Translate this page into:
Effect of serum albumin level on high-dose methotrexate induced toxicities in acute lymphoblastic leukemia patients
Address for correspondence: Mehdi Ali Mirza, Department of Pharmacology, ESIC Medical College and Hospital, Sanathnagar, Hyderabad, Telangana, India. Phone: +91-9160368850. E-mail: mehdialimirza@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Qassim Uninversity and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objectives:
Methotrexate (MTX) is a key therapeutic agent for leukemias. When given in high doses, leucovorin rescue is added to reduce its toxicity. It has been postulated that low albumin levels are associated with delayed clearance and increased toxicity of MTX. Hence, this prospective cohort study was proposed to evaluate the correlation between serum albumin level and HDMTX toxicity in acute lymphocytic leukemia (ALL) patients and to compare the MTX toxicity in hypo and normoalbuminemic patients.
Methods:
Forty-six ALL patients of either gender aged 2–40 years receiving HDMTX for 1st time were included in the study. The serum albumin levels were measured before chemotherapy before each cycle. The patients received 24-h infusion of HDMTX on days 8, 22, 36, and 50 (four cycles). The serum concentration of MTX was measured after first cycle only. The patients were followed for toxicities that were graded according to CTCAE-V4.0.
Results:
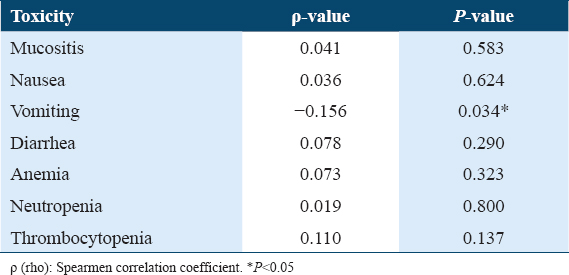
There was a negligible correlation between cumulative albumin levels of all four cycles and cumulative toxic events. The median toxic events were 19 (16–23). The Spearmen correlation coefficient ρ was 0.055 (P = 0.460). No association was found between albumin level and MTX toxicity in cycle wise analysis also. In each cycle, there was no significant difference in the toxicities between the hypo and normoalbuminemic patients. Only vomiting showed significant (P < 0.05) inverse correlation with albumin levels. Hypoalbuminemic patients showed significantly (P < 0.01) higher grade of nausea compared to normoalbuminemia.
Conclusion:
There was negligible correlation between albumin levels and MTX toxicity despite delayed clearance supporting the safety of MTX in mildly hypoabuminemic patients.
Keywords
Acute lymphoblastic leukemia
high-dose methotrexate
methotrexate induced toxicities
serum albumin
Introduction
Acute lymphoblastic leukemia (ALL) is a cancer of the lymphoid line of blood cells characterized by the development of large numbers of immature lymphocytes. Symptoms include feeling tired, pale skin color, fever, easy bleeding or bruising, enlarged lymph nodes, or bone pain. Diagnosis is typically based on blood tests and bone marrow examination.[1] Pediatric patients and young adolescents of ALL are found to be most responsive to present day chemotherapy. On the other hand, in young adults and older patients, clinical remission is rather difficult to obtain and early relapse is more common.[2] The Berlin, Frankfurt, and Munster (BFM-ALL) chemotherapy regimen has been used as a standard treatment for pediatric ALL, with promising outcomes in terms of remission and long-term survival.[3] This includes protocol-M, which consisted of four cycles of high-dose MTX given at a dose of 5 g/m2 once in 2 weeks along with daily 6-mercapatopurine (6-MP).[4] However, effectiveness of this chemotherapy is limited by its toxicity to other tissues in the body. Chemotherapy-related toxicities in general can occur acutely after administration, within hours or days or chronically from weeks to even years.[5] It was estimated that in 2020, about 1.93 million new cases of cancer and 10.0 million deaths occurred globally.[6]
MTX is an anti-folate cytotoxic drug. The inclusion of high-dose MTX with leucovorin rescue enhanced the efficacy of regimens treating lymphomas and acute leukemias.[7] Although, it is regularly administered to patients, it can cause significant renal toxicity including acute kidney injury in 2–12% of patients.[8,9] Non-renal adverse effects of MTX include emesis (10–30%),[10] myelosuppression, mucositis, dermatologic toxicities,[11] transient hepatotoxicity (60%),[12] transient disturbance of CNS (15%),[13] and also pulmonary toxicities (0.5%).[14]
Metabolic derangements due to tumor or pre-existing nephropathy and advancing age, body volume depletion, and raised urine acidity are linked with increased risk of MTX induced nephrotoxicity.[9] Pharmacogenomic factors like polymorphisms of SLCO1B1 gene that encodes a hepatic solute carrier and organic anion transporter mediates the disposition of MTX.[15] Further, mutation of methylene-tetrahydrofolate reductase (MTHFR) gene, coding the enzyme MTHFR, a key enzyme required to metabolize MTX plays a significant role.[16] Thus, all these factors demand careful dose adjustments.
It has been postulated that low albumin levels are associated with delayed clearance and increased toxicity. Thus, hypoalbuminemia affects the oncotic pressure, causing third space fluid collection. This leads to MTX retention, prolonged clearance, and raises its toxicity. Preclinical pharmacokinetic study by Choi et al.[17] using intravenous MTX in analbuminemic rats showed delayed MTX clearance compared to the control rats. In another retrospective human study, Reiss et al.[18] showed that hypoalbuminemia was associated with increased MTX clearance time in patients being treated for lymphoma and leukemia. From these studies, it was concluded that hypoalbuminemia leads to increased MTX toxicity. Moreover, patients with severe toxicity required significantly longer periods of hospitalization.
Corrective measures can be taken before MTX therapy if the correlation is established and quantified between albumin levels and MTX toxicity. These can be incorporated in future guidelines to minimize the adverse effects of high-dose MTX therapy. Very few prospective human studies correlating serum albumin levels with MTX toxicity are available. Hence, this prospective study was carried out to evaluate the effect of serum albumin levels on the extent of MTX toxicity through a range of toxic events outlined in Table 1.
Methods
A single-center, open-label, parallel, prospective, and observational cohort study was carried out. The protocol was approved by the NIMS Institutional Ethics Committee (Approval number EC/NIMS/2153/2018) and registered at Clinical Trial Registry India (CTRI No: CTRI/2018/03/012757). The research was conducted at the Department of Clinical Pharmacology and Therapeutics in collaboration with Department of Medical Oncology, Nizam’s Institute of Medical Sciences, Hyderabad, Telangana State, India. The study participants were recruited from March 1, 2018, to August 30, 2019, over the period of 18 months. Total 54 patients were recruited by including 3 patients/month, based on the outpatient load in Department of Medical Oncology. The patients of acute lymphoblastic leukemia receiving chemotherapy with high-dose MTX for 1st time (BFM-ALL protocol M) aged between 2 and 40 years of either gender were included in the study. Patients with impaired renal, hepatic functions, established ischemic heart disease, and cardiomyopathy were excluded from the study. For children below 7 years parental, written informed consent was obtained. For children aged between 7 and 12 years, oral consent was taken along with the parental informed consent. Children aged between 12 and 18 years (adolescents) child assent form was taken, along with written consent from parents before the study. Written informed consent was obtained from all patients above 18 years. The total treatment duration was 50 days of which the patients were followed on 0, 8, 22, 36, and 50 days for observing immediate and delayed MTX-induced toxicity post.
All the patients were admitted in medical oncology ward for MTX administration. The patient’s serum albumin levels were measured before starting of MTX chemotherapy before each cycle. Serum albumin was measured by bromocresol green method using fully automated equipment (Beckman Coulter). All the patients were divided into two groups depending on the baseline albumin level into hypoalbuminemic and normoalbuminemic group in each cycle. Albumin level <3.5 g/dl was considered as hypoalbuminemia. Albumin level from 3.5 to 5.4 g/dl was regarded as normoalbuminemia.[19] On day 1, all the patients were started tablet 6-MP 25 to 50 mg per day according to body surface area (BSA) and continued for up to 56 days. The first cycle of MTX was started on day 8 and repeated on days 22, 36, 50 (4 cycles). MTX dosage was calculated according to patients BSA following BFM ALL- Protocol. On day 8, patients were given high-dose MTX 5000 mg/m2 as intravenous infusion over a period of 24 h, in which initial 10% dose was given as bolus in the first half an hour. Simultaneously 12 mg of MTX was given by intrathecal route for CNS delivery. The serum concentration of MTX was measured for all patients after 24 h of stopping the infusion, in the first cycle only. Twenty-four hour serum MTX concentration >10 μmol/L was considered at increased risk of toxicity.[20] MTX concentration was estimated using commercially available Kit (Abbott) by fully automated analyzer (Architect system, Abbott).
The clearance of MTX was estimated for all patients. If the serum MTX levels are ≤ 0.05 μmol/L after 24 h, it was defined as cleared and if it was > 0.05 μmol/L, it is defined as not-cleared. Toxicity was graded according to common terminology criteria for adverse events (CTCAE) version 4.0.[21] Immediate toxicity was within 48 hours of MTX infusion and delayed toxicity was toxic events occurred that 48 h post-infusion to the start of the next cycle (day 10–day 21, day 24–35, day 38–49, and day 52–66). All the patients were followed up to 2 weeks after completion of the last cycle for delayed side effects. The safety parameter – CBP, RFT, and LFT were measured before each infusion of MTX. Any other side effects were recorded in the case record form.
The primary endpoint was to assess correlation between albumin levels and cumulative MTX toxicity. Secondary endpoints were: any correlation between serum albumin level and MTX toxicity in each cycle; correlation between serum albumin level and individual MTX toxicity. The changes in toxic events between hypoalbuminemia and normoalbuminemia patients in each cycle regarding number of events and grades of toxicities and any correlation between serum albumin levels and MTX serum concentration were evaluated. Proportion of patients showing delayed clearance of MTX in hypoalbuminemic and normoalbuminenic patients was estimated.
Statistical analysis was performed using IBM SPSS Version 20.0. Demographic data was presented as mean ± SD. The distribution of study variables such as serum albumin levels, serum MTX concentration, and number of MTX toxicity events was assessed using Shapiro–Wilk test. Descriptive data of study variables was presented as medians and ranges. The spearman correlation test was used to estimate correlation between serum albumin levels and MTX toxic events and also serum albumin levels and MTX concentrations. The toxicity difference between hypoalbuminemic patients and normoalbuminemic patients was compared using Mann–Whitney U-test. For ordinal data, the comparison between toxicity grades between hypoalbuminemic and normoalbuminemic patients was analyzed using Fisher’s exact test. P < 0.05 was considered statistically significant. Power of the study was 80%.
Results
From a total of 54 ALL patients who had received high-dose MTX, after screening 46 patients were enrolled. The participant flow diagram is represented in Figure 1 and demographic data in Table 2, respectively.
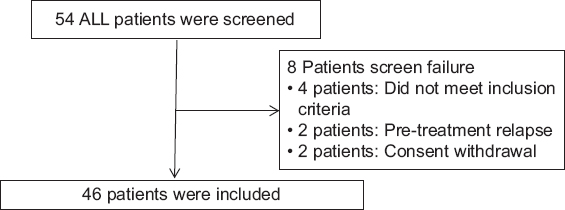
- The participants flow diagram
Correlation between serum albumin levels and MTX toxicity
There was a negligible correlation between serum albumin levels and cumulative toxic events (total number of toxic events) for all the cycles (n = 184). Median cumulative toxic events in all the cycles were 19 (16–23). The spearmen correlation coefficient ρ (rho) of 0.055 (P = 0.460) was observed. Scattered plot is shown in Figure 2.
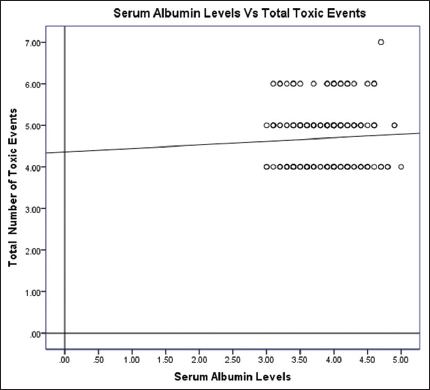
- Scattered plot for all the albumin levels and total number of toxic events
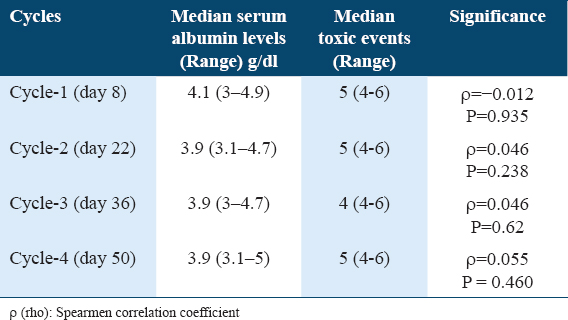
Cycle-wise correlation between serum albumin levels and MTX toxicity
In the cycle wise analysis too, we did not find association between serum albumin level and MTX toxicity. The values are given in Table 3.
Correlation between serum albumin levels and individual toxicities
Each single toxicity was correlated with serum albumin levels of all the patients in all four cycles. Vomiting showed significant (P < 0.05) inverse correlation with serum albumin levels, that is, as the serum albumin level decrease vomiting episodes increase (Figure 3).
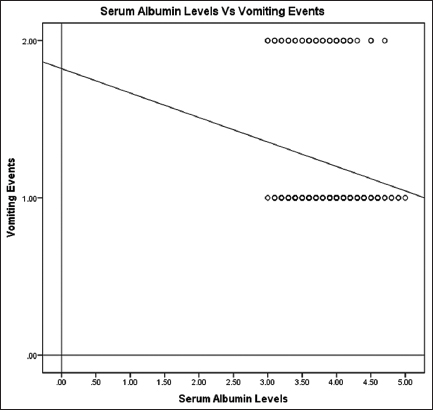
- Scattered plot total albumin levels and vomiting events
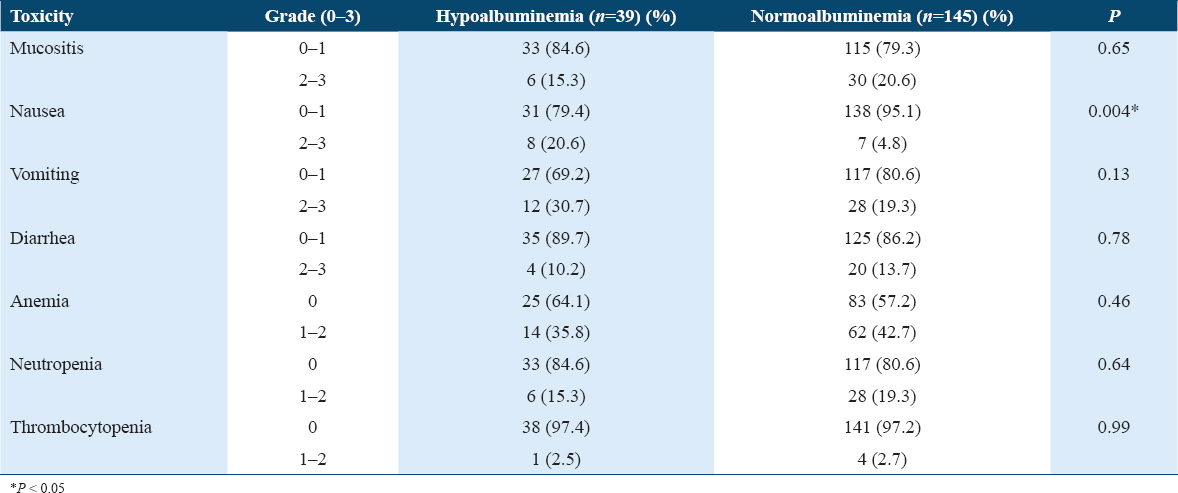
There was no significant correlation between mucositis, nausea, diarrhea, anemia, neutropenia, thrombocytopenia, and serum albumin levels (Table 1).
Comparison of toxic events between normal and hypoalbuminemic groups
We have compared the toxic events between hypoalbuminemic and normoalbuminemic patients in each cycle, but there was no significant difference between the groups. Median toxicities in each cycle in hypoalbuminemia and normoalbuminemia groups are presented in Table 4. The mean toxicities in each cycle in hypoalbuminemia and normoalbuminemia groups are given in Figure 4. Cycle 4 showed a trend in the increase in mean toxicities in hypoalbuminemic patients compared to normoalbuminemic patients. However, this was not statistically significant.
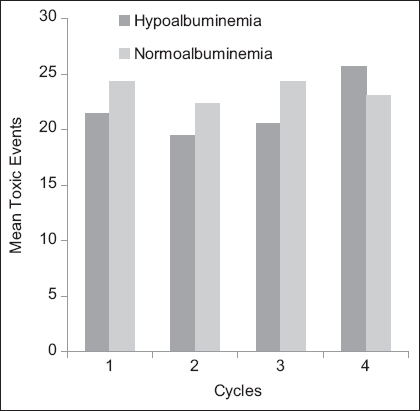
- Graphical representation of mean toxic events between hypoalbuminemic and normoalbuminemic groups at each cycle
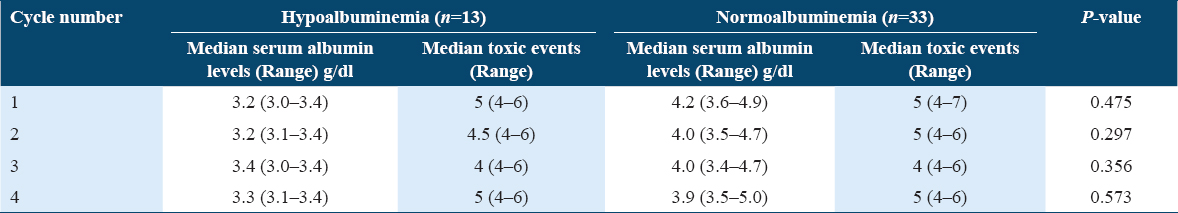
Patients with hypoalbuminemia had showed significantly (P < 0.05) higher grade of nausea compared to those with normoalbuminemia. The grades of other toxicity parameters were not statistically significant in both the groups. The grades of the toxicity and proportion of patients with hypoalbuminemia and normoalbuminemia are shown in Table 5.
Correlation between serum albumin levels and MTX concentrations
The median MTX concentration in the first cycle was 0.20 (0.02–6.58) μmol/L. There was a weak negative correlation between serum albumin levels and 24 h post-infusion serum MTX levels at day 8 (cycle-1). The spearman correlation coefficient ρ was −0.289 (P = 0.05). The scattered plot is represented in Figure 5.
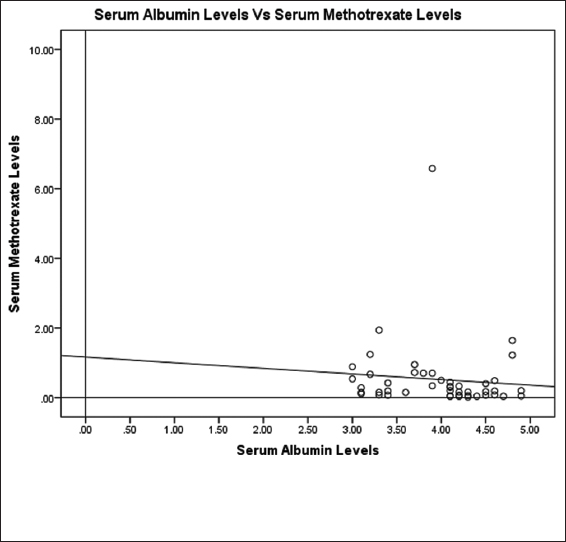
- Scattered plot showing baseline serum albumin levels and 24 h post-infusion serum methotrexate concentrations at day 8 (cycle-1)
Comparison of MTX clearance between hypoalbuminemic and normoalbuminemic groups
No clearance was observed in hypoalbuminemia group at 24 h and 21% clearance in normoalbuminemic patients. The data are represented in Table 6.

Adverse reactions
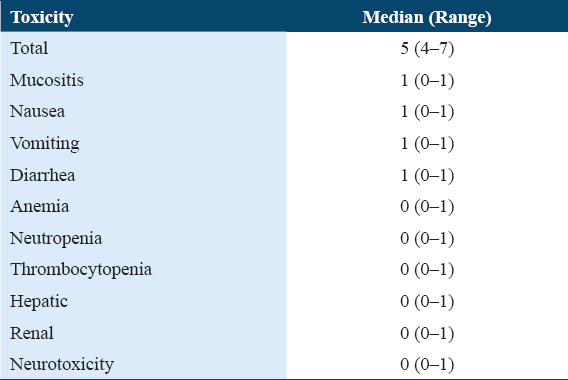
The median cumulative adverse effects were 19 (16–23) in 46 patients. The gastrointestinal side effects such as mucositis, nausea, vomiting, and diarrhea were reported in all 46 patients in all the cycles, either in the form of immediate or delayed toxicity. The myelosuppression events such as anemia, neutropenia, and thrombocytopenia were present in approximately equal proportions in both hypoalbuminemic and normoalbuminemic groups. No patient developed renal and neurotoxicity, and only one patient got hyperbilirubinemia during first three cycles of high-dose MTX. The cumulative median values of each individual toxicity in all 4 cycles (n = 46 patients X 4 cycles = 184) are shown in Table 7.
Discussion
This was a prospective observational study to evaluate the association between serum albumin levels and MTX toxicity in patients with ALL. Very few studies were found in literature on this topic.
The total duration of treatment for ALL is 2.5 to 3 years, this incorporates induction phase, consolidation phase, and maintenance phase. The patients with standard and moderate risk receiving high-dose MTX as a part of extra compartment therapy during consolidation phase (protocol-M) as per BFM-ALL protocol[3,4] were enrolled into our study. According to protocol-M, the patients received four cycles of high-dose MTX with the gap of 14 days and tablet 6-mercaptopurine orally for 56 days. During the induction and consolidation phase, the incidence and grades of toxicities is reported to be much higher.[22]
Our study demonstrated negligible correlation between serum albumin levels and MTX-induced cumulative toxic events. In a multicenter case–control study conducted by Alarcon et al.[23] in rheumatoid arthritis, patients with low-dose MTX showed that low levels of serum albumin had ten-fold higher odds for developing MTX toxicity. Moreover, this study was conducted with low-dose MTX. Our results were not consistent with this study. This might be due to leucovorin rescue that attenuated the MTX toxicity and pre-treatment with sufficient hydration and alkalization of urine to reduce the toxicity risk. To the best of our knowledge, this was the first study in ALL patients to evaluate the association between serum albumin levels and MTX toxicity.
An attempt was made to correlate pre-MTX serum albumin levels with overall MTX toxicity in each cycle. However, we did not find any correlation between serum albumin levels before each cycle and MTX toxicity in cycle wise analysis also.
However, we found significant inverse correlation between serum albumin levels and vomiting. There was no significant correlation with mucositis, diarrhea, anemia, neutropenia, thrombocytopenia, and hepatotoxicity.
All the patients were divided into hypoalbuminemic and normoalbuminemic groups depending on serum albumin levels before starting each cycle. Although the comparison of toxic events between the groups in each cycle was not statistically significant, there was an increase in the mean rank toxicities during the last cycle, in the hypoalbuminemia group.
When grades of the toxicity were compared, we found significantly higher grades (grade 2) with nausea, whereas lower grades were observed with other toxicities (grade-1). In the retrospective study of Reiss et al.,[18] the toxicity grades of MTX were compared with albumin levels in 167 patients. They reported that hyperbilirubinemia and anemia were of significantly higher grades in hypoalbuminemic groups. Our results were not consistent with this study. We found higher grades of nausea in our patients whereas they showed higher grades of anemia and bilirubin levels.
On measuring the clearance of MTX in our study, none of the patients with hypoalbuminemia had shown clearance of MTX at 24 h post-infusion. This confirmed the delayed MTX clearance in hypoalbuminemic group. In the normoalbuminemic group, 21% patients showed MTX clearance. Choi et al.[17] performed a pharmacokinetic study using intravenous MTX in rats. It showed delayed MTX clearance in analbuminemic rats compared to the control rats. In a retrospective study by Reiss et al.[18] for high-dose MTX, a significant relationship has been found between clearance of MTX and hypoalbuminemia patients. Our results were in agreement with the above studies.
The strength of this study: The inter-cycle bias in serum albumin levels was overcome by measuring serum albumin level before each cycle. Second, the bias due to nutritional status of the patients was overcome by advising all the patients a standard protein diet throughout the study period. Third, the patients receiving MTX and oral 6-MP only were selected, to avoid the bias due to multiple drugs used in chemotherapy.
The limitations of our study: The sample size was small. The number of Hypoalbuminemia patients (n=13) were less compared to normoalbuminemia patients (n=33).[24] Second, we did not consider the toxicity of 6-MP because all the patients received 6-MP. Third, causality assessment was not done, because all the patients were receiving two drugs, that is, MTX and 6 MP.
Conclusion
From the negligible correlation between serum albumin level and methotrexate cumulative toxicity events despite delayed clearance, we conclude that leucovorin rescue and pre-treatment with sufficient hydration and alkalization of urine attenuated the methotrexate toxicity. This may henceforth aid the physician to partake MTX therapy in an unrestricted manner. Our study also showed that there was a significant moderately inverse correlation between albumin levels and vomiting events. Higher grades of nausea were found in hypoalbuminemic group. Further, prospective studies should be conducted with larger sample size to support our findings.
Author’s Declaration Statement
Ethics approval and consent to participate
This study was conducted in accordance with the ethical principles of declaration of Helsinki (2013). The Institutional Ethics Committee approval (Approval number EC/NIMS/2153/2018) was taken before the study and registered at Clinical Trial Registry India (CTRI No: CTRI/2018/03/012757). Written informed consent was obtained from all the participants.
Availability of data and material
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Competing Interests
Authors declare no conflicts of interest.
Funding Statement
No external funding was received for this project. It is investigator initiated academic study.
Author Contribution
MAM contributed in the design of the study as well as interpretation of the data. MAM, DA and MLK participated in data collection and analysis, and manuscript writing.
Acknowledgements
The author would like to thank all the patients who participated in this study.
REFERENCES
- Treatment of pediatric acute lymphoblastic leukemia. Pediatr Clin North Am. 2015;62:61-73.
- [Google Scholar]
- Augmented Berlin-Frankfurt-Munster therapy abrogates the adverse prognostic significance of slow early response to induction chemotherapy for children and adolescents with acute lymphoblastic leukemia and unfavorable presenting features:A report from the Children's cancer group. J Clin Oncol. 1997;15:2222-30.
- [Google Scholar]
- Risk-adjusted therapy of acute lymphoblastic leukemia can decrease treatment burden and improve survival:treatment results of 2169 unselected pediatric and adolescent patients enrolled in the trial ALL-BFM 95. Blood. 2008;111:4477-89.
- [Google Scholar]
- The economic burden of toxicities associated with cancer treatment:Review of the literature and analysis of nausea and vomiting, diarrhoea, oral mucositis and fatigue. Pharmacoeconomics. 2013;31:753-66.
- [Google Scholar]
- Global cancer statistics 2020:GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-49.
- [Google Scholar]
- High dose methotrexate with leucovorin rescue Rationale and spectrum of antitumor activity. Am J Med. 1980;68:370-6.
- [Google Scholar]
- Glucarpidase, leucovorin, and thymidine for high-dose methotrexate-induced renal dysfunction:Clinical and pharmacologic factors affecting outcome. J Clin Oncol. 2010;28:3979-86.
- [Google Scholar]
- Preventing and managing toxicities of high-dose methotrexate. Oncologist. 2016;21:1471-82.
- [Google Scholar]
- Antiemetics:American society of clinical oncology clinical practice guideline update. J Clin Oncol. 2011;29:4189-98.
- [Google Scholar]
- High-dose methotrexate-induced renal dysfunction:Is glucarpidase necessary for rescue? J Clin Oncol1. 2011;29:e180.
- [Google Scholar]
- Methotrexate-induced neurotoxicity and leukoencephalopathy in childhood acute lymphoblastic leukemia. J Clin Oncol. 2014;32:949-59.
- [Google Scholar]
- Germline genetic variation in an organic anion transporter polypeptide associated with methotrexate pharmacokinetics and clinical effects. J Clin Oncol. 2009;27:5972-8.
- [Google Scholar]
- The role of MTHFR and RFC1 polymorphisms on toxicity and outcome of adult patients with hematological malignancies treated with high-dose methotrexate followed by leucovorin rescue. Cancer Chemother Pharmacol. 2012;69:691-6.
- [Google Scholar]
- Pharmacokinetics of intravenous methotrexate in mutant Nagase analbuminemic rats. Biopharm Drug Dispos. 2007;28:385-92.
- [Google Scholar]
- Hypoalbuminemia is significantly associated with increased clearance time of high dose methotrexate in patients being treated for lymphoma or leukemia. Ann Hematol. 2016;95:2009-15.
- [Google Scholar]
- Amino acids, peptides and proteins. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics 2018:373-403.
- [Google Scholar]
- High-dose methotrexate with citrovorum factor rescue:Predictive value of serum methotrexate concentrations and corrective measures to avert toxicity. Cancer Treat Rep. 1977;61:779-83.
- [Google Scholar]
- Common Terminology Criteria for Adverse Events (CTCAE) Version 5 2017
- Pharmacogenetics and induction/consolidation therapy toxicities in acute lymphoblastic leukemia patients treated with AIEOP-BFM ALL 2000 protocol. Pharmacogenomics J. 2017;17:4-10.
- [Google Scholar]
- Risk factors for methotrexate-induced lung injury in patients with rheumatoid arthritis A multicenter, case-control study. Methotrexate-lung study group. Ann Intern Med. 1997;127:356-64.
- [Google Scholar]







