Translate this page into:
Association of serum hepcidin with prostate-specific antigen levels in men from high Andean cities of Peru
Address for correspondence: Diana Alcantara-Zapata, Endocrinology and Reproduction Laboratory, Research and Development Laboratories (LID), Faculty of Sciences and Philosophy, Universidad Peruana Cayetano Heredia, Lima 15102, Peru. Telephone: +51 (01) 319-0000. Extension: 233212 or 233213. E-mail: diana.alcantara.z@upch.pe
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Qassim Uninversity and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective:
The prostate-specific antigen (PSA) is the primary biomarker to diagnose prostate cancer. Hepcidin has been reported as an alternative for this diagnosis; however, it is unclear how PSA and hepcidin function at high altitude (HA). This study aims to assess the association between hepcidin with PSA in HA residents chronically exposed to hypobaric hypoxia.
Methods:
We retrospectively examined data of 70 healthy males (aged 18–65-years-old) from four different altitudes cities in Peru: Lima (<150 m), Huancayo (2380 m), Puno (3800 m), and Cerro de Pasco (4320 m). Serum hepcidin, testosterone, and PSA were analyzed by chemiluminescence immunoassay. HA parameters (hemoglobin [Hb], pulse oxygen saturation [SpO2], and chronic mountain sickness [CMS] score) were also included in the study. Bivariate analyses and a multivariate linear mixed model were used to evaluate the association between hepcidin and PSA, adjusted by HA parameters, age, and body mass index (BMI).
Results:
Cases of excessive erythrocytosis (EE) (Hb >21 g/dL) were observed in the three highest cities. Hepcidin was positively correlated with Hb, CMS score, and BMI (P ≤ 0.05). Hepcidin was higher in Huancayo with respect to Puno, while PSA was lower in Cerro de Pasco in regard to Puno and Lima (P ≤ 0.05). Neither hepcidin nor PSA was increased by altitude in each city (P > 0.05). We did not find an association between hepcidin and PSA, even adjusted by age, BMI, Hb, and SpO2 (P ≤ 0.05).
Conclusion:
These findings showed no association between hepcidin and PSA levels in healthy residents at HA.
Keywords
Hepcidin
high altitude
hypoxia
prostate-specific antigen
prostat
Introduction
People living at high altitude (HA) show different mechanisms to adapt to hypoxic environments to compensate for the decrease in partial pressure of oxygen.[1,2] In this acclimatization process, the activation of the endocrine system plays a crucial role.[3] For instance, the sex hormone testosterone is activated in a physiological response to HA exposure regulating hemoglobin (Hb) levels to stimulate erythropoiesis.[4] However, in maladapted individuals, it may cause excessive erythrocytosis (EE) (Hb ≥ 21 g/dL), a solid indicator of chronic mountain sickness (CMS).[5]
Nevertheless, the liver hormone hepcidin controls iron homeostasis and contributes to HA adaptation by sustaining iron availability, which is critical for Hb synthesis during erythropoiesis.[6]
Hepcidin reduces the release of intracellular free iron from the reticuloendothelial system and inhibits intestinal iron absorption without affecting iron reserve. This mechanism acts by blocking the iron transporter ferroportin.[7,8] However, hepcidin synthesis is not restricted only to the liver since its expression was too reported in the prostate epithelial cells in an autocrine regulation.[9,10] Moreover, in patients with prostate cancer, hepcidin expression contributes not only to tumor-associated anemia but also to tumor growth, angiogenesis, and metastasis.[11] This process is mediated by inhibiting ferroportin and increasing intracellular iron retention.[6,12,13] Thence, the proposal of hepcidin as a new prognostic factor for prostate cancer.[11,14]
On the other hand, a biomarker currently used for diagnosing prostate cancer is the prostate-specific antigen (PSA). This biomarker has been slightly studied in HA residents,[15] and it is unknown how it could predict prostate disease in highlands. Scientific literature reported that in healthy HA landers exposed to chronic hypobaric hypoxia, the levels of PSA were lower than sea level landers.[16] Also, in miners with normal values of PSA (≤4 ng/mL) exposed to intermittent hypobaric hypoxia, the PSA levels were lower when the altitude difference between their works and their camps was extensive.[17] Notwithstanding, the accuracy of PSA in predicting prostate illness in men exposed to hypobaric hypoxia is unclear, and complementary biomarkers might be required.
This article analyzed how hepcidin is associated with PSA in healthy men at three high-altitude cities and one sea-level city to test whether hepcidin could be a complementary biomarker in the diagnosis of prostate disease. We hypothesized that hepcidin levels correlate with PSA at low and HAs.
Materials and Methods
Study design and subjects of study
This cross-sectional study evaluated the serum levels of hepcidin and PSA in men from four cities at different altitudes in Peru. Secondary data from the project of the Research Circle in Plants with Effects on Health (N°010-2014-FONDECYT) were analyzed. The data were collected between December 2014 and February 2015 from 70 healthy volunteers from three high-altitude cities: Huancayo (3200 m), Puno (3800 m), and Cerro de Pasco (4340 m), and one city from see-level: Lima (150 m).
To be included in the study, the subjects met the following requirements: men from 18 to 65-years-old, having at least 10 years as a permanent resident in their city, not presenting metabolic illness, and not receiving any medication.
This project was approved by the Universidad Peruana Cayetano Heredia Research Ethics Committee and the National Institute of Health (NIH), belonging to the Peruvian Ministry of Health (Project identification codes 61697 and 63654).
Biological measurements and questionnaires
Biological measurements
Serum hepcidin, PSA, and testosterone were assessed by the Chemiluminescence immunoassay method.[18] The Hb concentration was measured on-site with a HemoCue (Anglholm, Sweden) system. Pulse oxygen saturation (SpO2) was evaluated in the second left finger by a Nellcor N-20 oximeter (Pleasanton, CA). Polycythemia and EE were considered as having Hb 17–20 g/dL and ≥ 21 g/dL, respectively.[5]
Anthropometric measurements
Anthropometric measurements such as body weight and height were performed on each volunteer. Body weight (kg) was measured while the participants wore light clothing and no shoes with a TANITA Body Composition Analyzer BF 350 (Tanita Corporation, Tokyo, Japan). Height was determined with a stadiometer to the nearest 0.1 cm. With these data, body mass index (BMI) was calculated in kg/m2 in three categories: Normal (18–24.9 kg/m2), overweight (25–29 kg/m2), and obese (≥30 kg/m2).[19]
CMS Score
All volunteers completed a test of seven signs and symptoms of CMS based on the “Qinghai CMS Score,” which is based on the following symptoms: breathlessness and/or palpitations, sleep disturbance, cyanosis, dilatation of veins, paresthesia, headache, tinnitus, and a final value of 3 if the Hb value is ≥ 21 g/dL.[20]
Statistical analysis
The characteristics of the participants were presented as absolute frequencies and percentages for categorical variables and median and interquartile ranges for quantitative variables. The χ2 test, Fisher’s exact test (for categorical variables), and Kruskal–Wallis test (for continuous variables) were used to compare groups.[21] The Wilcoxon rank-sum evaluated differences between high-altitude cities (Cerro de Pasco, Puno, and Huancayo) with sea-level (Lima). Hepcidin levels were associated with HA parameters (serum total PSA, testosterone, hepcidin, and CMS score) by the Spearman correlation. Multivariable linear mixed-effect models with a random intercept effect for cities were used to determine the association between hepcidin and PSA, adjusting for age, BMI, Hb, and SpO2 in a stepwise process as previous study.[16] A “null” model was initially done to quantify the correlation in hepcidin among individuals from the same city, using the intraclass correlation coefficient. Model 1 incorporated PSA and might be considered the “benchmark” model with which the other three models would be compared. A value of P ≤ 0.05 was considered statistically significant. Data were analyzed utilizing the R studio software version 4.1.0. (RStudio PBC, Massachusetts, USA).
Results
The characteristics of the participants are shown in Table 1. The mean age was 37 years, with a higher proportion of obese men in Huancayo city (38.5%). Hb increased with altitude and were higher in Huancayo (19.4 g/dL), Puno (17.6 g/dL), and Cerro de Pasco (18.6 g/dL) than Lima (15.3 g/dL). However, the Hb levels in Puno (3800 m) were lower than in Huancayo (2380 m) and Cerro de Pasco (4320 m). Only Hb in Huancayo and Cerro de Pasco was not statistically different (p ≤ 0.05).
The proportion of polycythemia (Hb 17–20 g/dL) was 61.5%, 31.8%, and 43.5% for Huancayo, Puno, and Cerro de Pasco, respectively. The proportion of EE (≥21 g/dL) was 7.7%, 4.5%, and 8.7% for Huancayo, Puno, and Cerro de Pasco, respectively. All high-altitude cities had a CMS score of 2 (Puno) or 3 (Huancayo and Cerro de Pasco), while Lima had 0.
The testosterone levels dropped from 6.5 ng/mL in Lima (low altitude) to 3.6 ng/mL in Huancayo, 2.2 ng/mL in Puno but 5.3 ng/mL in Cerro de Pasco without a tendency to altitude. Although significant only at P = 0.133, the hepcidin levels in Huancayo (23.4 ng/mL) and Cerro de Pasco (18.6 ng/mL) tended to be higher than in Puno (16.3 ng/mL) and in Lima (15.6 ng/mL).
The PSA levels were higher at sea-level and dropped from 0.4 ng/mL in Lima to 0.3 ng/mL in Puno and 0.2 ng/mL in Huancayo and Cerro de Pasco (P < 0.05). Hepcidin levels did not differ statistically among cities a different altitude, although they tended to be higher at HA.
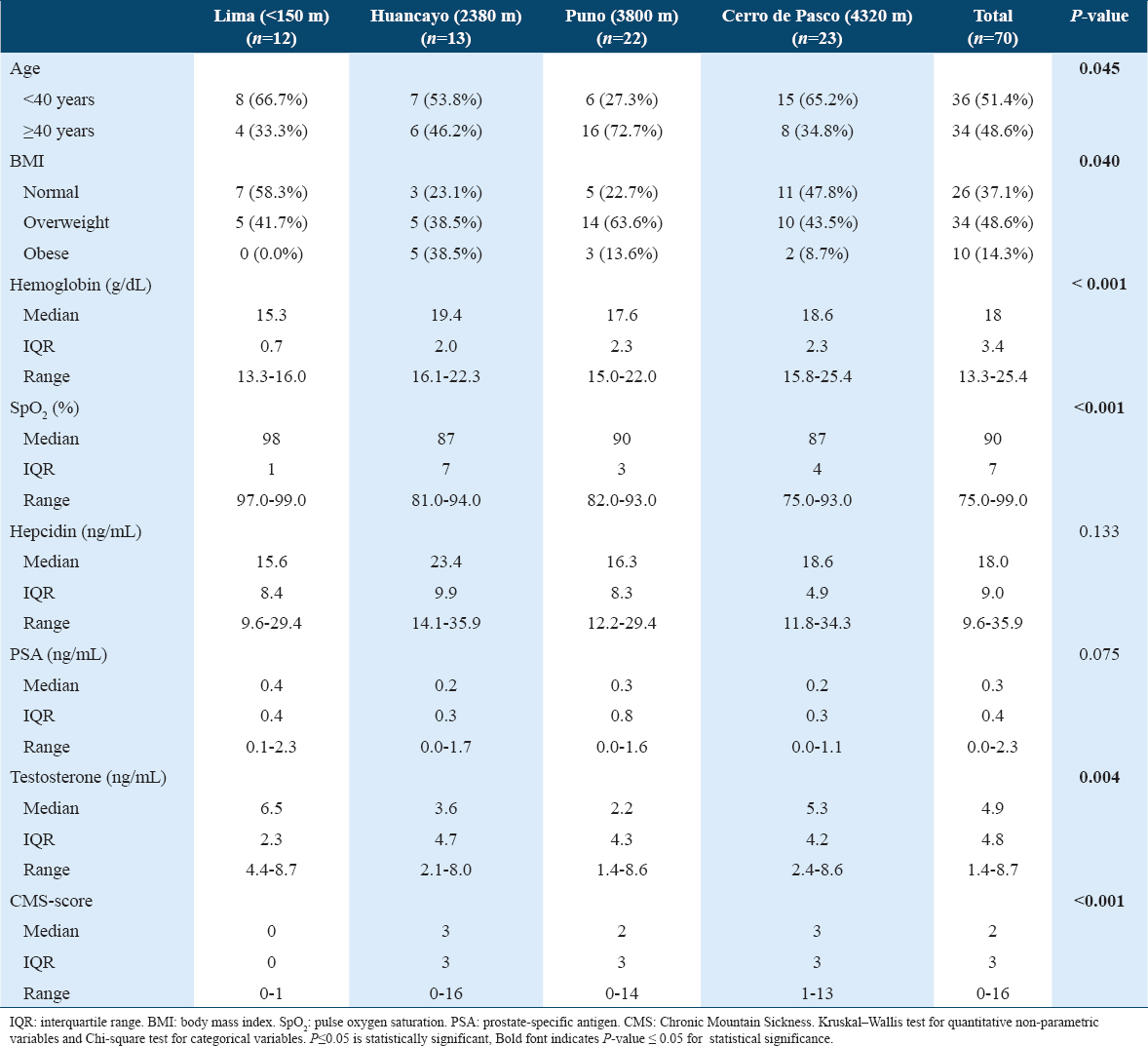
Bivariate association analysis of hepcidin and PSA by city and age [Figure 1] showed no significant correlation between hepcidin concentration and PSA levels at different altitudes. However, hepcidin positively correlated with Hb, CMS score, and BMI (p ≤ 0.05) but not with testosterone or partial pressure of oxygen (SpO2) (p > 0.05) [Figure 2].
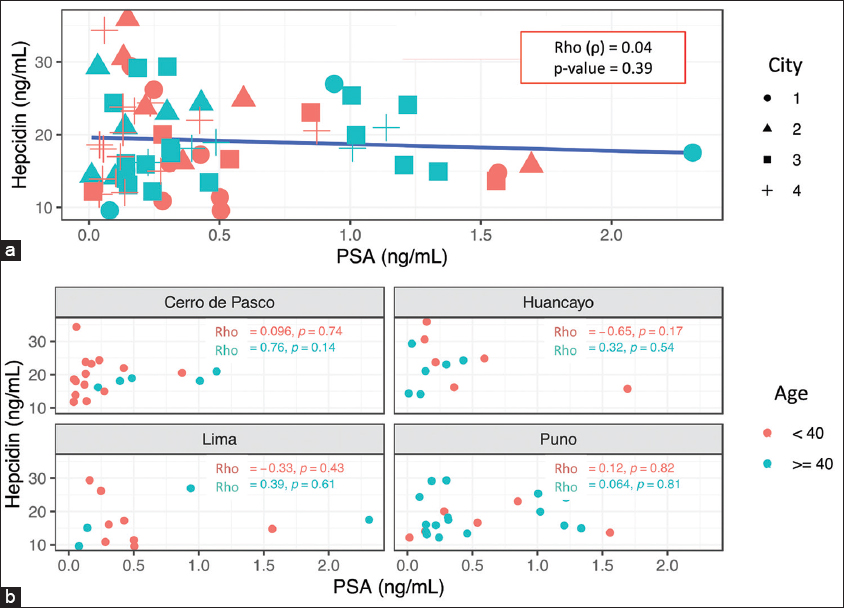
- (a and b) Association between hepcidin and PSA by categorized age and city. City: 1: Lima (<150 m), 2: Huancayo (2380 m), 3: Puno (3800 m), 4: Cerro de Pasco (4320 m). Categorized age: <40 years; ≥40 years. Rho (ρ): Spearman coefficient. PSA: Prostate-specific antigen. Statistical significance: P ≤ 0.05
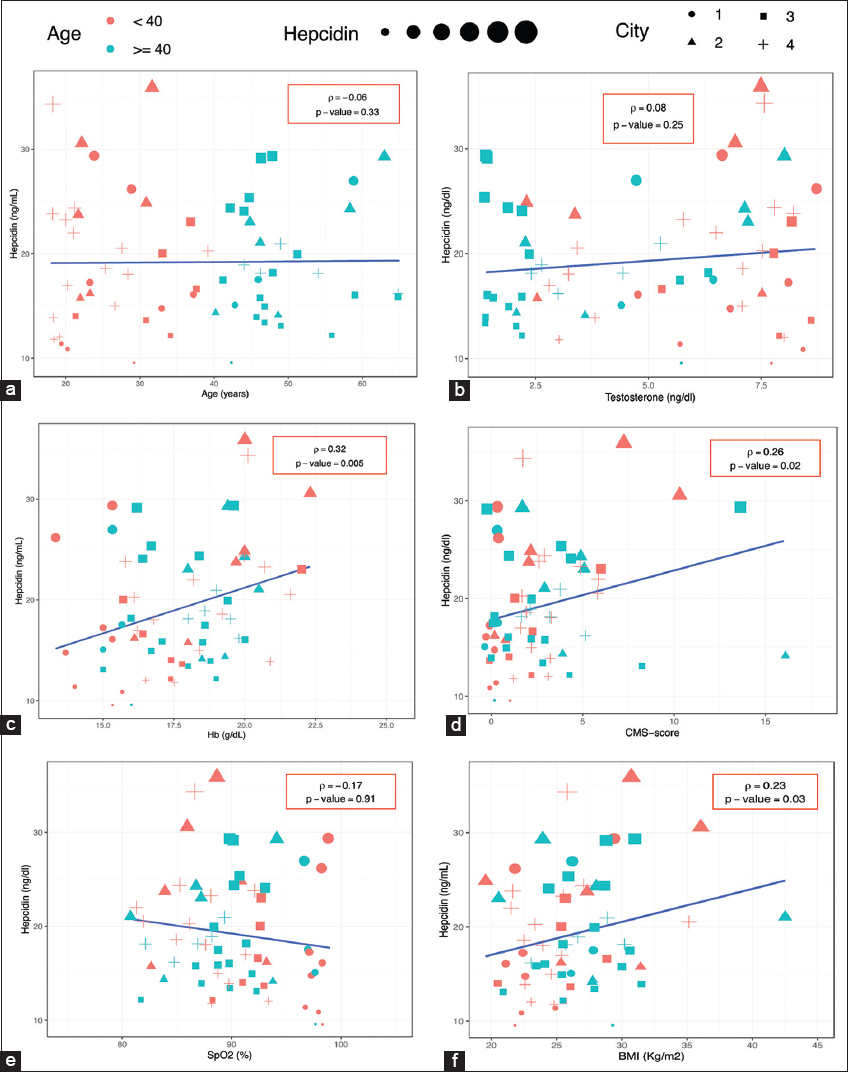
- Correlation between hepcidin and biological parameters by age. City: 1: Lima (<150 m), 2: Huancayo (2380 m), 3: Puno (3800 m), 4: Cerro de Pasco (4320 m). (a) Hepcidin versus Age, (b) Hepcidin versus Testosterone, (c) Hepcidin versus Hemoglobin, (d) Hepcidin versus CMS-score, (e) Hepcidin versus SpO2, (f) Hepcidin versus BMI. Hb: Hemoglobin; CMS: Chronic mountain sickness; SpO2: Pulse oxygen saturation, BMI: Body mass index. Rho (ρ): Spearman coefficient. Statistical significance: P ≤ 0.05
Furthermore, we performed a linear mixed-effect analysis [Table 2]. There were five missing observations for hepcidin (n = 65). The null model showed the intraclass correlation coefficient was 0.03, which indicates that only 3% of the total variance in hepcidin was due to the variability of each city. We found no association between hepcidin and PSA, even adjusted by covariates (age, BMI, Hb, and SpO2) (Models 1–4). Even though, BMI and Hb are positively correlated with hepcidin (Model 3 and 4), these two variables did not contribute to the association between hepcidin and PSA.
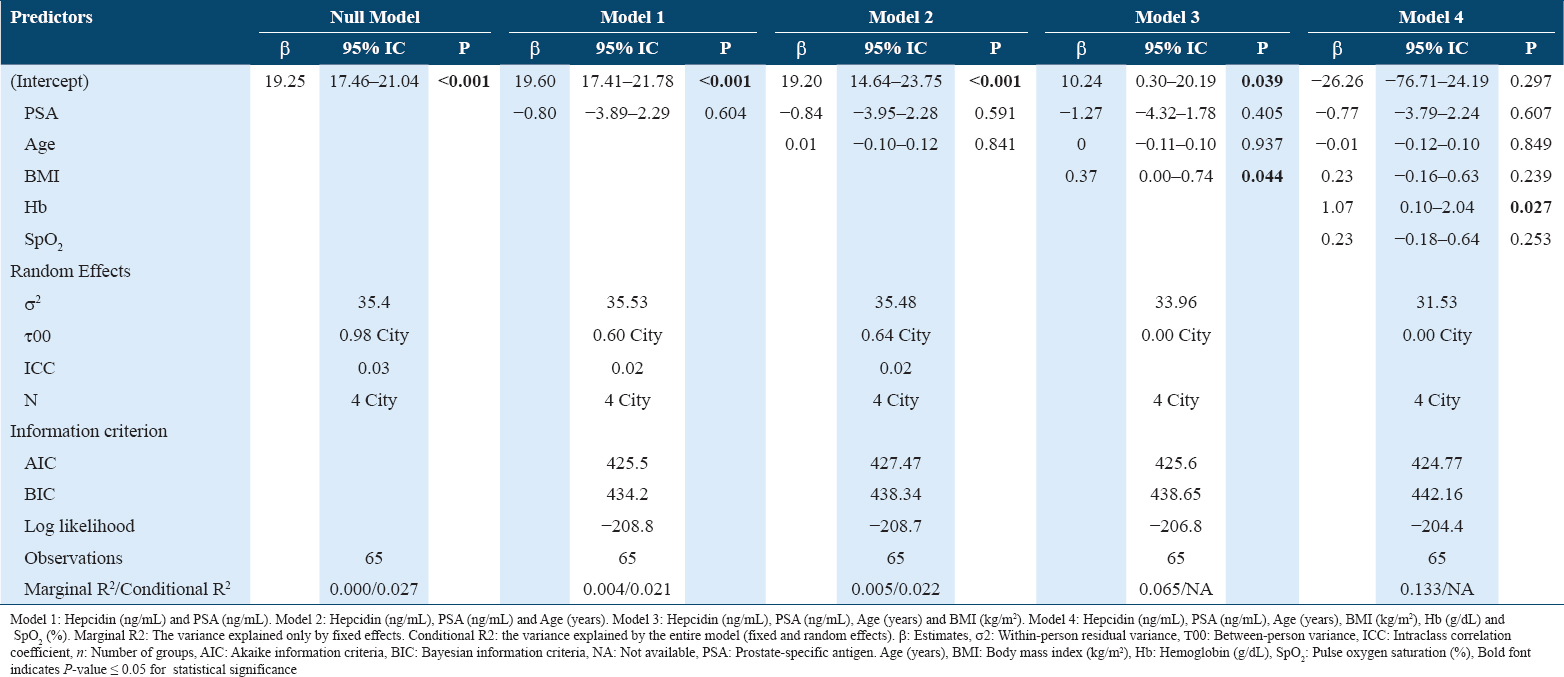
In this analysis, the linear mixed-effect models did not include testosterone due to collinearity with hemoglobin.
Discussion
This pioneer investigation analyzes the relationship between hepcidin and PSA in high-altitude landers. Our study explored this association based on the fact that prostate malignancy is related to oxygen scarcity in the tissue microenvironment due to low oxygen availability and increased oxygen consumption resulting from rapid cell proliferation rates.[22,23]
Another fact that supported our hypothesis is that hepcidin can be secreted by prostate cancer cells.[12,24] Synthesis and secretion of hepcidin from prostate epithelial cells are markedly increased in this organ cancer status.[6] In addition, it has been reported that when troops are rapidly deployed to HA, energy consumption is affected and an equivalent activity at HA requires greater effort and more energy than it does at sea level.[25] Likewise, higher levels of serum and urine hepcidin are presented in athletes after prolonged periods of training,[26] especially during the first 3 h post exercise under hypoxic conditions.[27] Nevertheless, acclimatization to HA also involves a negative energy balance that suppresses appetite and reduces energy intake.[28]
Lifelong exposure to altitude is accompanied by a lower risk for obesity, whereas altitude sojourns are generally associated with a loss of body mass.[29] However, in our study, the higher number of obesity men was at HA cities.
We expected a negative correlation between hepcidin and PSA, not only by hypoxia but also by obesity (which is a BMI category), because it is negatively correlated with PSA.[30] However, we did not find a statistical association between hepcidin and PSA, or a trend with altitude. This probably due to the age of the participants, who were relatively young population, and their age distribution did not show a positive correlation with hepcidin; even the analysis by altitude city.
Obesity has the critical ability to predict stress-associated diseases through triglycerides and cholesterol biomarkers.[31] In this regard, obesity can be considered as a low grade inflammatory state, which stimulates the production of inflammatory markers such as CRP that can up-regulate hepcidin synthesis[32] and related with prostatic diseases,[33] which unfortunately were not analyzed in this study.
Considering that, in this study, there was a significant prevalence of obesity in the high cities (Huancayo, Puno and Cerro de Pasco), we analyzed the relationship between hepcidin and BMI and found a positive association. However, we did not observe a significant correlation between hepcidin and PSA by BMI categories, nor significant correlation between PSA and BMI. Although, we did observe a higher concentration of PSA at sea level (without obese cases) compared to the higher altitude cities, contrarily to the known negative association between PSA and BMI.[34,35]
Because extended sojourns to HA, results in increased erythropoiesis and an increased physiological requirement for iron,[28] we also investigated whether other biomarkers influence the association between hepcidin and PSA. In this sense, the multivariable linear mixed model showed that altitude accounts for only 3% of the variability of hepcidin. Thus, erythropoietin levels, ferritin, iron availability, inflammation markers,[36] and external factors not evaluated in this study might influence our results. Concerning to inflammation, the increased levels of hepcidin in response to exercise may be induced by stress and inflammatory signal.[37]
Indeed, like others before,[38] we show a positive association between hepcidin and Hb after adjusting for BMI, age, and SpO2 as well high and significant correlation with CMS score. This observation is surprising because elevated erythropoiesis demands more iron than usual, where hepcidin diminishes, and the erythroblast-derived hormone erythroferrone suppresses hepcidin expression to meet the elevated iron demand.[36] In addition, increased Hb levels induce higher hepcidin levels, since no iron is required for erythropoiesis.[5,39] However, in CMS patients, basal hepcidin levels were similar to those observed at low and HAs.[8]
For example, the Ethiopian population has steady-state hepcidin levels that do not differ between low and HA, suggesting that natives at HA are not iron deficient.[38] The erythropoietic activity in these people seems to be relatively stable, although elevated. The erythroid regulator signals an imbalance between erythropoietic drive and iron supply rather than absolute marrow activity.[8]
Moreover, in HA the dietary iron and iron recycling from macrophages cannot match the increased requirements for iron.[7] Likewise, in acute hypoxia, these results make us hypothesize that chronic hypoxia might induce a rapid mobilization of iron from storage cells to plasma to ensure enough iron for the mounting red blood cell hemoglobinization.[40] Another hypothesis is that plasmatic hepcidin would not suppress iron-replete individuals chronically adapted to HAs.[38]
In this sense, a man well-adapted to HA should have hepcidin levels as normal as those at sea-level. Therefore, an elevated PSA concentration or a high prevalence of prostate cancer would not be expected. Unfortunately, the scientific evidence on prostate diseases at HA is still insufficient and our results did not clarify this hypothesis.
Further, it was also surprising to find a positive association of hepcidin with CMS score, and with Hb. In this regard, a manifestation of EE (Hb >21g/dL),[41] a sign of CMS, has been associated with higher levels of testosterone.[42]
Testosterone, which also plays an essential role in prostate growth and tumor hypoxia,[43,44] could suppress hepcidin and consequently increase hematocrit in a manner analogous to testosterone therapy,[45] and independently of its effects on erythropoietin or hypoxia-sensing mechanisms.[46] Alternatively, testosterone could increase iron bioavailability and Hb up to EE,[5] and it could also activate ferroportin, transferrin, and transferrin receptor (TR), improving iron bioavailability for erythropoiesis.[47]
Despite these facts, in our results, testosterone did not impact hepcidin, Hb, or CMS as did hepcidin with Hb and CMS score.
Disturbances in hepcidin regulation contribute to the pathogenesis of iron disorders in two ways: One by deficiency, which causes iron overload in hereditary hemochromatosis and non-transfused β-thalassemia; and the other one by overproduction, which is associated with iron-restricted anemia’s seen in patients with chronic kidney disease, chronic inflammatory diseases, and some cancers[48] and several agents aimed at manipulating this pathway have been designed, with some already in clinical trials.[49] However, these pathologies have only been evaluated based on hepatic synthesis, the main determinant of plasma hepcidin concentrations.
Furthermore, regarding molecular studies in prostate cells that showed higher hepcidin levels,[12] their approach allows no differentiation between prostatic and hepatic hepcidin secretion.
Murine studies suggest that hepcidin mRNA levels in the pancreas are 1000 times lower than in the liver.[50] Considering the organ size of the human liver (ca. 1.5 kg) and pancreas (ca. 0.1 kg)[51] and the total number of hepcidin-producing cells, we predict that serum hepcidin levels obtained in our study are predominantly liver-derived. Thus, we cannot affirm that the analyzed serum hepcidin samples come only from the prostate. In addition, prostate hepcidin has an autocrine function,[6] and all its mechanisms developed in prostate cancer cells, such as decreased cell surface ferroportin and increased intracellular iron retention, would only act at the cellular level within the prostate. However, it is not yet known whether differences in total hepcidin and alterations in Hb could influence prostate metabolic pathways.
The limitations in our study are related to the sample size, the lack of measurements of erythropoietin, ferritin, iron availability, and inflammation markers. The time to withdraw the serum testosterone samples (which is hormone time-dependent), and the fact that we have tested this association in healthy men but not in prostate cancer (PCa) patients, are additional limitations.
Even though one research reported higher hepcidin levels correlating with higher PSA levels in prostate patients,[11] we support the hypothesis that hepcidin concentration may depend on the personal and physiologic characteristics to adapt to hypobaric hypoxia. Moreover, we cannot exclude the possibility that this condition would be worst in PCa patients who reside at HA. Further research is needed to demonstrate whether hepcidin would be a good candidate as an adjunctive biomarker in the diagnosis of prostatic diseases in HA residents.
Conclusion
Summing up the aforementioned limitations with our findings and additional analysis (Appendix), it would be difficult to affirm that hepcidin would be a promising biomarker for prostatic illness at HA.
Authors’ Declaration Statements
Ethics approval and consent to participate
All procedures carried out in studies involving human participants were in accordance with the ethical standards of the Institutional and National Research Committee and with the 1964 Declaration of Helsinki and its subsequent amendments or comparable ethical standards. This study was approved by the Ethical Committee of Cayetano Heredia University and the NIH, belonging to the Peruvian Ministry of Health (Project identification codes 61697 and 63654). Written consent form was obtained from all participants.
Availability of data and material
The data that support the findings of this study are not available due to ethical restrictions.
Consent for publication
All authors of this study given their consent for publication.
Competing interest
The authors declare they do not have any conflict of interest.
Funding statement
The present study received a grant from CONCYTEC/CIENCIACTIVA, Perú (N°010-2014-FONDECYT “CIENTIFICOS INC-CIRCULOS DE INVESTIGACION EN CIENCIA Y TECNOLOGIA” to the Research Circle in Plants with Effects on Health. UPCH-SIDISI codes: 61697 and 63654.
Acknowledgments
The authors acknowledge support during fieldwork from Ivan Condori and Alisson Zevallos from Lima. Yeraldine Cardenas, Edith Crispin and Fanny Ugarte from Huancayo. Lidia Caballero, Harnold Portocarrero and Ruben Flores from Puno. Almicar Tinoco, Jhomar Cordova, Julio Chammi, Jesus Leandro, and Alfredo Melo from Cerro de Pasco.
Authors’ contributions
D.A-Z. designed the study and participated in preparing the manuscript, the statistical analysis, and the interpretation of results. G.F.G. participated in the fieldwork supervision, the discussion, and the interpretation of results. M.T. contributes to the preparation of the manuscript, the discussion, and the interpretation of results. All the authors revised and approve the final version of the manuscript.
References
- Physiological and biological responses to short-term intermittent hypobaric hypoxia exposure:From sports and mountain medicine to new biomedical applications. Front Physiol. 2018;9:814.
- [Google Scholar]
- Altitude adaptation through hematocrit changes. J Physiol Pharmacol. 2007;58:811-8.
- [Google Scholar]
- Decreased plasma soluble erythropoietin receptor in high-altitude excessive erythrocytosis and chronic mountain sickness. J Appl Physiol. 2014;117:1356-62.
- [Google Scholar]
- Serum testosterone levels and excessive erythrocytosis during the process of adaptation to high altitudes. Asian J Androl. 2013;15:368-74.
- [Google Scholar]
- Higher androgen bioactivity is associated with excessive erythrocytosis and chronic mountain sickness in Andean Highlanders:A review. Andrologia. 2015;47:729-43.
- [Google Scholar]
- Hepcidin regulation in prostate and its disruption in prostate cancer. Cancer Res. 2015;75:2254-63.
- [Google Scholar]
- Adaptation of iron requirement to hypoxic conditions at high altitude. J Appl Physiol. 2015;119:1432-40.
- [Google Scholar]
- Hepcidin is localised in gastric parietal cells, regulates acid secretion and is induced by Helicobacter pylori infection. Gut. 2012;61:193-201.
- [Google Scholar]
- GDF15 and hepcidin as prognostic factors in patients with prostate cancer. J Mol Biomark Diagn. 2014;5:1-8.
- [Google Scholar]
- Hepcidin and iron metabolism in the pathogenesis of prostate cancer. J BUON. 2017;22:1328-32.
- [Google Scholar]
- Role of hepcidin and iron metabolism in the prostate cancer. Oncol Lett. 2018;15:9953-8.
- [Google Scholar]
- Chronic hypoxia, physical exercise and PSA:Correlation during high-altitude trekking (2004 K2 expedition) Urol Int. 2007;78:305-7.
- [Google Scholar]
- Prostatic-specific antigen levels in men from two andean cities of peru. High Alt Med Biol. 2018;19:213-4.
- [Google Scholar]
- Effects of chronic intermittent hypobaric hypoxia on prostate-specific antigen (PSA) in Chilean miners. Occup Environ Med. 2021;78:753-60.
- [Google Scholar]
- Research progress on chemiluminescence immunoassay combined with novel technologies. TrAC Trends Anal Chem. 2020;124:115780.
- [Google Scholar]
- Association between BMI and semen quality:An observational study of 3966 sperm donors. Hum Reprod. 2019;34:155-62.
- [Google Scholar]
- Consensus statement on chronic and subacute high altitude diseases. High Alt Med Biol. 2005;6:147-57.
- [Google Scholar]
- Extending the mann-whitney-wilcoxon rank sum test to survey data for comparing mean ranks. Stat Med. 2021;40:1705-17.
- [Google Scholar]
- High altitude exposure affects male reproductive parameters:Could it also affect the prostate? Biol Reprod. 2022;106:385-96.
- [Google Scholar]
- Combined mr imaging of oxygen consumption and supply reveals tumor hypoxia and aggressiveness in prostate cancer patients. Cancer Res. 2018;78:4774-85.
- [Google Scholar]
- Iron metabolism:A double-edged sword in the resistance of glioblastoma to therapies. Trends Endocrinol Metab. 2015;26:322-31.
- [Google Scholar]
- Effects of a 7-day military training exercise on inflammatory biomarkers, serum hepcidin, and iron status. Nutr J. 2013;12:141.
- [Google Scholar]
- Live high, train low-influence on resting and post-exercise hepcidin levels. Scand J Med Sci Sport. 2017;27:704-13.
- [Google Scholar]
- High-altitude acclimatization suppresses hepcidin expression during severe energy deficit. High Alt Med Biol. 2020;21:232-6.
- [Google Scholar]
- Serum prostate-specific antigen concentration and hemodilution among Chinese middle-aged obese men:A hematocrit-based equation for plasma volume estimation is induced. Cancer Epidemiol Biomarkers Prev. 2012;21:1731-4.
- [Google Scholar]
- Physiological biomarkers of chronic stress:A systematic review. Int J Health Sci (Qassim). 2021;15:46-59.
- [Google Scholar]
- Study of serum hepcidin as a potential mediator of the disrupted iron metabolism in obese adolescents. Int J Health Sci. 2015;9:171-8.
- [Google Scholar]
- Investigating the link between benign prostatic hypertrophy, BMI and Type 2 diabetes mellitus. Diabetes Metab Syndr Clin Res Rev. 2017;9:148-52.
- [Google Scholar]
- Relationship between prostate-specific antigen and hematocrit:Does hemodilution lead to lower PSA concentrations in men with a higher body mass index?Urology. . 2010;75:648-52.
- [Google Scholar]
- Visceral adiposity index is associated with benign prostatic enlargement in non-diabetic patients:A cross-sectional study. Aging Male. 2018;21:40-7.
- [Google Scholar]
- Iron homeostasis in host defence and inflammation. Nat Rev Immunol. 2016;15:500-10.
- [Google Scholar]
- Association of serum hepcidin levels with aerobic and resistance exercise:A systematic review. Nutrients. 2021;13:393.
- [Google Scholar]
- Plasma hepcidin of Ethiopian highlanders with steady-state hypoxia. Blood. 2014;122:1989-92.
- [Google Scholar]
- Suppression of plasma hepcidin by venesection during steady-state hypoxia. Blood. 2016;127:1206-7.
- [Google Scholar]
- Modulation of hepcidin production during hypoxia-induced erythropoiesis in humans in vivo:Data from the HIGHCARE project. Blood. 2011;117:2953-9.
- [Google Scholar]
- SENP1, but not fetal hemoglobin, differentiates Andean highlanders with chronic mountain sickness from healthy individuals among Andean highlanders. Exp Hematol. 2016;44:483-90e2.
- [Google Scholar]
- High serum testosterone levels are associated with excessive erythrocytosis of chronic mountain sickness in men. Am J Physiol Endocrinol Metab. 2009;296:E1319-25.
- [Google Scholar]
- Prostatic ischemia induces ventral prostatic hyperplasia in the SHR;possible mechanism of development of BPH. Sci Rep. 2014;4:3822.
- [Google Scholar]
- Hypoxia enhances ligand-occupied androgen receptor activity. Biochem Biophys Res Commun. 2012;418:319-23.
- [Google Scholar]
- Testosterone induces erythrocytosis via increased erythropoietin and suppressed hepcidin:Evidence for a new erythropoietin/hemoglobin set point. J Gerontol A Biol Sci Med Sci. 2014;69:725-35.
- [Google Scholar]
- Testosterone administration inhibits hepcidin transcription and is associated with increased iron incorporation into red blood cells. Aging Cell. 2013;12:280-91.
- [Google Scholar]
- Effect of testosterone on hepcidin, ferroportin, ferritin and iron binding capacity in patients with hypogonadotropic hypogonadism and Type 2 diabetes. Clin Endocrinol (Oxf). 2016;85:772-80.
- [Google Scholar]
- Hepcidin-ferroportin axis in health and disease. In: Litwack G, ed. Vitamins and Hormones. United States: Academic Press; 2019. p. :17-45.
- [Google Scholar]
- Normal organ weights in men:Part II-the brain, lungs, liver, spleen, and kidneys. Am J Forensic Med Pathol. 2012;33:368-72.
- [Google Scholar]
- Pancreas volume in health and disease:A systematic review and meta-analysis. Expert Rev Gastroenterol Hepatol. 2018;12:757-66.
- [Google Scholar]







