Translate this page into:
The pattern of cytomegalovirus replication in post-renal transplant recipients with pre-emptive therapy strategy during the 1st year of post-transplantation
Address for correspondence: Rohitha Muthugala, Department of Virology, National Hospital Kandy, Kandy 20000, Sri Lanka. Phone: +94779229529. E-mail: rohithavm@yahoo.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Qassim Uninversity and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objectives:
The prevalence and reactivating pattern of cytomegalovirus (CMV) among renal transplant recipients in Sri Lanka is scarce. The study was aimed to describe the replication patterns of CMV in post-renal transplant recipients who were on pre-emptive therapy and identify the risk factors and time period for CMV reactivating during the 1st year of transplantation and provide an insight into the selection of pre-emptive therapy in the local setting.
Methods:
A retrospective and cohort study was conducted, enrolling renal transplant recipients who have completed routine 1-year follow-up for pre-emptive management at the National Hospital, Kandy, from January 2016 to January 2021. CMV quantitative polymerase chain reaction results and demographic data of enrolled recipients were analyzed to investigate the CMV replication pattern and risk factors. Categorical data were analyzed using Pearson’s Chi-square test, considering P < 0.05 statistically significant. Continuous variables were presented as percentages.
Results:
Two hundred and fifty-one renal transplant recipients’ data were included in the study. Of them, 75.70% were male patients, and the mean age of the study population was 43.25 years. CMV DNAemia incidence was 56.57% during the 1st year of post-renal transplantation. Only 9.16% had developed more than 104 IU/mL or significant DNAemia. Sex and donor type were not risk factors for CMV reactivation. However, the recipient’s age was significantly associated with CMV viraemia among renal transplant recipients.
Conclusion:
Considering the low incidence of significant viraemia among the study population, pre-emptive treatment would be the cost-effective strategy for management of the post-renal transplant recipients in local settings.
Keywords
Cytomegalovirus DNAemia
cytomegalovirus
pre-emptive therapy
renal transplant recipients
replication pattern
Introduction
Cytomegalovirus (CMV) belongs to the Herpesviridae family and has the ability to remain latent in CD34+ myeloid progenitors, CD14+ monocytes, dendritic cells, and megakaryocytes after primary infection and reactivate when the host is immunocompromised.[1] Reactivation or new infection of CMV is one of the most common infectious sources associated with high morbidity and mortality among immunocompromised patients, especially after solid organ transplantations.[2,3]
In solid organ transplant recipients, CMV infection may occur as a primary infection when the recipient is seronegative for CMV immunoglobulin G (IgG) (Donor positive: D+/Recipient Negative: R−) or as a reactivation when the recipient is seropositive for CMV IgG (D+/R+ or D−/R+). CMV replication due to primary infection or reactivation in transplant recipients can appear as an infection when there is evidence of viral replication without symptoms or as a disease with the evidence of viral infection in the presence of symptoms such as fever, malaise, leukopenia, and/or thrombocytopenia or as an invasive end-organ disease.[4] The direct effects of CMV infection can lead to CMV disease and the indirect effects of CMV infection can induce rejection and lead to chronic allograft nephropathy, allograft loss or secondary infections.[4] The direct effect of the virus replication causes CMV disease, including fever, neutropenia syndrome symptoms, and invasive organ diseases such as pneumonia, enteritis, meningitis, and encephalitis. On the other hand, indirect effects are due to immunomodulatory substances, such as cytokines, chemokines, and growth factors, being released in response to viral replication, deepening immunosuppression and increasing the risk of other opportunistic infections. In addition, viral infection may alter the expression of surface antigens causing graft rejection.[5] Two management strategies have been adopted for the prevention of the CMV disease, namely, universal prophylaxis and pre-emptive therapy.[4,6] Universal prophylaxis entails the routine administration of antiviral drugs for all the RT recipients or high-risk RT recipients immediately after the renal transplantation and continues for a fixed period, usually for 3–6 months. Aciclovir, intravenous ganciclovir, valacyclovir, and valganciclovir have been assessed to use as antiviral drugs in universal prophylaxis. Of them, valganciclovir and ganciclovir are recommended as the first-line and second-line drugs in universal prophylaxis, while aciclovir and valacyclovir are not recommended for prophylaxis of CMV as their low efficacy.[7,8] Although prophylaxis reduces CMV disease, it is associated with risks of side effects, antiviral resistance, and late-onset CMV disease.[9,10] Moreover, antiviral drugs are expensive and not widely available in local settings. Pre-emptive therapy avoids the above drawbacks, but it requires frequent monitoring and does not prevent complications of asymptomatic CMV replication.
Pre-emptive therapy aims to early diagnose of viral replication and prevent progression to the clinical disease. This management strategy needs frequent laboratory monitoring of viral markers (CMV DNA or CMV pp65 protein). When the viral load in the peripheral blood is above the predetermined value, antiviral treatment is begun and continued at the treatment dose until two negative polymerase chain reaction (PCR) results are obtained. Therefore, pre-emptive therapy avoids drug use with patients who do not need antiviral treatments. Moreover, it will lower the antiviral drugs’ cost and side effects and decrease the antiviral resistance.
Although the reactivating pattern of CMV in renal transplant recipients has been well documented in other countries, the data on the prevalence and the reactivating pattern of CMV among renal transplant recipients in the local setting is scarce. Therefore, the study was conducted to describe the reactivating patterns of CMV in post-renal transplant recipients who were on pre-emptive therapy and identify the risk factors and time period for CMV reactivating during the 1st year of transplantation.
Materials and Methods
The Nephrology and Transplant Unit, National Hospital, Kandy, follows CMV pre-emptive therapy with regular monitoring of viral load. Preventive protocol for CMV disease management at the unit includes pre-emptive therapy for the RT recipients at risk for reactivation of CMV (D+/R+, D−/R+) by monitoring of CMV DNAemia at frequent recommended intervals, 1, 2, 3, 6, 9, and 12 months in the 1st year of transplantation and prophylaxis for the patients at risk for primary infections (D+/R−).
A retrospective and cohort study was conducted using laboratory and demographic data of renal transplant recipients who have completed routine 1-year follow-up for pre-emptive management at the National Hospital, Kandy, from January 2016 to January 2021. Renal transplant recipients who did not complete the routine 1-year follow-up preemptive procedure or recipients under antiviral prophylaxis were excluded from the study.
The serial EDTA plasma samples were collected at frequent intervals of 1, 2, 3, 6, 9, and 12 months after transplantation. CMV DNA in the samples was extracted using a locally validated commercial viral nucleic acid extraction kit as the manufacturer’s instructions (SpinStar™ Viral Nucleic Acid Kit, Malaysia). The presence of the virus and viral load in serial plasma were tested and quantified as routine laboratory investigations using IVD approved, locally validated real-time PCR assay kit following the manufacturer’s instructions (Real Star, Altona Diagnostics, Germany). In addition, data were extracted from laboratory investigation request forms.
Laboratory and demographic data were analyzed to investigate the CMV reactivating pattern and to identify risk factors. Categorical data were analyzed using Pearson’s Chi-square test, considering P < 0.05 statistically significant. Continuous variables were presented as percentages.
Ethical clearance was obtained from the faculty of Allied Health Sciences, University of Peradeniya (AHS/ERC/2021/087) and permission to use the laboratory data was obtained from the Director of National Hospital, Kandy. However, individual consent from each patient was not required as this was a retrospective data analysis, and in agreement on patients’ identities will not be disclosed.
Results
Two hundred fifty-one renal transplant recipients’ data were analyzed. Of them, 190 (75.70%) were males with a mean age of 43.25 years (Range = 18–63 years, IQR = 52–34). The average sample number received from a patient was 5.27, and six samples, five samples, four samples, and three samples were received from 121, 71, 51, and 8 patients, respectively. A total of 1324 samples were analyzed, including 237 tests in the 1st month, 209 tests in the 2nd month, 229 tests in the 3rd month, 234 tests in the 6th month, 207 tests in the 9th month, and 208 tests in the 12th month. Of 251 renal transplant recipients, only 241 patients completed the 1-year follow-up and the rest (10) participated in the follow-up for 6 months only.
Of the 251 renal transplant recipients, 142 (56.57%) were positive for CMV DNAemia, and the rest were negative during the 1st year of post-renal transplantation. The mean age of the CMV DNAemia detected patients was 45.10 years (IQR: 54–37; range: 18–63 years). Patients were divided into four groups based on their age such as <30 years, 31–40 years, 41–50 years, and >50 years [Table 1]. CMV replication was higher with increased age, and age was a significant risk factor for CMV replication. CMV DNAemia was detected in 56.31% of males and in 57.38% of females. However, there was no association between CMV DNAemia and gender, or the donor type, live, or deceased donor.
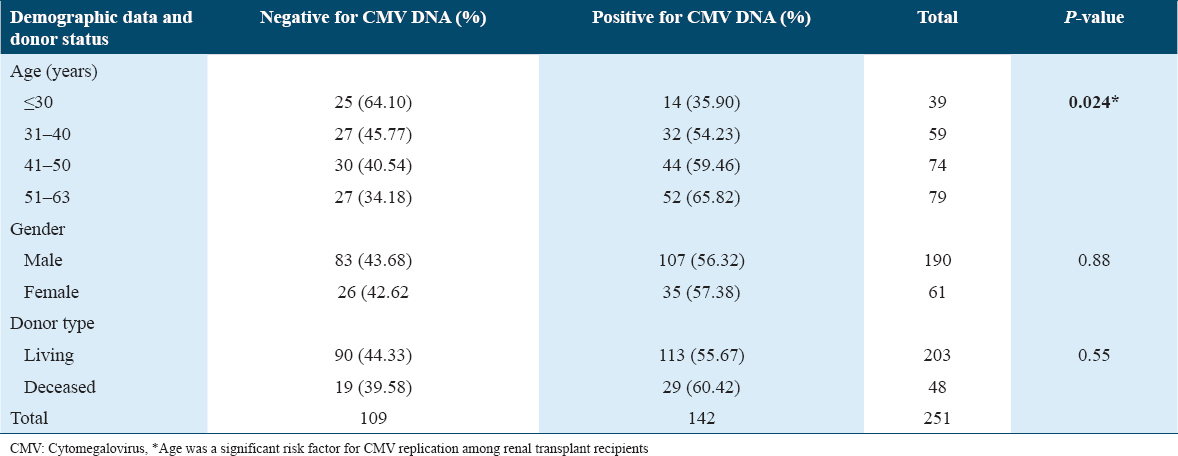
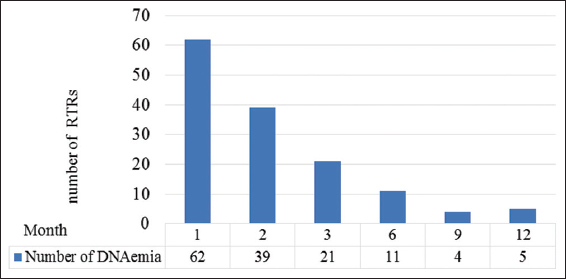
In CMV DNAemia detected population, the highest number of initial CMV detection noted in the 1st month of the post-transplant period (n = 62, 43.67%), followed by in 2nd (n = 39, 27.46%) and 3rd month (n = 21, 14.79%). In subsequent months, a gradual reduction was noted [Figure 1].
- Initial detection of cytomegalovirus DNAemia in renal transplant recipients within the 1st year of transplant
Among CMV viral activity detected patients (n = 142), 23 (16.20%) had significant or high levels of CMV DNAemia (more than or equal to 104 IU/mL), 34 (23.94%) renal transplant recipients had alert levels of CMV DNAemia (equal to 103 IU/mL) and the rest of the recipients (n = 85, 59.86%) had low level of DNAemia (≤102 IU/mL). From the whole study population (n = 251), only 9.16% (n = 23) had significant detection of high viral load. Fifteen patients with significant CMV DNAemia had resolved the infection within 4 months, while eight patients had low-level DNAemia during the follow-up period after the intervention.
In the low CMV DNAemia population, transient low DNAemia was detected in 68 (80.0%) and persistent low-level DNAemia in 17 (20.0%) patients. There was no statistically significant difference in age, gender, and donor type among outcome groups. A comparison of demographic data among outcome groups is shown in Table 2.
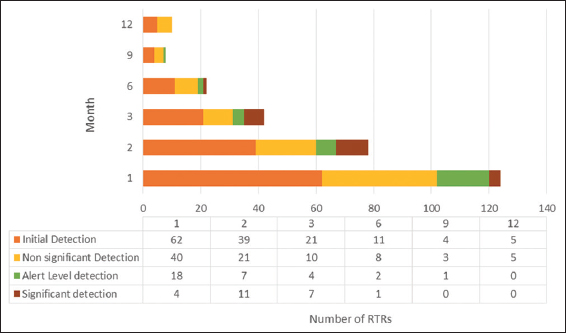
The 2nd and 3rd months following renal transplantation had a greater number of recipients with significant DNAemia than the other months, and all with significant levels of viral load showed in the first 6 months. The first (n = 40, 47.06%) and second (n = 21, 24.71%) months following transplantation had a greater number of low levels of DNAemia than the other months. There was a gradual decrease in the number of recipients positive for CMV DNA with time [Figure 2].
- The number of renal transplant recipients according to the cytomegalovirus DNAemia detection status with months
Discussion
The incidence of CMV reactivation among renal transplant recipients varies between 10% and 80% in different study populations, and CMV infection is common up to the first 100 days of post-transplantation.[5,11-15] Occurrence of CMV infection was 41.6%, 63.2%, 53.2%, 43.1%, 48%, and 38.5% in Seoul, Korea,[16] Brazil,[3] Matera, Italy,[17] Rome, Italy,[18] Iran,[15] and in Khartoum State, Sudan,[5] respectively, in patients with pre-emptive therapy. The reasons for this variation were due to the infected CMV strains of the donor and the recipients, the type of diagnostic test methods, and the immunosuppression drugs used.[19] In this study, the cumulative incidence of CMV viral replication among renal transplant recipients who were under pre-emptive therapy was 56.57% and this was comparable with the other studies in the literature. Of them, 86% of renal transplant recipients were positive for CMV DNAemia within the first 3 months following transplantation, while 14% were positive within 4–12 months of transplantation. This is comparable to the previous findings of De Matos et al., and Cordero et al., where 75% and 84% of transplant recipients showed early infections, respectively.[3,12] However, 14% of late reactivation cases were reported, highlighting the importance of continuous monitoring after the 3rd month of transplantation. Current practice of the study center after the 3 months of the renal transplantation is to assess the viral load in the plasma at 6, 9, and 12 months of renal transplantation. However, immidiate antiviral treatments are indicated when the recipient develops symptoms of viral disease during the pre-emptive therapy monitoring period. At the same time, DNAemia level is assessed.
In the study population, only 9.16% had developed significant CMV DNAemia. Of them, 96% were positive with significant or high viral load within first 3 months after renal transplantation, and none of the patients was positive with significant or high viral load after 6 months of transplantation. This finding was comparable to the Jung et al. where 13.25% (84/634) recipients had significant antigenemia while overall CMV infection was 41.64% in the study population.[16]
Universal prophylaxis entails the routine administration of antiviral drugs for all renal transplant recipients or high-risk RT recipients immediately after the renal transplantation and continues for a fixed, more extended period, usually for 3–6 months.[4] However, in our study, CMV reactivation with significant DNAemia was only 9.16% of the population, and 90.84% did not require antiviral treatment. Only 22 recipients (8.87%) of the study population had CMV reactivation with high or significant viral load during the first 3 months following kidney transplantation, and the most common viral load was 102–103, which accounted for 20% of the total population within the first 3 months. Moreover, 43.43% of renal transplant recipients never had a detectable CMV DNAemia during the study period. Therefore, the need for the use of antiviral drugs was reduced in a significant number of patients with this pre-emptive therapy management. Thus, this pre-emptive therapy management leads to selective drug use, reduced drug cost, and toxicities in the study population.
For the management of renal transplant recipients with prophylaxis, recipient should receive valganciclovir 450 mg 2 times/day for 3–6 months. However, when the renal transplant recipient is managed with pre-emptive therapy, they need to do serial qPCRs in defined intervals, and it is cost-effective compared to spending money for prophylaxis in the local setting. Moreover, the risk of developing antiviral resistance due to prolonged exposure to the antiviral is a major issue with the RTRs population. On top of that, the toxicity of the antiviral drugs and the availability of antiviral medications in the local setting are other issues with prophylaxis. Considering the fact of the toxicity, the potential for the emergence of antiviral resistance CMV, availability, and the cost of antiviral drugs, in combination with the above low level of significant CMV activity, pre-emptive therapy can be the best choice for the management of RTRs with seropositive for CMV infection. However, a major concern with pre-emptive therapy is that it may not prevent the indirect effect of CMV infection. A recently published meta-analysis study showed no difference between the two strategies on acute graft rejection and graft loss, and this study encourages using pre-emptive therapy instead of universal prophylaxis.[20]
Our study results indicated that there is no significant association of the gender of the recipient and donor type for the CMV activity. These findings are comparable with some studies in the literature.[12,17,21] However, in some studies, deceased donor transplantation is a risk factor for CMV incidence.[3] Another study showed a high risk of developing CMV disease in recipients receiving the graft from deceased donors and more than 60-year-old donors.[22] Age was a significant risk factor for the CMV activity in our studies group, which is comparable with Matos et al. and a few other studies where there was a risk of developing CMV disease with older age.[3,21,23]
Long-term monitoring is essential to study the effectiveness of pre-emptive therapy for preventing the indirect effect of CMV infection, and the lack of follow-up data after 1 year of renal transplantation is a limitation of our study.
Conclusion
Frequent intensive monitoring and active intervention for significant DNAemia can reduce the incidence of CMV disease among CMV seropositive renal transplant recipients. Due to the low number of CMV-reactivated patients had been found in the study population within the first 3 months, pre-emptive treatment can be suggested for RTRs during the first 3 months rather than using costly, toxic antiviral prophylaxis in Sri Lankan community. However, it is advisable to standardize the cutoff value for significant CMV DNAemia to initiate the treatment with antiviral drugs. Further large-scale studies are needed to assess the efficacy of pre-emptive therapy on the indirect effect of CMV viral replication.
Ethics Approval and Consent to Participate
Ethical clearance was obtained from the faculty of Allied Health Sciences, University of Peradeniya (AHS/ERC/2021/087), and permission to use the laboratory data was obtained from the Director of National Hospital, Kandy. However, individual consent from each patient was not required as this was a retrospective data analysis and in agreement on patients’ identities will not be disclosed.
Availability of Data and Material
The data of this study are available with the corresponding author.
Competing Interests
The authors declare that there are no competing interests.
Funding Statement
No external funding source was utilized for this study.
Authors’ Contributions
KD: Analysis and interpretation of data and writing of the original manuscript, RT: Analysis of data, LR: Acquisition of data, AW and NN: Concept, patient care, review, and editing, RM: Study design, review, and editing. All authors approved the final version of the manuscript.
Acknowledgments
We acknowledge all the staff members of the Department of Virology and Nephrology and Transplant Unit, National Hospital, Kandy, for the support given to conduct the study.
References
- Cytomegalovirus infection in renal transplantation:Clinical aspects, management and the perspectives. Einstein (Sao Paulo). 2015;13:142-8.
- [Google Scholar]
- Cytomegalovirus infection and disease in the new era of immunosuppression following solid organ transplantation. Transpl Infect Dis. 2009;11:195-202.
- [Google Scholar]
- Cytomegalovirus infection after renal transplantation:Occurrence, clinical features, and the cutoff for antigenemia in a university hospital in Brazil. Infect Chemother. 2017;49:255-61.
- [Google Scholar]
- Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96:333-60.
- [Google Scholar]
- Molecular detection and glycoprotein B (UL55) genotyping of cytomegalovirus among Sudanese renal transplant recipients. Biomed Res Int. 2022;2022:5403694.
- [Google Scholar]
- Summary of the British transplantation society guidelines for the prevention and management of CMV disease after solid organ transplantation. Transplantation. 2011;92:1181-7.
- [Google Scholar]
- Efficacy and safety of conventional antiviral agents in preventive strategies for cytomegalovirus infection after kidney transplantation:A systematic review and network meta-analysis. Transpl Int. 2021;34:2720-34.
- [Google Scholar]
- The third international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2018;102:900-31.
- [Google Scholar]
- Risk factors and outcomes of ganciclovir-resistant cytomegalovirus infection in solid organ transplant recipients. Clin Infect Dis. 2017;65:57-63.
- [Google Scholar]
- Cytomegalovirus post kidney transplantation:Prophylaxis versus pre-emptive therapy? Transpl Int. 2015;28:1351-6.
- [Google Scholar]
- The impact of cytomegalovirus infection ≥1 year after primary renal transplantation. Clin Transplant. 2010;24:572-7.
- [Google Scholar]
- Cytomegalovirus disease in kidney transplant recipients:Incidence, clinical profile, and risk factors. Transplant Proc. 2012;44:694-700.
- [Google Scholar]
- Prevalence and clinical impact of cytomegalovirus infection and disease in renal transplantation:Ten years of experience in a single center. Transplant Proc. 2012;44:2715-7.
- [Google Scholar]
- The prevention of cytomegalovirus disease in renal transplantation. Am J Kidney Dis. 1990;16:175-88.
- [Google Scholar]
- The incidence of cytomegalovirus glycoprotein B Genotypes in kidney transplant recipients in Iran. Int J Organ Transplant Med. 2018;9:173-7.
- [Google Scholar]
- The effect of cytomegalovirus antigenemia titer on the efficacy of preemptive therapy for the prevention of cytomegalovirus disease after kidney transplantation. Transplant Proc. 2010;42:804-10.
- [Google Scholar]
- The effects of preemptive therapy using a very low threshold of pp65 antigenemia to prevent cytomegalovirus disease in kidney transplant recipients:A single-center experience. Transplant Proc. 2013;45:182-4.
- [Google Scholar]
- Pre-emptive therapy for the treatment of cytomegalovirus after kidney transplantation. Transplant Proc. 2017;49:638-41.
- [Google Scholar]
- Cytomegalovirus infections in solid organ transplantation:A review. Infect Chemother. 2013;45:260-71.
- [Google Scholar]
- Comparison of universal prophylaxis and preemptive approach for cytomegalovirus associated outcome measures in renal transplant patients:A meta-analysis of available data. Transpl Infect Dis. 2019;21:e13016.
- [Google Scholar]
- Cytomegalovirus viraemia and mortality in renal transplant recipients in the era of antiviral prophylaxis. Lessons from the Western Australian experience. BMC Infect Dis. 2017;17:501.
- [Google Scholar]
- Age matters:Older age as a risk factor for CMV reactivation in the CMV serostatus-positive kidney transplant recipient. Eur J Clin Microbiol Infect Dis. 2020;39:455-63.
- [Google Scholar]







