.01, 0.35 ± 0.01, 1.95 ± 0.89, 1.69 ± 0.67, and 1.44 ± 0.45%, respectively. It indicated that gradual increase of dose of PFEE increased the band density of proteins (%) except Bcl2, Pro-caspase 3, and Pro-caspase 9. In these three cases, reverse trend was noticed. We found that treatment with PFEE decreased the expression of gene Bcl-2, while increased the expression of pro-apoptotic genes Caspase 3 and Caspase 9 but decreased the pro-caspase 3 and 9 [Table 6] in HeLa cells in vitro. Moreover p53 gene, one of important cancer guard genes showed high expression, that is, it was upregulated by PFEE. Using western blotting, it was observed that the levels of p53 protein and its downstream target proteins were up regulated. The expression of Bax was increased gradually as concentrations increased [Table 6].
Effect of PFEE on intracellular ROS generation
To investigate the intracellular levels of ROS, the cell-permeable probe H2DCFDA was utilized. Non-fluorescent H2DCFDA, which is hydrolyzed to DCFH inside the cells, yields highly fluorescent DCFDA in the presence of intracellular H2O2 and associated peroxides. Whether or not PFEE increased or decreased the generation of ROS was subsequently examined. As shown in Figure 5, PFEE treatment increased the generation of ROS in HeLa cells, as determined by monitoring DCF fluorescence, when the dose of PFEE was gradually increased.
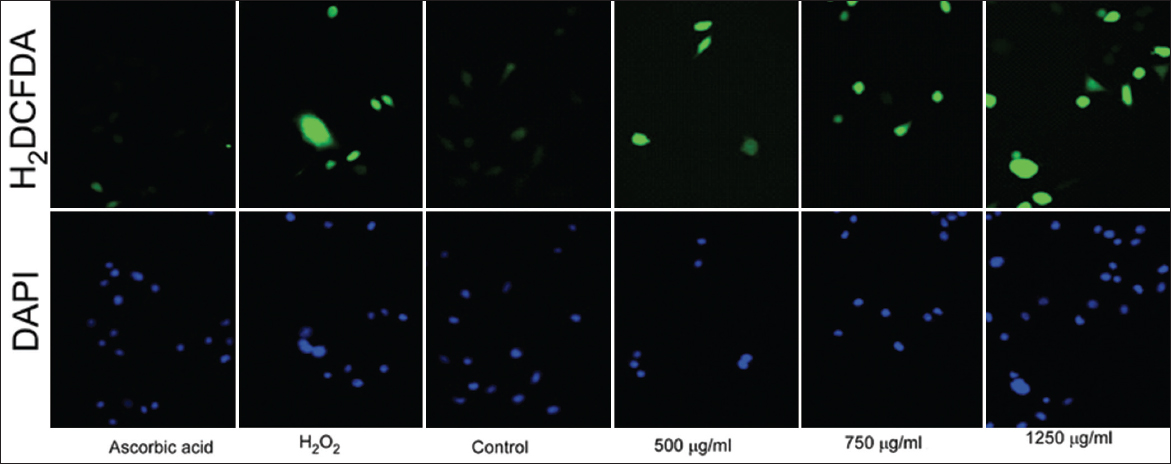
- Demonstrates intracellular ROS generation by PFEE in HeLa cell. Treated cells showed high-intensity green fluorescent as compared to control cell, here ascorbic acid and H2O2 used as a negative and positive control
Effect of PFEE on the MMP
Changes in the MMP are key events in measuring levels of cell apoptosis. Therefore, alterations in the MMP were investigated in the present study using the JC1 dye, after 12 h of PFEE treatment in the HeLa cell line. Figure 6 revealed that non-treated cells exhibited a high level of JC1 aggregates (indicated by the red fluorescence), whereas cells that were treated with PFEE exhibited a red-to-green (monomeric) shift in the fluorescence. This experiment also revealed that the MMP change was concentration-dependent.
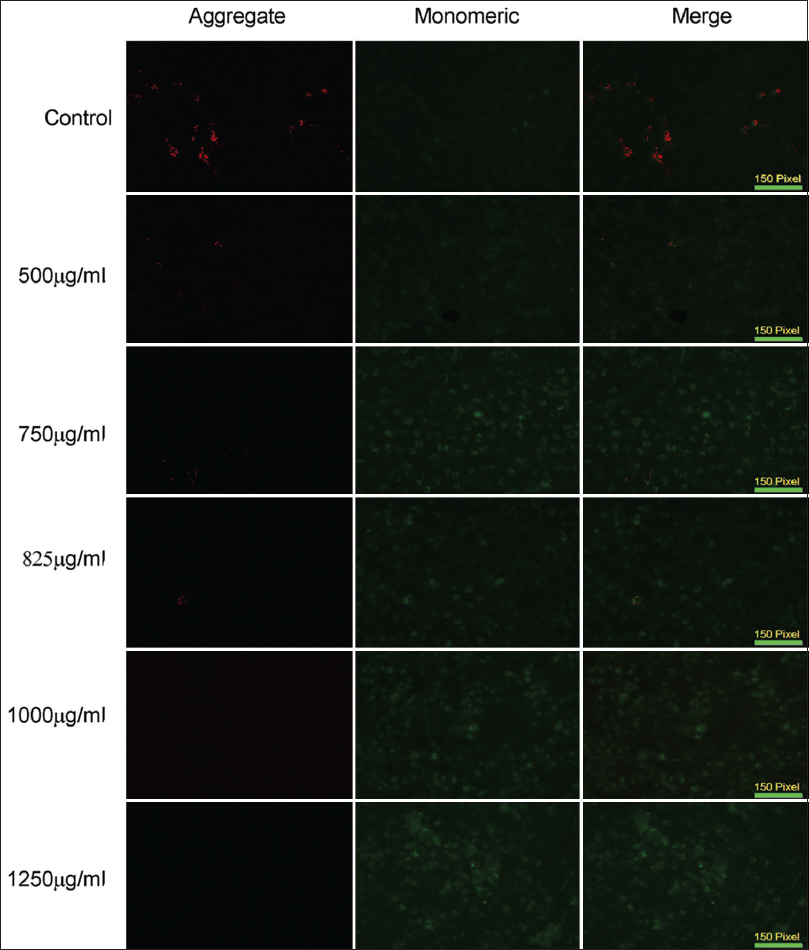
- Fluorescence microscopic (10×) images of HeLa cells after JC1 staining. Control cell showed no MMP change as they are mostly red (JC1 aggregate), but treated cells are mostly green (JC1 monomeric)
DNA fragmentation assay
DNA fragmentation of HeLa cells treated with PFEE was easily detected as DNA laddering, prominent in the agarose gel electrophoresis experiments [Figure 7]. Increases in the concentration of PFEE led to increase in the extent of the laddering.
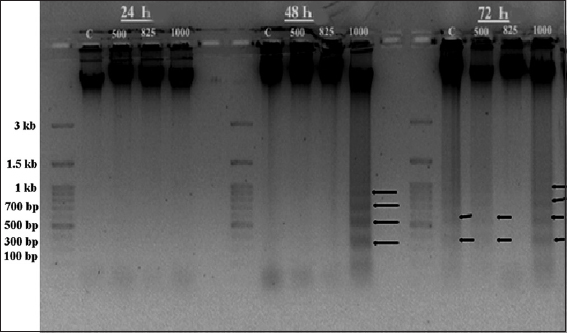
- Agarose gel (1.5%) electrophoresis of isolated HeLa cell DNA for 24,48 and 72 h. Control (c) lane showed no fragmented DNA but 1000 μg/ml showed heavily fragmented DNA in the form of ladder after 72 h.
Anti-migration effects mediated by PFEE against HeLa cells as determined from the wound healing/scratch assay experiment
Cell migration is an important property of cancer cells. The effect of PFEE on cell migration was examined using a wound healing/scratch assay in vitro. Images were captured to show the migration of cells, and these were presented in Figure 8a and b. Cells in the control experiment (untreated/DMEM vehicle) filled the scratched space after the 24 h period of incubation, whereas treatment with PFEE reduced cell migration, as evidenced by the wider open space left between the two sides of the cells [Figure 8a]. In the case of treatment with 500 μg/ml PFEE, 52% of the cells migrated to the scratch, whereas on 1000 μg/ml PFEE treatment, only ~25% cells migrated into the scratched area [Figure 8b]. These results demonstrated that the inhibitory effect of PFEE on cell migration was dose-dependent.
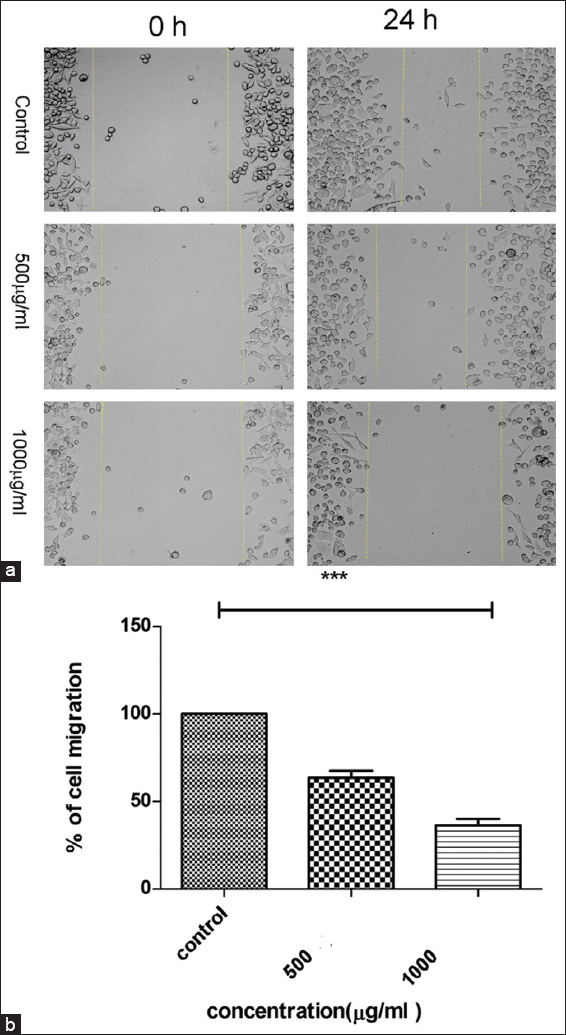
- Effect of PFEE on migration of HeLa Cells in scratch method. (a) At 0 h no cell migration in control and treated (500 and 1000 μg/ml) and after 24 h control showed scratch/distance is almost filled by migrated cells but treated showed some cells are migrated in the scratch. (b) Showed bar graph of percentage of cell migrated (Y axis) against concentration of PFEE (X axis). Data are representative of three independent experiments and bar graph shows mean ± SEM (**P < 0.05, ***P < 0.001, not significant).
Effect of PFEE treatment on anti-colonization in HeLa cells
Subsequently, the ability of PFEE to inhibit colony formation of the HeLa cells on 6-well plates was examined. Figure 9 revealed that PFEE gradually decreased the capability of these cancer cells to form colonies. When the dose of PFEE was gradually increased up to a maximum dose of 125 μg/ml, a minimum number of cells were identified upon 14 day(s)’ treatment [Figure 9a]. The percentages of colony formation with respect to untreated (control) were calculated for each concentration of PFEE. At the lower concentration (50 μg/ml), the percentage of colony formation was 53.25, and this decreased concomitantly with an increase in the concentration of PFEE. At the highest concentration (1,250 μg/ml), colony formation was reduced to only 12.45% [Figure 9b]. Anchorage-independent growth of cancer cells in vitro is one of the most important properties that cancer cells have, and colony formation is also associated with the in vivo oncogenic potential of cancer cells.
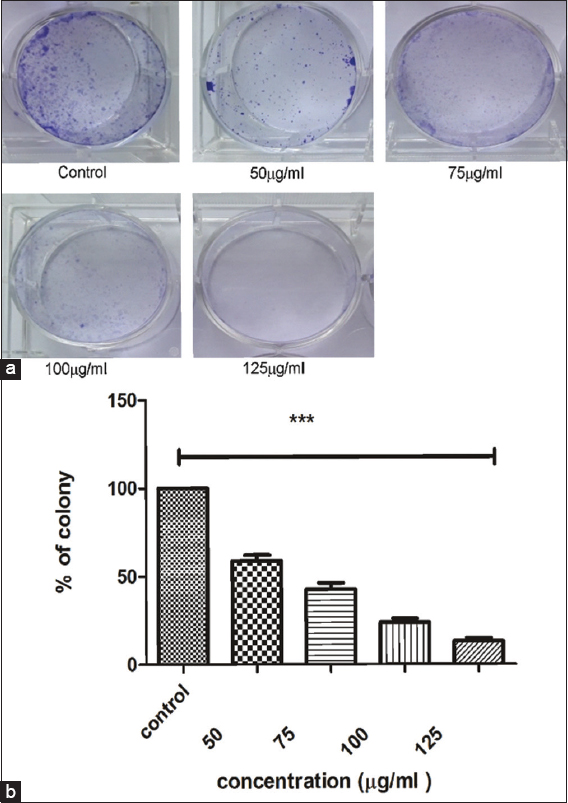
- Effect of PFEE on colony formation of HeLa cells in vitro. (a) Showed dose dependent inhibition of clonogenicity in HeLa cell. (b) Showed bar graph of percentage of colonies (Y axis) against concentration of PFEE (X axis). Data are representative of three independent experiments and bar graph shows mean ± SEM (**P < 0.05, ***P < 0.001, ns not significant)
Antitumor effects of PFEE treatment in HeLa-implanted mice
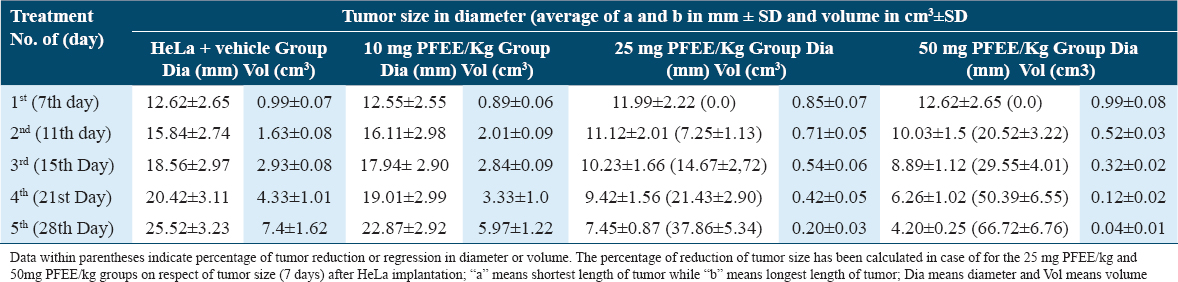
Growth of the tumors in Hela cell-implanted mice is shown in Figure 10, and data of this experiment have been plotted in line plot [Figure 11]. Tumors in the HeLa + vehicle group increased in size after 7 days (12.62 ± 2.65mm3), reaching a maximum of 25.52 ± 3.23 mm3 after 28 days. The tumor sizes for the 50 mg/kg PFEE treatment groups on days 11, 15, 21, and 28 were 10.03 ± 1.5, 8.89 ± 1.12, 6.26 ± 1.02, and 4.20 ± 0.25 mm3, respectively [Table 7]. When the weight of the tumors in this group was measured on day 28, following the sacrifice of the mice, the tumors were of the minimum weight (280 ± 14.32 mg), whereas, in the HeLa + vehicle control group (negative), the tumors attained the maximum size (2230 ± 30 mg). Treatment with 50 mg/kg PFEE led to a marked reduction in the size (66.72%) and weight (87.44%) of the tumors. However, the reduction of tumor growth in the HeLa + PFEE10 treatment group (22.87 ± 2.92 mm3) did not reach a significant level compared with the HeLa+ vehicle group (25.52 ± 3.23 mm3). The visceral organs (kidney, liver, spleen, and lungs) were morphologically observed, and they were revealed to be normal and intact: No disorganization, no lesions and no abnormal growth were identified.
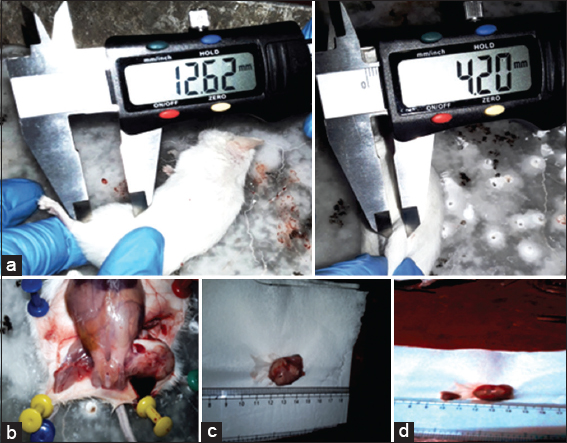
- Effects of PFEE on tumor growth. PFEE suppresses the growth of cervical cancer tumors in Swiss albino mice. Mice were inoculated subcutaneously in the right flank with 2 × 106 HeLa cells. Tumor volume was measured twice/week using a caliper and calculated as (width) 2 × length/2. Representative images were captured at the end of therapy, (a) shows measurement of tumor treated (50mg PFEE/Kg) and control (HeLa+ vehicle), (b) sacrificed mouse (dissected), (c) tumor from PFEE (10 mg/kg) treated mouse, (d) tumor from PFEE (50 mg/kg) treated mouse
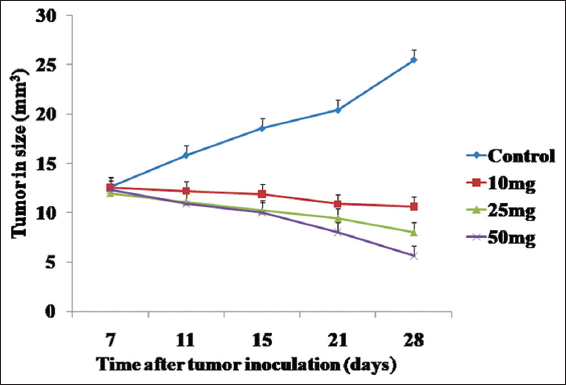
- Graphical representation (line plot) of the effects of PFEE on tumor growth in vivo mg/kg indicates mg PFEE per kg body weight of mice. The data represented as mean ±SD for the three different experiments performed in triplicate. Error bars of ± SD are inserted in figure
Discussion
The antioxidant chemical content and antioxidant property of any food are now prime choice of customers and many mushrooms have antioxidant chemicals and antioxidant property. The nutritional and functional values of mushroom depend on the antioxidant content.[52] Here two extracting procedures such as dip and Soxhlet method were applied but the yield of PFEE by two methods were more or less similar. The several qualitative tests for mycochemistry of PFEE exhibited that it was positive for terpenoids, alkaloids, flavonoids, tannins, and phenolic compounds but negative for Phlobatannins. TPC of fruiting body and mycelial extracts of Pleurotus sp was of 4.62 ± 0.08 and 2.02 ± 0.02 mg GAE/g extract, respectively,[11] while Tsai et al.[53] recorded higher content of fruit body of this mushroom (7.11 ± 0.24 mg GAE/g ethanolic extracts). In our strain of P. florida TPC was more or similar to other (7.98±0.78 mg GAEs/g). Differences in the phenolic content may be related to the extraction method, species, strain, etc., of mushroom.[54] In the literature; total flavonoid content of methanolic extract of fruit body of P. ostreatus, Pleurotus sajor caju, and Pleurotus sapidus were 1.82, 1.88, and 1.39 mg QE/g, respectively, and it varied due to species of mushroom[55] but total flavonoid content of P. ostreatus was below 1mg.g-1[56] In our experiment, total flavonoids were greater than earlier workers (2.031.85 ± 0.024 mg QEs/g) but our species were P. florida and solvent was ethanol. The detailed chemistry and biological effects of flavonoids and phenolic acids have been studied by some workers.[57,58] The scavenging activity of flavonoids against several free radicals and maximum kinds of oxidizing molecules, including singlet oxygen have been observed.[59] Furthermore, flavonoids have ability to alter peroxidation kinetics and reduce the fluidity of membranes.[60,18] Research revealed that flavonoids were prospective compounds against cancer as they inhibited cancer cell proliferation, triggered apoptosis, checked angiogenesis, and metastasis for anti-cancer therapy. The presence of ascorbic acid in different species of Pleurotus was reported to be variable, if we go through the literature survey. Tsai et al.[53] recorded that its content was relatively higher in P. citrinopileatus (6.76 mg/g) and P. ostreatus (6.55 mg/g), P. eryngii (5.88 mg/g) but in other species such as P. sajor-caju, Pleurotus flabellatus, and P. florida, its presence was lower (4.20, 4.15, 3.89, and 3.76 mg/g, respectively) and even not presence in some extracts such as ethanolic and hot water extracts of P. ferulae and P. ostreatus fruit bodies.[53] In our PFEE of P. florida, its presence was very low in amount (0.167 ± 0.03 mg AAEs/g). From this study, it was found that P. florida contains good amount of antioxidant chemicals such as phenolic compound, flavonoids, and ascorbic acids. Furthermore, PFEE showed good activity of free-radical scavenging (antioxidant) potentiality. The mushroom products among the various natural compounds have been recognized as the useful candidates for the searching of effective antioxidant with radical scavenging potentiality[61-63] and Vieira et al.[64] also recorded antioxidant content and activity of effects of the oyster mushroom P. ostreatus. Antioxidant properties of different edible mushroom species including P. eryngii have been recorded by some workers.[12] The antioxidant capacity of the methanolic extracts of P. florida and Calocybe indica was determined by the DPPH method and different concentrations of P. florida and C. indica (200–1000 μg/ml) showed maximum DPPH RSA of 37.04 ± 0.15 and 28.04 ± 0.41%, respectively.[65] The IC50 values of fruiting body extracts of wild Pleurotus (0.45 ± 0.04 μg/ml) which was more higher than those of mycelial extracts.[66] Similarly, Reis et al.[67] recorded that fruiting body extracts of P. ostreatus had much higher DPPH scavenging potentiality than its mycelial extracts. Here, our PFEE of cultivated P. florida (fruit body) exhibited its IC50 activity as 41.17 ± 1.42 μg/ml which was far lower than other reports. Many authors have suggested that mushrooms rich in antioxidant activity play an important role in cancer prevention.[68-70] Xu[71] proposed phenolic compounds to be the major effective anticancer compounds. Phenolic compounds have been found to inhibit TNF-α gene expression and their production.[72,73] Differences in the antioxidant activity may be related to the extraction method, species, strain, etc., of mushroom.[54] Furthermore, it should be noted that temperature influences antioxidant property of substance. In general, the activity of antioxidants has been found due to heating; but, changes in temperature may affect the mode of mechanism of some antioxidants depending on the environment in which they are present.[74] Preserving the mushroom or plant products at different low temperatures (20°C, 4°C, −10°C, −20°C, and −40°C) before estimation of antioxidant, has an effect on activity of antioxidant.[75-77] As from reports of earlier workers, the reducing the storage temperature of frozen food caused the antioxidant activity to fall down. According to Sharpe et al.,[54] the stability for antioxidant activity of antioxidant-rich mushroom extracts on the shelf of market may lose much of their antioxidant activity.
The in vitro anticancer effects of the ethanolic extracts of other species of Pleurotus, such as P. sapindus, on HeLa cells and several other human cancer cell lines, including MGC-803 (gastric cancer) and A549 and SPC-a-1 (lung cancer) cells,[78] and of P. ferulae on B16F10 (melanoma) and BGC-823 (gastric cancer) cells,[49] have been reported. The methanolic extract of P. ostreatus was shown to suppress the proliferation of breast cancer (MCF-7, MDA-MB-231) and colon cancer (HT-29, HCT-116) cells.[13] The cytotoxicity assay in the present study revealed a maximum inhibition of HeLa cell growth of 84.65% upon PFEE treatment, and cell morphological changes were observed, from the normal spindle shape to either rounded or irregular shapes; furthermore, decreases in the confluence level were also observed based on the phase contrast microscopy analysis. Similarly, Jedinak et al.[13] reported that P. ostreatus changed the morphology of MCF-7 and HT-29 cells, although their study was based on a methanolic extract of the fruit body. Bhat et al.[79] obtained a water-soluble extract from P. florida, prepared gold nanoparticles (AuNPs) and applied them to HeLa and other cell lines for 24 h. Their results revealed that water extract (10–30 μg/ml) had no effect on HeLa and other cell lines, although their AuNPs (30 μg/ml) exhibited good cytotoxicity on all cell lines. In the present study, the cytotoxicity of the ethanolic extract from the same mushroom species was investigated at a dose of 1250 μg/ml for 24 h. This revealed an inhibition rate of 75.22% against HeLa cells. Therefore, in comparison with the study by Bhat et al.,[79] and without AuNP preparation of PFEE, our PFEE yielded an improved percentage of inhibition after 24 h treatment at highest dose. Inconsistent PFEE concentrations for different experiments have been used, that is, one kind of limitations of our study.
Chromatin condensation and DNA cleavage are major indicators of apoptosis.[80] Induction of apoptosis is suggested to be one of the major modes of action of chemotherapeutic anti-cancer drugs on malignant cells.[81-83] Ethanolic extracts of P. ferulae were reported to induce apoptosis through caspase 3 activity and by reduction of the MMP.[49] Only one previously published report has demonstrated that PFEE induced apoptosis in the T24 cell line.[84,85] which also suggested that water and methanolic extracts of C. indica could provide a promising agent in the treatment of human sarcoma and breast cancer. In the present study, treatment of the HeLa cells with PFEE led to an induction of apoptosis, as determined by nuclear condensation and the irregular shape of the cells (based on Hoechst staining). Loss of the mitochondrial trans-membrane potential (Δψm), leading to damage of the mitochondrial membrane, is the most important step for the mitochondria-dependent apoptotic pathway. In the present study, PFEE reduced the MMP and led to mitochondrial membrane damage, suggesting that the mitochondrial pathway was involved in PFEE-induced apoptosis. In this experiment, we have not work on isolated single mitochondria for MMP study. Wang et al.[49] also reported a loss of MMP in the B16F10, BGC 823, and GES-1 cell lines when treated with the ethanolic extract of P. ferue. A carboxy methylated P-glucan from the sclerotia of P. tuberregium exhibited anti-proliferative, apoptotic activity against human breast carcinoma MCF-7 cells in vitro.[86,87] Several groups have reported DNA fragmentation of different cancer cells by different mushroom extracts, as evidenced by treatment with ethyl acetate extract (10 g/ml) of Cyathus striatus for 24 h on HPAF-II and PL45 cells,[88] the extract of Agaricus blazei against human leukemia NB-4 and K-562 cells[89], extract from button mushrooms on LNCaP cells,[90] and three other mushroom extracts on COLO-205 cells.[91] The results from our DNA laddering assay have, therefore, corroborated the results from previously published studies.
There are two types of death pathways in apoptosis: The extrinsic pathway and the intrinsic mitochondria-mediated pathway. The intrinsic pathway triggers the release of pro-apoptotic mitochondrial proteins into the cytosol. Consequently, this leads to activation of caspase-dependent and -independent pathways, there by inducing cell apoptosis. In the present study, it was observed that PFEE increased the apoptosis of cells in vitro. Comparison of the effects of polysaccharides and ethanol extracts of Tremella mesenterica revealed that only the ethanol extract induced apoptosis in A549 cells. This was shown to be mediated through activation of a mitochondrial pathway: Disruption of the MMP, the production of ROS, and the activation of caspase-3 protein in A549 cells.[92]
Gu and Belury[16] investigated selective induction of apoptosis in murine skin carcinoma CH72 cells by an ethanol extract of Lentinula edodes. Cordycepin from Cordyceps militaris induced apoptosis of human leukemia cells through a signaling cascade involving a ROS-mediated caspase pathway.[93] According to Xu,[71] intracellular ROS were produced in cancer cells by phenolic compounds. The results of experiments performed by Ahmad et al.[94] in androgen-dependent ALVA-41, and androgen-independent PC-3, prostate cancer cells demonstrated that intracellular ROS increased caspase-3 activity led to a release of cytochrome-c from mitochondria, and subsequently induced apoptosis. Selenium-containing compounds were shown to cause apoptosis and block cell cycle in LNCaP prostate cancer cells through alteration of the cellular redox status by elevating intracellular ROS.[95,96] Already marketed anti-cancer drugs, such as cisplatin and paclitaxel, kill cancer cells through an elevated generation of intracellular ROS.[97-99] Xu[71] observed an increase in the levels of ROS upon treatment with brown button mushroom ethyl acetate extract (BBEA) from Agaricus bisporus in LNCaP cells. In the present study, the treated cells exhibited higher levels of intracellular ROS formation compared with untreated cells, and therefore ROS formation is one of the important factors contributing to an upregulation of caspases-3 and -9, and to apoptosis. The results regarding ROS formation corroborated those of previously published studies performed with different cell lines, and involving different mushroom extracts. To the best of our knowledge, intracellular ROS production by PFEE in the HeLa cell line has not been previously investigated. Our findings revealed that the anti-cancer bioactivity of PFEE was mediated through an induction of apoptosis, through ROS formation, and through MMP reduction. These conclusions were also supported by our protein expression data, which revealed a downregulation of the anti-apoptotic gene, Bcl-2, and an upregulation of the pro-apoptotic genes, Bax, caspase 3 and caspase 9, and an upregulation of the tumor suppressor p53 gene. p53 is one of the pivotal molecules involved in apoptosis induction. Numerous studies have raised the possibility that cells lacking p53 activity due to mutation may be more resistant to cancer chemotherapy.[100-102] The quantification and statistical analysis of the blotting assay has not been done presently. In the cell cycle experiments performed in the present study, PFEE arrested cell cycle at the Go/G1 checkpoint of HeLa cells. Similarly, Jiang and Sliva[103] observed that the methanolic extract of myco-complex induced significant cell cycle arrest at the G2/M phase. Furthermore, cell cycle arrest at G2/M was induced by A. blazei in gastric epithelial cells,[104] and by cordycepin isolated from C. sinensis in bladder cancer cells,[44] whereas methanolic extract of P. ostreatus induced cell cycle arrest at the G0/G1stage of breast cancer cells (MDA-MB-231 and MCF-7), and of colon cancer cells (HCT-116 and HT-29).[16] Similarly, Wang et al.[49] demonstrated that ethanolic extract of P. ferulae arrested the cell cycle of melanoma cancer B16F10 cells at the G0/G1 stage. It is noteworthy that different extracts from G. lucidum demonstrated specific effects on cell cycle progression. Thus, extracts from G. lucidum were shown to induce cell cycle arrest at the G0/G1 phase in breast cancer cells,[105-107] whereas arrest at the G2/M phase was induced in prostate,[105] hepatoma,[108] and bladder[109] cancer. Commercial chemotherapeutic drug such as metformin or irinotecan triggered cell cycle arrest at G1 and S phases of HCT116 and SW480 (colorectal cancer cell lines).[110] On the other hand, Dudhgaonkar et al.[111] isolated tri-terpenes from G. lucidum, and observed that they induced cell cycle arrest at the G0/G1 phase, their study comprising specific biologically active compounds, as well as particular cells.
Colony formation is a key attribute of cancer cells in acquiring metastatic potential. Ganoderic acids from Ganoderma lucidum led to an inhibition of cell proliferation, as well as colony formation with invasive breast cancer cells.[112] P. ostreatus aqueous extract exhibited a greater reduction in the number of colonies of COLO-205 (oral cancer) cells (43.8 ± 3.5% [P < 0.01] compared with 100% proliferation of untreated cells), whereas aqueous extracts from Auricularia polytricha and Macrolepiota procera also exhibited a reduction in colony formation potential, to 59.9 ± 2.6% (P < 0.01) and 47.7% (P < 0.01) colony formation, respectively.[91] In the present study, PFEE inhibited the colony formation of HeLa cells significantly compared with the negative control (DMEM vehicle), in agreement with other groups’ studies. Similarly, migration of cancer cells is also an important factor associated with the metastatic behavior of cancer, and likewise, in the present study, PFEE’s treatment inhibited the migration of cells, as demonstrated in the scratch wound assay experiment.
A study of mice in vivo with implanted BGC-823 cancer cells revealed that the weight and volume of the tumors were reduced after 15 days’ treatment with P. ostreatus mycelium polysaccharide 2 (POMP2).[113] In another study, polysaccharides were extracted from Lepista sordida, and when applied against laryngo carcinoma, these inhibited cell growths in vitro and caused a reduction in tumor size in vivo.[114] The compound ergosterol was isolated from Agaricus brasiliensis, and led to a retardation in tumor growth in sarcoma 180-bearing mice. In studies in vivo, ergosterol was shown to inhibit neovascularization, as well as being potentially an inhibitor of angiogenesis.[9,115] A marked reduction in tumor fluid volume, and changes in various other properties, were identified in Dalton’s lymphoma ascites-bearing mice following C. indica (milky mushroom) treatment, in comparison with negative control group mice.[116] In the present study, 50 mg PFEE/kg body weight of mice also led to a marked reduction in tumor size and volume in HeLa cell-implanted mice.
Conclusions
Considering the various points included in the above discussion, it may be concluded that PFEE containing high antioxidant content and activity, inhibited the proliferation of HeLa cells, induced mitochondrial apoptosis, and arrested cell cycle at the G0/G1 phase. Furthermore, it inhibited colony formation and migration of these cancer cells, blocking metastasis and markedly reducing the tumor size and weight of HeLa-implanted mice. Taken together, these results suggest that the P. florida mushroom may be a novel promising agent for the treatment of cervical cancer.
Limitations
This study limited to extraction of mushroom fruit body using ethanolic solvent and EE was tested for antioxidant content, activity and anticancer activity against HeLa cell line and also as antitumor activity against HeLa implanted mice.
Recommendations for Future Studies
Future analytical chemistry studies for the active chemicals responsible for the anticancer potentials of PFEE and human trial of PFEE are likely recommended to identify a novel class of mushroom species that have broad-spectrum anti-tumor activity for cervical cancer management.
Authors’ Declaration Statements
Ethics approval and consent to participate
All experimental protocols were approved by the Institutional Animal Ethics Committee, Government of India (approval no:12/P/S/IAEC/2018).
Availability of data and material
The data used in this study are available and will be provided by the corresponding author on a reasonable request.
Competing interests
The authors declare that there are no conflicts of interest.
Funding statement
DHESTBT, Govt of West Bengal funded for this research (Grant no.840sanc/ST/P/S&T/1G-11/2015). Funder has no role for designing of research.
Authors’ Contributions
SKG contributed in the design of the study as well as interpretation of the data. KP, TS, TB, and SP participated in data collection and analysis, and manuscript writing
Acknowledgments
The authors are grateful to DHESTBT, Govt. of West Bengal for giving fund for conduction of this research.
References
- Functional Foods:The Consumer, the Products and the Evidence. United Kingdom: Royal Society of Chemistry; 1998. p. :215.
- Evaluation of toxicity of Bilsaan stem bark extracts in Swiss Albino mice:Evaluation of acute toxicity of Bilsaan. Int J Health Sci. 2019;13:31-6.
- [Google Scholar]
- Biochemical studies on the protective effect of honey against doxorubicin-induced toxicity in BALB/C mice. Int J Health Sci (Qassim). 2020;14:31-7.
- [Google Scholar]
- Antitumor polysaccharides from mushrooms:A review on their isolation process, structural characteristics and antitumor activity. Trends Food Sci Technol. 2007;18:4-19.
- [Google Scholar]
- Medicinal mushroom modulators of molecular targets as cancer therapeutics. Appl Microbiol Biotechnol. 2005;67:453-68.
- [Google Scholar]
- Wild and commercial mushrooms as source of nutrients and nutraceuticals. Food Chem Toxicol. 2008;46:2742-7.
- [Google Scholar]
- Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl Microbiol Biotechnol. 2002;60:258-74.
- [Google Scholar]
- Indian medicinal mushrooms as a source of antioxidant and antitumor agents. J Clin Biochem Nutr. 2007;40:157-62.
- [Google Scholar]
- Cancer and its remedy by mushrooms. In: Chanda S, Rakshit A, eds. Biotechnology in Human Welfare. Kolkata: Avenel Press; 2018. p. :29-41.
- [Google Scholar]
- Methanol extract of the oyster mushroom, Pleurotus florida, inhibits inflammation and platelet aggregation. Phytother Res. 2004;18:43-6.
- [Google Scholar]
- Antioxidant properties of different edible mushroom species and increased bioconversion efficiency of Pleurotus eryngii using locally available casing materials. Food Chem. 2013;138:1557-63.
- [Google Scholar]
- Pleurotus ostreatus inhibits proliferation of human breast and colon cancer cells through p53-dependent as well as p53-independent pathway. Int J Oncol. 2008;33:1307-13.
- [Google Scholar]
- Anticancer Ability of Mushroom Polysaccharides:PhD Thesis, School of Chemical Sciences, The University of Auckland, Symonds Street, Auckland 1142 :283.
- Selective induction of apoptosis in murine skin carcinoma cells (CH72) by an ethanol extract of Lentinula edodes. Cancer Lett. 2005;220:21-8.
- [Google Scholar]
- Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm J. 2013;21:143-52.
- [Google Scholar]
- Flavonoids as prospective compounds for anti-cancer therapy. The Int J Biochm Cell Biol. 2013;45:2821-31.
- [Google Scholar]
- Antioxidant capacity and the correlation with major phenolic compounds, anthocyanin, and tocopherol content in various extracts from the wild edible Boletus edulis mushroom. Biomed Res Int. 2013;2013:313905.
- [Google Scholar]
- Awareness of cervical cancer among women in Malaysia. Int J Health Sci (Qassim). 2018;12:42-8.
- [Google Scholar]
- ICO Information Centre on HPV and Cancer 2014
- Epidemiology of cervical cancer with special focus on India. Int J Womens Health. 2015;7:405-14.
- [Google Scholar]
- Projection of new cancer cases in the state of West Bengal, India-2020. Int J Med Biomed Stud. 2021;5:109-20.
- [Google Scholar]
- Identification of the Larger Fungi. Hulton, Amersham: Educational Publications Ltd; 1973. p. :282.
- Die Röhrlinge und Blätterpilze (Polyporales, Boletales, Agariales, Russulales) In: Gams H, ed. Kleine Kryptogamenflora, Band II b/2. Basidiomyceten, 2. Teil, 5. Stuttgart: Aufl, Gustav Fischer Verlag; 1983. p. :1-532.
- [Google Scholar]
- Manual of Indian Edible Mushrooms. New Delhi: Today and Tomorrow Printers and Publishers; 1985. p. :267.
- A rapid DNA isolation procedure from small quantities of fresh leaf tissues. Phytochem Bull. 1987;19:11-5.
- [Google Scholar]
- ITS primers with enhanced specificity for Basidiomycetes-application to the identification of mycorrhizae and rusts. Mol Ecol. 1993;2:113-8.
- [Google Scholar]
- Preliminary phytochemical and elemental analysis of aqueous and fractionated pod extracts of Acacia nilotica (Thorn mimosa) Vet Res Forum. 2014;5:95-100.
- [Google Scholar]
- Preliminary phytochemical screening, quantitative analysis of alkaloids, and antisoxidant activity of crude plant extracts from Ephedra intermedia indigenous to Balochistan. Sci World J. 2017;2017:5873648.
- [Google Scholar]
- Phytochemical evaluation, antimicrobial activity, and determination of bioactive components from leaves of Aegle marmelos. Biomed Res Int. 2014;2014:497606.
- [Google Scholar]
- Study of total phenol, flavonoids contents and phytochemical screening of various leaves crude extracts of locally grown Thymus vulgaris. Asian Pac J Trop Biomed. 2013;3:705-10.
- [Google Scholar]
- Qualitative phytochemical screening and in vitro antimicrobial effects of methanol stem bark extract of Ficus thonningii (Moraceae) Afr J Tradit Complement Altern Med. 2009;6:289-95.
- [Google Scholar]
- Screening of phytochemicals antioxidant and inhibitory effect on alpha-amylase by ethanolic extract of Elaeocarpus Ganitrus (Bark) Int J Pharma Sci Res. 2017;8:5270-5.
- [Google Scholar]
- Phenolic content and antioxidant activity of olive extracts. Food Chem. 2001;73:73-84.
- [Google Scholar]
- Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005;91:571-7.
- [Google Scholar]
- A new colorimetric technique for the estimation of Vitamin C using Folin phenol reagent. Anal Biochem. 1982;127:178-82.
- [Google Scholar]
- Kinetics and mechanisms of antioxidant activity using the DPPH. Free radical method. LWT Food Sci Technol. 1997;30:609-15.
- [Google Scholar]
- Culture of Animal Cells. In: A Manual for Basic Technique (4th ed). New York: Wiley-liss, Ajohuwiley and Sons. Inc. Publication; 2000. p. :235-58.
- [Google Scholar]
- Rapid colorimetric assay for cellular growth and survival:Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55-63.
- [Google Scholar]
- Anticancer activity of solvent extracts of Hexogonia glabra against cervical cancer cell lines. Asian Pac J Cancer Prev. 2020;21:1977-86.
- [Google Scholar]
- Use of Hoechst 33342 staining to detect apoptotic changes in bovine mononuclear phagocytes infected with Mycobacterium avium subsp. paratuberculosis. Clin Diag Lab Immunol. 2001;8:460-4.
- [Google Scholar]
- Analysis of cell cycle by flow cytometry. In: Schönthal AH, ed. Checkpoint Controls and Cancer. Methods in Molecular Biology. New Jersey: Humana Press; 2004. p. :281. 301-11
- [Google Scholar]
- Induction of G2/M arrest and apoptosis in human gastric epithelial AGS cells by aqueous extract of Agaricus blazei. Oncol Rep. 2006;16:1349-55.
- [Google Scholar]
- Measuring survival of adherent cells with the colony-forming assay. Cold Spring Harb Protoc. 2016;2016:pdb-rot087171.
- [Google Scholar]
- Comparative assessment of the apoptotic potential of silver nanoparticles synthesized by Bacillus tequilensis and Calocybe indica in MDA-MB-231 human breast cancer cells:Targeting p53 for anticancer therapy. Int J Nanomedicine. 2015;10:4203-23.
- [Google Scholar]
- Evaluation of the anti-genotoxicity and growth performance impacts of green algae on Mugil cephalus. Life Sci J. 2013;10:1543-54.
- [Google Scholar]
- Hyperketonemia decreases mitochondrial membrane potential and its normalization with chromium (III) supplementation in monocytes. Mol Cell Biochem. 2011;349:77-82.
- [Google Scholar]
- Suppression of tumor growth by Pleurotus ferulae ethanol extract through induction of cell apoptosis, and inhibition of cell proliferation and migration. PLoS One (9):e102673.
- [Google Scholar]
- Antiproliferative, apoptotic, and antimigration property of ethyl acetate extract of Calocybe indica against HeLa and CaSki cell lines of cervical cancer, and its antioxidant and mycochemistry analysis. Middle East J Cancer. 2020;11:454-68.
- [Google Scholar]
- Potential antitumor activity of nonsteroidal anti-inflammatory drugs against Ehrlich ascites carcinoma in experimental animals. Int J Health Sci. 2019;13:11-7.
- [Google Scholar]
- Bioactive of fruiting bodies and submerged culture mycelia of Agaricus brasiliensis (A. blazei) and their antioxidant properties. LWT Food Sci Technol. 2012;46:493-9.
- [Google Scholar]
- Flavour components and antioxidant properties of several cultivated mushrooms. Food Chem. 2009;113:578-84.
- [Google Scholar]
- Comparison of antioxidant activity and extraction techniques for commercially and laboratory prepared extracts from six mushroom species. J Agric Food Res. 2021;4:100130.
- [Google Scholar]
- Study on in vitro antioxidant potentialome of some edible cultivated Pleurotus species (Oyster mushroom) Indian J Nat Prod Resour. 2016;5:56-61.
- [Google Scholar]
- Antioxidant properties of phenolic compounds occurring in edible mushrooms. Food Chem. 2011;128:674-8.
- [Google Scholar]
- Chemistry and biological activities of flavonoids:An overview. Sci World J. 2013;2013:162750.
- [Google Scholar]
- The chemistry and biological effects of flavonoids and phenolic acids. Ann N Y Acad Sci. 1998;854:435-42.
- [Google Scholar]
- Modulation of liposomal membrane fluidity by flavonoids and isoflavonoids. Arch Biochem Biophys. 2000;373:102-9.
- [Google Scholar]
- In-vitro and in-vivo antioxidant effects of the oyster mushroom Pleurotus ostreatus. Food Res Int. 2011;44:851-61.
- [Google Scholar]
- Free radical scavenging activities of mushroom polysaccharide extracts. Life Sci. 1997;60:763-71.
- [Google Scholar]
- Antioxidant and free radical scavenging activities of edible mushrooms. J Food Lipids. 2002;9:35-43.
- [Google Scholar]
- Antioxidant activities, total phenolics and metal contents in Pleurotus ostreatus mushrooms enriched with iron, zinc or lithium. LWT Food Sci Technol. 2013;54:421-5.
- [Google Scholar]
- Antioxidant activity of oyster mushroom (Pleurotus florida [Mont.singer) and milky mushroom (Calocybe indica P and C) Int J Curr Pharm Res. 2016;8:1-4.
- [Google Scholar]
- Production of fruiting body and antioxidant activity of wild Pleurotus. HAYATIJ Biosci. 2016;23:191-5.
- [Google Scholar]
- Antioxidant properties and phenolic profile of the most widely appreciated cultivated mushrooms:A comparative study between in vivo and in vitro samples. Food Chem Toxicol. 2012;50:1201-7.
- [Google Scholar]
- Therapeutic effects of substances occurring in higher Basidiomycetes mushrooms:A modern perspective. Crit Rev Immunol. 1999;19:65-96.
- [Google Scholar]
- The use of mushroom glucans and proteoglycans in cancer treatment. Altern Med Rev. 2000;5:4-27.
- [Google Scholar]
- Antiproliferative and apoptotic effect of methanolic extract of edible mushroom Agaricus bisporus against HeLa, MCF-7 and MDA-MB-231 cell lines of human cancer and chemoprofile by GC-MS. Plant Cell Biotechnol Mol Biol. 2020;21:109-22.
- [Google Scholar]
- Anti-cancer Effects of PHENOLIC-rich Extracts of Button Mushrooms (Agaricus bisporus) PhD Thesis. USA: Department of Food Science, The Graduate School, The Pennsylvania State University; 2013. p. :135.
- Antioxidant potentiality of Pleurotus ostreatus (MTCC142) cultivated on different agro wastes. Asian J Plant Sci Res. 2015;5:22-7.
- [Google Scholar]
- Modulating TNF-a signaling with natural products. Drug Discov Today. 2006;11:725-32.
- [Google Scholar]
- Effect of temperature on the antioxidant activity of phenolic acids. Czech J Food Sci. 2012;30:171-5.
- [Google Scholar]
- Ellagic acid, Vitamin C, and total phenolic contents and radical scavenging capacity affected by freezing and frozen storage in raspberry fruit. J Agric Food Chem. 2000;48:4565-70.
- [Google Scholar]
- Changes in total anthocyanin content and antioxidant activity in sweet cherries during frozen storage, and air-oven and infrared drying. Fruits. 2016;71:281-8.
- [Google Scholar]
- Investigation of antioxidant activities of Pleurotus ostreatus stored at different temperatures. Food Sci Nutr. 2018;6:1040-4.
- [Google Scholar]
- The experimental study on the antitumor effects of Pleurotus sapidus in vitro. Acta Nutri Sin. 2002;24:139-43.
- [Google Scholar]
- Photo-bio-synthesis of irregular shaped functionalized gold nanoparticles using edible mushroom Pleurotus florida and its anticancer evaluation. J Photochem Photobiol B Biol. 2013;125:63-9.
- [Google Scholar]
- Apoptosis, A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239-57.
- [Google Scholar]
- Apoptosis enzyme-linked immunosorbent assay distinguishes anticancer drugs from toxic chemicals and predicts drug synergism. Chem Biol Interact. 2003;145:89-99.
- [Google Scholar]
- Anticancer potential evoked by Pleurotus florida and Calocybe indica using T 24 urinary bladder cancer cell line. Afr J Biotechnol. 2011;10:7279-85.
- [Google Scholar]
- Study of anticancer effect of Calocybe indica mushroom on breast cancer cell line and human Ewings sarcoma cancer cell lines. N Y Sci J. 2015;8:10-5.
- [Google Scholar]
- Cell-cycle arrest and apoptosis induction in human breast carcinoma MCF-7 cells by carboxymethylated beta-glucan from the mushroom sclerotia of Pleurotus tuberregium. Carbohydr Polym. 2006;66:455-62.
- [Google Scholar]
- Bioactivities and health benefits of mushrooms mainly from China. Molecules. 2016;21:1-16.
- [Google Scholar]
- Extracts of Cyathus striatus Mushrooms, Pharmaceutical Compositions Comprising them and a new Cyathus striatus Strain. 8871197B2. U. S. Patent 2014
- [Google Scholar]
- Phenolic compound concentration and antioxidant activities of edible and medicinal mushrooms from Korea. J Agric Food Chem. 2008;56:7265-70.
- [Google Scholar]
- White button mushroom (Agaricus bisporus) exhibits antiproliferative and proapoptotic properties and inhibits prostate tumor growth in athymic mice. NutrCancer. 2008;60:744-56.
- [Google Scholar]
- Mushroom extracts induce human colon cancer cell (COLO-205) death by triggering the mitochondrial apoptosis pathway and Go/G1-phase cell cycle arrest. Arch Iran Med. 2005;18:284-95.
- [Google Scholar]
- p53 is important for the anti-invasion of ganoderic acid T in human carcinoma cells. Phytomedicine. 2011;18:719-25.
- [Google Scholar]
- Induction of apoptosis by cordycepin via reactive oxygen species generation in human leukemia cells. Toxicol In Vitro. 2011;25:817-24.
- [Google Scholar]
- Intracellular hydrogen peroxide production is an upstream event in apoptosis induced by down-regulation of casein kinase 2 in prostate cancer cells. Mol Cancer Res. 2006;4:331-8.
- [Google Scholar]
- Redox mediated effects of selenium on apoptosis and cell cycle in the LNCaP human prostate cancer cell line. Cancer Res. 1985;45:4229-36.
- [Google Scholar]
- Differential involvement of reactive oxygen species in apoptosis induced by two classes of selenium compounds in human prostate cancer cells. Int J Cancer. 2007;120:2034-43.
- [Google Scholar]
- Arsenic trioxide selectively induces acute promyelocytic leukemia cell apoptosis via a hydrogen peroxide-dependent pathway. Blood. J Am Soc Hematol. 1999;94:2102-11.
- [Google Scholar]
- Improvement of the therapeutic index of anticancer drugs by the superoxide dismutase mimic mangafodipir. J Natl Cancer Inst. 2006;98:236-44.
- [Google Scholar]
- Dual role of hydrogen peroxide in cancer:Possible relevance to cancer chemoprevention and therapy. Cancer Lett. 2007;252:1-8.
- [Google Scholar]
- Prognostic value of p53 gene mutations in a large series of node-negative breast cancer patients. Cancer Res. 1998;58:1451-5.
- [Google Scholar]
- Apoptosis, p53, and tumor cell sensitivity to anticancer agents. Cancer Res. 1999;59:1391-9.
- [Google Scholar]
- Novel medicinal mushroom blend suppresses growth and invasiveness of human breast cancer cells. Int J Oncol. 2010;37:1529-36.
- [Google Scholar]
- Induction of G2/M arrest and apoptosis in human gastric epithelial AGS cells by aqueous extract of Agaricus blazei. Oncol Rep. 2006;16:1349-55.
- [Google Scholar]
- Ganoderma lucidum suppresses growth of breast cancer cells through the inhibition of Akt/NF-kappa B signaling. Nutr Cancer. 2004;49:209-16.
- [Google Scholar]
- Ganoderma lucidum inhibits proliferation of human breast cancer cells by down-regulation of estrogen receptor and NF-kappaB signaling. Int J Oncol. 2006;29:695-703.
- [Google Scholar]
- Ganoderma lucidum extract induces cell cycle arrest and apoptosis in MCF-7 human breast cancer cell. Int J Cancer. 2002;102:250-3.
- [Google Scholar]
- Triterpene-enriched extracts from Ganoderma lucidum inhibit growth of hepatoma cells via suppressing protein kinase C, activating mitogen-activated protein kinases and G2-phase cell cycle arrest. Life Sci. 2003;72:2381-90.
- [Google Scholar]
- Ganoderma lucidum extracts inhibit growth and induce actin polymerization in bladder cancer cells in vitro. Cancer Lett. 2004;216:9-20.
- [Google Scholar]
- Effect of metformin on irinotecan-induced cell cycle arrest in colorectal cancer cell lines HCT116 and SW480. Int J Health Sci. 2021;15:34-41.
- [Google Scholar]
- Suppression of the inflammatory response by triterpenes isolated from the mushroom Ganoderma lucidum. Int Immunopharmacol. 2009;9:1272-80.
- [Google Scholar]
- Ganoderic acids suppress growth and invasive behavior of breast cancer cells by modulating AP-1 and NF-kB signaling. Int J Mol Med. 2008;21:577-84.
- [Google Scholar]
- Antitumor activity of polysaccharide extracted from Pleurotus ostreatus mycelia against gastric cancer in vitro and in vivo. Mol Med Rep. 2015;12:2383-9.
- [Google Scholar]
- Antitumor activity of polysaccharides from Lepista sordida against laryngocarcinoma in vitro and in vivo. Int J Biol Macromol. 2013;60:235-40.
- [Google Scholar]
- Isolation of an antitumor compound from Agaricus blazei Murill and its mechanism of action. J Nutr. 2001;131:1409-13.
- [Google Scholar]
- Evaluation of anti-cancer activity and anti-oxidant status of Calocybe Indica (Milky Mushroom) on Dalton's lymphoma ascites induced mice. Aust J Basic Appl Sci. 2014;8:466-75.
- [Google Scholar]







