Translate this page into:
Green synthesis of silver nanoparticles from Euphorbia milii plant extract for enhanced antibacterial and enzyme inhibition effects
Address for correspondence: Saud Bawazeer, Department of Pharmaceutical Science, Faculty of Pharmacy, Umm Al-Qura University, Makkah, P.O. Box 751, Saudi Arabia. E-mail: ssbawazeer@uqu.edu.sa
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Qassim Uninversity and was migrated to Scientific Scholar after the change of Publisher.
Abstract
ABSTRACT
Objectives:
Silver nanoparticles (AgNPs) are gaining increasing attention in biomedical applications due to their unique properties. Green synthesis methods are environmentally friendly and have demonstrated potential for AgNP production. This study explores the green synthesis of AgNPs using the methanolic extract of Euphorbia milii, a plant known for its medicinal properties. The primary objectives of this research were to synthesize AgNPs using E. milii extract, characterize the nanoparticles (NPs) using various techniques, and evaluate their antibacterial and enzyme inhibitory activities.
Methods:
E. milii plant extract was utilized for the green synthesis of AgNPs. The characterization of the NPs was performed through ultraviolet-visible spectroscopy (UV-Vis), Fourier-transform infrared spectroscopy, scanning electron microscopy, and energy-dispersive X-ray spectroscopy (EDX). Antibacterial activity was assessed against Staphylococcus aureus, while enzyme inhibitory assays were conducted against urease, α-glucosidase, carbonic anhydrase II, and xanthine oxidase.
Results:
The synthesized AgNPs exhibited significant antibacterial effects, with a remarkable 20-mm zone of inhibition against S. aureus, surpassing the efficacy of the plant extract alone. Furthermore, the AgNPs demonstrated remarkable enzyme inhibition, achieving impressive percentages of 77.98% against α-glucosidase and 88.54% against carbonic anhydrase II. Half-maximal inhibitory concentration values for enzyme inhibition were highly promising, including 78.09 ± 1.98 μM for α-glucosidase, 0.22 ± 0.10 μM for carbonic anhydrase II, and 7.11 ± 0.55 μM for xanthine oxidase.
Conclusion:
In this study, AgNPs were successfully synthesized using E. milii extract and characterized using various techniques. The AgNPs exhibited significant antibacterial and enzyme-inhibitory activities, showcasing their potential for biomedical applications.
Keywords
Antibacterial activity
enzyme inhibition
Euphorbia milii
green synthesis
silver nanoparticles

Introduction
Nanoparticles (NPs) are an emerging field in today’s era with a wide range of applications.[1-3] Silver NPs (AgNPs) have appeared as a promising class of NPs with wide applications in a variety of fields, including medicine, electronics, and environmental remediation.[4-7] Their unique characteristics, like their high surface-to-volume ratio and enhanced reactivity, contribute to their exceptional antimicrobial and enzyme inhibitory activities. These properties have attracted significant attention in the search for novel therapeutics and catalysts.[8] However, the conventional methods of synthesizing AgNPs often involve the use of toxic chemicals and high-energy processes, which can have adverse effects on both the environment and living things.[9,10]
To address these concerns, the field of green synthesis has gained prominence as an eco-friendly and sustainable alternative for the fabrication of NPs.[11] Green synthesis involves the utilization of natural resources, such as plant extracts, for the synthesis of NPs, in which these extracts are used as stabilizing and reducing agents.[12] Plant extracts possess a rich repertoire of bioactive compounds, including polyphenols, flavonoids, and terpenoids, which can do the reduction of metal ions and provide stability to the resulting NPs.[13-15] Moreover, green synthesis offers several advantages, including low cost, ease of scalability, and reduced environmental impact.[16]
Euphorbia milii, commonly known as the crown of thorns, is a medicinal plant widely distributed in tropical and subtropical regions.[17] It has been used conventionally for its therapeutic properties, including wound healing, anti-inflammatory, and antimicrobial activities.[18-20] The presence of bioactive compounds in E. milii makes it a promising candidate for the green synthesis of AgNPs and their biological activities.[19] Various medicinal plants have been used for the green synthesis of AgNPs.[15,21,22] The strong reducing capability and stabilizing potential of E. milii plant extract, AgNPs can be synthesized in a sustainable and environmentally friendly manner.
The novelty of this work is to synthesize AgNPs using the methanolic extract of the E. milii plant. Green synthesis represents an innovative and eco-friendly approach, utilizing natural extracts as reducing and stabilizing agents for NP formation and avoiding the use of harmful chemicals prevalent in conventional synthesis methods. The rationale behind selecting E. milii lies in its abundance of bioactive compounds that may play a pivotal role in the reduction and stabilization of silver ions, leading to the formation of NPs with enhanced biomedical applications.
In this study, we aim to explore the ecofriendly green synthesis of silver NPs using the methanolic extract of E. milii and evaluate their antibacterial and enzyme inhibitory activities. The green synthesized AgNPs will be characterized by ultraviolet (UV), Fourier-transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), and energy-dispersive X-ray spectroscopy (EDX). The bactericidal activity of the AgNPs will be assessed against the pathogenic bacterium Staphylococcus aureus, a common cause of various infections. In addition, the enzyme inhibitory potential of the AgNPs will be investigated against key enzymes, including urease, α-glucosidase, carbonic anhydrase II, and xanthine oxidase.
Experimental
Plant collection identification and extract preparation
E. milii is an ornamental plant cultivated for decorative purposes and was gifted by Dr. Abdur Rauf of the Department of Chemistry at the University of Swabi, Khyber Pakhtunkhwa, Pakistan. The plant was identified and confirmed by Dr. Abdur Rashid, a taxonomist, and a voucher specimen (UOP-545) was deposited in the herbarium of the Department of Botany at the University of Peshawar, Pakistan. The plant material, comprising leaves and stems, was carefully selected and collected in a sterile container. Upon collection, the plant material was transported to the laboratory and washed thoroughly with distilled water to remove any surface contaminants. Subsequently, the plant material was air-dried under shade to preserve its bioactive components, as reported in the literature.[23,24]
Grinding and extraction
The dried plant material was ground into a fine powder. The obtained powder was stored in an airtight container for further use. For the extraction process, 10 g of the powdered plant material was added to 100 mL of methanol in a round-bottom flask. The flask was then sealed and subjected to periodic stirring at room temperature for 12 h. After the extraction period, the mixture was filtered using a filter paper to obtain the methanolic extract of E. milii.
Synthesis of AgNPs
The green synthesis of AgNPs was carried out using the methanolic extract of E. milii, as described in the literature.[23,25,26] Briefly, 10 mL of the extract was mixed with 90 mL of aqueous silver nitrate (AgNO3) solution at a concentration of 1 mM. The mixture was stirred continuously at room temperature for several hours until a color change from pale yellow to dark brown was seen, showing the reduction of silver ions and the formation of AgNPs.[27]
Instrumental characterization
The synthesized AgNPs were characterized extensively using different techniques to assess their size, morphology, and elemental composition. UV spectroscopy was performed by recording the absorbance spectrum of the AgNPs using a UV-visible spectroscopy (UV-Vis) spectrophotometer. The FTIR KBr pellet method was performed to obtain spectra to identify the functional groups present in the plant extract and their potential role in AgNP stabilization and reduction. SEM was utilized to examine the morphology and texture of the AgNPs. EDX analysis was conducted to check the elemental makeup of the AgNPs.
Bactericidal activity
The antibacterial activities of the synthesized AgNPs were assessed against the pathogenic bacterium S. aureus using the agar-well diffusion method. Sterile agar plates were prepared, and wells were created using a sterile cork borer. A standardized suspension of S. aureus was spread uniformly on the agar surface. Subsequently, 100 μL of the synthesized AgNPs solution was employed to the wells. The plates were incubated at the appropriate temperature for 24 h, and the inhibition zones were measured to evaluate the antibacterial activity of the AgNPs.[28]
Enzyme inhibitory activity
The enzyme inhibitory capabilities of the synthesized AgNPs were examined against selected enzymes, including urease, α-glucosidase, carbonic anhydrase II, and xanthine oxidase. For each enzyme, the appropriate assay method was followed to evaluate the inhibitory effects of the AgNPs.[29-32] The percentage inhibition was calculated by comparing the enzyme activity in the presence and absence of the AgNPs. The half-maximal inhibitory concentration (IC50) values, representing the concentration required to inhibit 50% of enzyme activity, were determined using a suitable concentration range of the AgNPs.
Statistical analysis
The findings in this study are presented as the mean value with the corresponding standard error of the mean (SEM) to assess statistical significance (P < 0.05 or 0.01). Statistical analyses were conducted using the GraphPad Prism software.
Results
Biogenic synthesis of AgNPs
In this study, the methanolic extract of the E. milii plant was employed as the green reducing agent for the synthesis of AgNPs. The plant extract contains a rich repertoire of bioactive compounds, such as flavonoids, polyphenols, and terpenoids, which can serve as potent reducing agents capable of converting silver ions (Ag+) into AgNPs (Ag0). The process is initiated by mixing the plant extract with an aqueous silver nitrate (AgNO3) solution. The bioactive compounds in the extract interact with Ag+ ions, leading to the reduction of Ag+ to Ag0. This reduction reaction is followed by the nucleation and growth of Ag0 particles, ultimately resulting in the formation of AgNPs. In addition, the various functional groups found in the plant extract play a crucial role in stabilizing the synthesized AgNPs, preventing their aggregation, and maintaining their colloidal stability.[33]
Characterizations
UV-Vis spectroscopy
UV-Vis spectroscopy is a fundamental technique used to analyze and confirm the formation of NPs. In this study, the synthesized AgNPs exhibited a characteristic peak at 430 nm, which confirmed the successful synthesis of AgNPs. The absorption peak observed in the UV-Vis spectrum relates to the surface plasmon resonance (SPR) of the AgNPs. The SPR phenomenon is a result of the collective oscillation of conduction electrons in the NPs on excitation by light. The position and intensity of the SPR peak can provide valuable information about the size, shape, and aggregation state of the NPs. In our case, the observed peak at 430 nm suggests the presence of well-dispersed AgNPs with a particular size and shape, as shown in Figure 1a. The UV-Vis spectroscopy results are consistent with previous studies on AgNPs, where the SPR peak in the range of 400–450 nm is typically observed.[34,35] The specific peak observed in our study further confirms the successful formation of AgNPs using the methanolic extract of the E. milii plant.
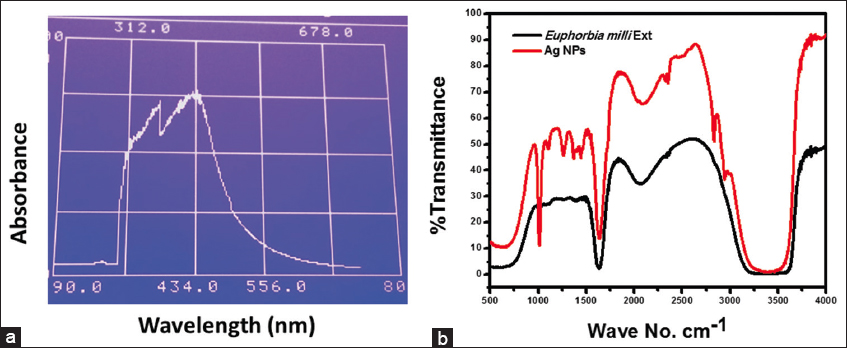
- Ultraviolet-visible spectroscopy spectrum of AgNPs (a) and fourier-transform infrared spectroscopy spectrum of plant extract and AgNPs (b). AgNPs: Silver nanoparticles
FTIR spectroscopy
FTIR spectroscopy is a crucial tool for determining the functional groups present in compounds. In this study, the FTIR spectra were recorded for both the E. milii plant extract and the synthesized AgNPs to analyze their respective functional groups. The FTIR spectrum of both AgNPs and the plant extract exhibited some common peaks, indicating the presence of similar functional groups. A broad peak at 3406 cm−1 was observed in both the plant extract and AgNPs, suggesting the presence of O-H stretching vibrations of alcohol or phenolic groups. However, it is worth noting that the broadening of this peak in the AgNPs was somewhat weaker compared to the plant extract. This difference in broadness may indicate a modification in the hydrogen bonding or interactions of the functional groups upon the formation of AgNPs. Another common feature observed in both the plant extract and AgNPs was a small peak at 2044 cm−1. This peak corresponds to the stretching vibrations of C≡N groups, which may indicate the presence of nitrile functional groups. In addition, a sharp peak at 1628 cm−1 was observed in both the plant extract and AgNPs, which can be attributed to the stretching vibrations of C=O bonds, showing the presence of carbonyl groups. In the plant extract, additional small peaks at 1377 cm−1 and 1257 cm−1 were observed, indicating the presence of C-H bending vibrations and C-O stretching vibrations, respectively. However, in the AgNPs, the intensity of these peaks increased, suggesting a potential interaction or adsorption of these functional groups on the surface of the NPs. Interestingly, a sharp and narrow peak at 1016 cm−1 was observed exclusively in the FTIR spectrum of AgNPs. This peak could be attributed to the stretching vibrations of metal-oxygen (Ag-O) bonds, indicating the formation of AgNPs. The FTIR spectrum of the plant extract and AgNPs is shown in Figure 1b.
SEM
SEM, also known as scanning electron microscopy, is a technique commonly used to characterize the surface features of materials. In this study, SEM imaging was employed to examine the morphology of the synthesized silver NPs in solution form. Both high- and low-resolution SEM images were captured to provide a comprehensive understanding of the AgNPs’ surface characteristics [Figure 2]. The SEM images of the AgNPs revealed a rough texture and distinctive features, which can be attributed to the occurrence of the NPs in the solution. The rough surface morphology observed in the SEM images suggests the stabilization of the AgNPs, possibly due to the interaction between the NP surfaces and the surrounding medium. These interactions may involve the adsorption of stabilizing agents or the occurrence of functional groups from the plant extract used in the green synthesis process.
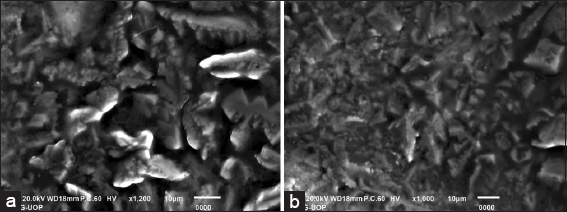
- SEM images of silver nanoparticles. High resolution (a) and low resolution (b)
EDX spectroscopy
The EDX analysis of the green-synthesized AgNPs derived from E. milii extract revealed the occurrence of various elements. Carbon and oxygen were the predominant elements, comprising 49.09% and 38.49% of the composition, respectively, as shown in the inset of Figure 3. The presence of these elements suggests the involvement of organic compounds, potentially derived from biomolecules present in the extract. In addition, aluminum and potassium were detected in smaller amounts, indicating the occurrence of trace elements or impurities. Notably, the analysis confirmed the successful synthesis of AgNPs, as a small amount of silver (0.13%) was observed. These results highlight the potential role of organic compounds in the extract in the reduction of silver ions and subsequent NP formation.
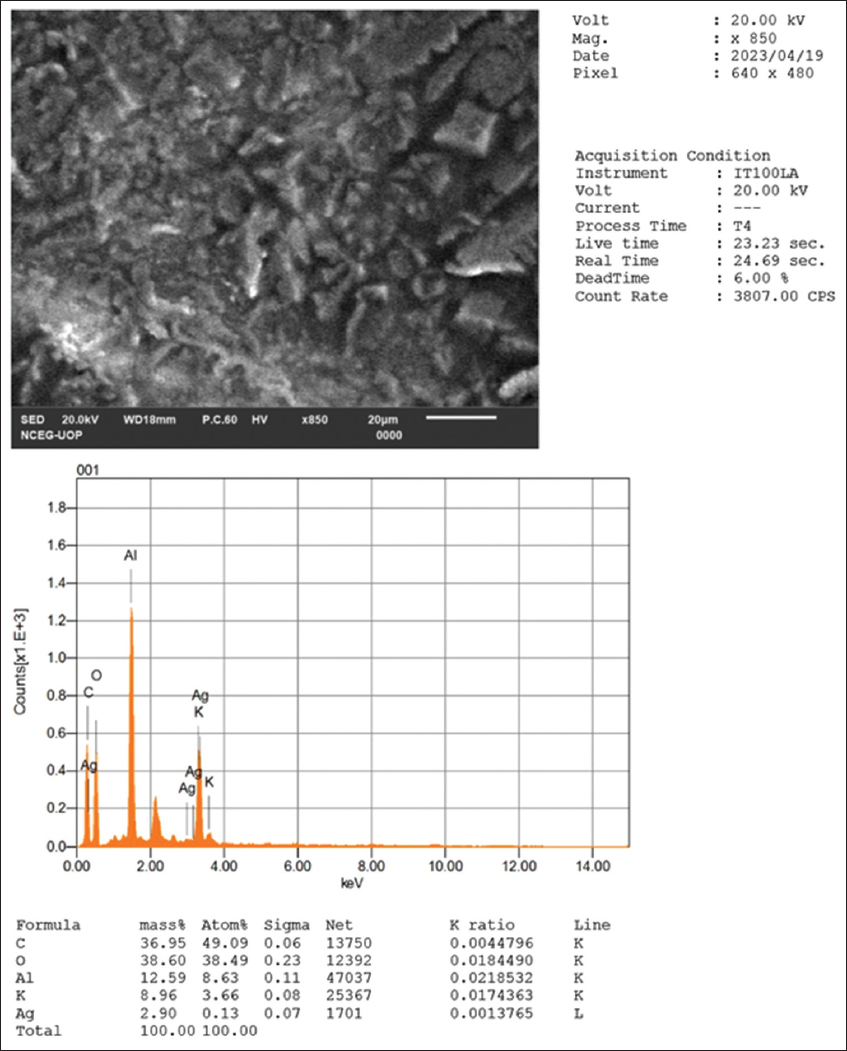
- EDS spectrum of the synthesized silver nanoparticles
Biological activities
The antibacterial activity of the prepared AgNPs was evaluated against the pathogenic bacterium S. aureus using the agar-well diffusion method. The experiment included a negative control using distilled water, a positive control using the standard antibiotic drug linezolid, and an additional control using the E. milii extract without AgNPs. The results of the agar-well diffusion method demonstrated the inhibitory effect of both the standard drug linezolid and the synthesized AgNPs against S. aureus. The zone of inhibition observed for linezolid was 25 mm, indicating its potent antibacterial activity. The AgNPs also exhibited significant antibacterial activity, with a zone of inhibition measuring 20 mm. These results indicate that the synthesized AgNPs possess promising antimicrobial properties. Furthermore, the Euphorbia extract without AgNPs showed a smaller zone of inhibition, measuring 18 mm. This suggests that the extract itself may possess some inherent antibacterial activity, albeit weaker than that of the synthesized AgNPs or linezolid. The negative control using distilled water did not exhibit any inhibitory effect, confirming the absence of contamination or interference. Table 1 shows the zone of inhibition in mm.

Enzymes inhibition
The enzymatic inhibitory activities of the tested extracts and AgNPs were evaluated against four enzymes: urease, α-glucosidase, carbonic anhydrase II, and xanthine oxidase. The results, including the concentration used, percentage inhibition, and IC50 values, are presented in Table 2.
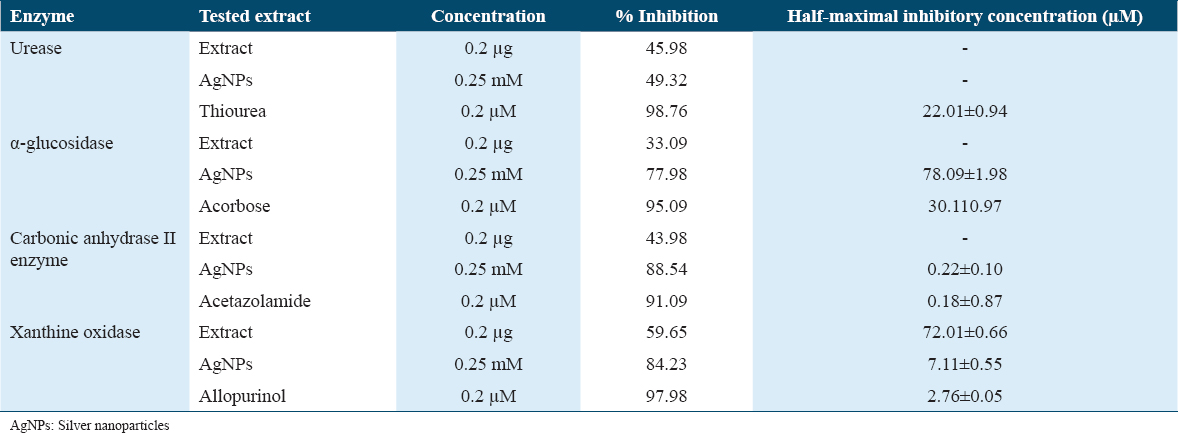
Urease activity was significantly inhibited by both the extract and AgNPs. The extract exhibited a 45.98% inhibition at a concentration of 0.2 μg, while the AgNPs showed a higher inhibition of 49.32% at a concentration of 0.25 mM. Notably, the standard inhibitor thiourea demonstrated potent urease inhibition with 98.76% efficacy and an IC50 value of 22.01 ± 0.94 μM. The α-glucosidase activity was also effectively inhibited by the extract and AgNPs. The extract displayed a moderate inhibition of 33.09% at 0.2 μg concentration, while the AgNPs exhibited a significant inhibition of 77.98% at 0.25 mM concentration. The standard inhibitor, acarbose, showed robust inhibition with 95.09% efficacy and an IC50 value of 30.11 ± 0.97 μM.
Carbonic anhydrase II enzyme activity was notably inhibited by both the extract and AgNPs. The extract exhibited a 43.98% inhibition at a concentration of 0.2 μg, while the AgNPs showed a higher inhibition of 88.54% at a 0.25 mM concentration. The standard inhibitor, acetazolamide, demonstrated potent inhibition with 91.09% efficacy and an IC50 value of 0.18 ± 0.87 μM.
In the case of xanthine oxidase activity, both the extract and AgNPs demonstrated significant inhibitory effects. The extract exhibited a 59.65% inhibition at a concentration of 0.2 μg, while the AgNPs showed a higher inhibition of 84.23% at a 0.25 mM concentration. The standard inhibitor, allopurinol, displayed strong inhibition with 97.98% efficacy and an IC50 value of 2.76 ± 0.05 μM.
Discussion
The biogenic synthesis of AgNPs involves a sustainable and eco-friendly approach, utilizing natural extracts or biomaterials as reducing and stabilizing agents for the formation of NPs.[15,25] The outcomes of this research will shed light on the effectiveness of E. milii plant extract in synthesizing AgNPs with enhanced antimicrobial and enzyme inhibitory properties. The green synthesis approach offers a sustainable alternative to conventional methods, reducing the reliance on toxic chemicals and minimizing the ecological footprint.[26,33] The results from this study will contribute to the emerging body of knowledge on green synthesis techniques and broaden the scope of AgNPs’ applications in biomedicine and enzyme inhibition. The green synthesis approach not only ensures the sustainable production of AgNPs but also offers the potential for enhanced biomedical applications due to the presence of bioactive components from the plant extract, which may impart additional therapeutic properties to the NPs.
This eco-friendly approach using E. milii plant extract represents a promising approach with potential biomedical applications. The successful synthesis and characterization of AgNPs were achieved through the reduction of silver ions (Ag+) by the bioactive compounds present in the plant extract. The presence of various functional groups in the extract played a crucial role in stabilizing the AgNPs and preventing their aggregation.[23,36] The synthesized AgNPs displayed a characteristic peak at 430 nm in the UV-Vis spectrum, confirming their successful formation and well-dispersed nature. The observed antibacterial activity of the synthesized AgNPs can be attributed to the inherent properties of AgNPs, including their high surface area, which allows for enhanced interaction with bacterial cells. The NPs may penetrate the bacterial cell wall, leading to cellular damage and inhibition of bacterial growth.[37,38] The antibacterial mechanism of AgNPs involves the release of silver ions, which exhibit antimicrobial effects by interfering with essential cellular processes. The slightly lower zone of inhibition observed for the synthesized AgNPs compared to the standard drug linezolid could be attributed to differences in their mechanisms of action and concentrations used. Linezolid is a well-established antibiotic specifically designed to target bacterial ribosomes, while AgNPs may have a broader mode of action against various cellular components. Furthermore, the AgNPs exhibited substantial inhibitory effects against enzymes such as urease, α-glucosidase, carbonic anhydrase II, and xanthine oxidase. The results indicate that the tested extracts and AgNPs possess potential enzymatic inhibitory activities. The AgNPs consistently demonstrated higher inhibitory effects compared to the extract for all enzymes tested. This could be attributed to the increased surface area and improved bioavailability of the NPs, allowing for enhanced interaction with the enzymes.[39] Furthermore, the IC50 values provide information on the concentration of the extract or AgNPs required to achieve 50% inhibition of the respective enzyme activity. Lower IC50 values indicate greater potency. Notably, some of the standard inhibitors, such as thiourea, acarbose, acetazolamide, and allopurinol, displayed higher inhibitory activities with lower IC50 values compared to the tested extracts and AgNPs. This highlights the potential of these compounds as reference standards for comparison. The observed enzymatic inhibitory activities of the tested extracts and AgNPs suggest their potential therapeutic applications. Inhibition of urease, α-glucosidase, carbonic anhydrase II, and xanthine oxidase is associated with various physiological processes and can be targeted for the treatment of diseases such as diabetes, cancer, and gout.
Conclusion
The green synthesis of AgNPs using the methanolic extract of the E. milii plant resulted in the production of NPs with significant antibacterial and enzyme inhibitory activities. The synthesized AgNPs exhibited a notable inhibition zone against S. aureus, surpassing the activity of the plant extract alone. Furthermore, the AgNPs demonstrated potent enzyme inhibition against urease, α-glucosidase, carbonic anhydrase II, and xanthine oxidase. These results highlight the potential of the E. milii plant extract for synthesizing AgNPs with valuable biomedical applications. The green synthesis approach offers an eco-friendly and sustainable method for producing NPs, making it a promising avenue for further exploration in nanotechnology and biomedical sciences.
Acknowledgment
The author is greatly acknowledging the Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Umm Al-Qura University, Makkah, for providing experimental facilities.
Conflict of Interest
The author declares no conflict of interest.
Consent for Publication
All the authors have agreed to the published version of the manuscript.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Availability of Data and Materials
Not applicable.
Funding
Not applicable.
Author Contributions
SB: conceptualization, investigation, writing-original draft, writing-review, and editing. All authors read and approved the submitted version.
References
- Cellulose acetate composite films fabricated with zero-valent iron nanoparticles and its use in the degradation of persistent organic pollutants. Appl Organomet Chem. 2020;34:e5892.
- [Google Scholar]
- Melia Azedarach impregnated Co and Ni zero-valent metal nanoparticles for organic pollutants degradation:Validation of experiments through statistical analysis. J Mater Sci Mater Electron. 2020;31:16938-50.
- [Google Scholar]
- An overview of iron oxide (F|ne3O4) nanoparticles:From synthetic strategies, characterization to antibacterial and anticancer applications. Crystals. 2022;12:1809.
- [Google Scholar]
- Synthesis and application of silver nanoparticles (Ag NPs) for the prevention of infection in healthcare workers. Int J Mol Sci. 2019;20:3620.
- [Google Scholar]
- Recent advances in green synthesis of Ag NPs for extenuating antimicrobial resistance. Nanomaterials (Basel). 2022;12:1115.
- [Google Scholar]
- Enhanced catalytic reduction/degradation of organic pollutants and antimicrobial activity with metallic nanoparticles immobilized on copolymer modified with NaY zeolite films. J Mol Liq. 2022;359:119246.
- [Google Scholar]
- Biomass impregnated zero-valent Ag and Cu supported-catalyst:Evaluation in the reduction of nitrophenol and discoloration of dyes in aqueous medium. J Organomet Chem. 2021;938:121756.
- [Google Scholar]
- Synthesis and characterization of stable silver nanoparticles, Ag-NPs:Discussion on the applications of Ag-NPs as antimicrobial agents. Physica B:Condensed Matter. 2019;554:21-30.
- [Google Scholar]
- Synthesis of silver nanoparticles by chemical and biological methods and their antimicrobial properties. J Exp Nanosci. 2016;11:714-21.
- [Google Scholar]
- Anchoring Zero-valent Cu and Ni nanoparticles on carboxymethyl cellulose-polystyrene-block polyisoprene-block polystyrene composite films for nitrophenol reduction and dyes degradation. J Polym Environ. 2023;31:608-20.
- [Google Scholar]
- Green synthesis of nanoparticles and its potential application. Biotechnol Lett. 2016;38:545-60.
- [Google Scholar]
- Advances in green synthesis of nanoparticles. Artif Cells Nanomed Biotechnol. 2019;47:844-51.
- [Google Scholar]
- Plant-extract-assisted green synthesis of silver nanoparticles using Origanum vulgare Lextract and their microbicidal activities. Sustainability. 2018;10:913.
- [Google Scholar]
- Phytochemical analysis and antibacterial activity of Nicotiana tabacum and Nicotiana rustica. RADS J Biol Res Appl Sci. 2021;12:60-5.
- [Google Scholar]
- Bioactivities of the green synthesized silver nanoparticles reduced using Allium cepa L aqueous extracts induced apoptosis in colorectal cancer cell lines. J Nanomater. 2022;2022:1-13.
- [Google Scholar]
- Green Synthesis of Nanoparticles:Their Advantages and Disadvantages. In: AIP Conference Proceedings. Maryland: AIP Publishing; 2016.
- [Google Scholar]
- Occurrence of Euphorbia ringspot virus in Euphorbia milii cv. splendens in Venezuela. J Phytopathol. 2011;159:66-8.
- [Google Scholar]
- Traditional uses, pharmacological, and phytochemical studies of Euphorbia:A review. Curr Top Med Chem. 2022;22:1553-70.
- [Google Scholar]
- Highly stable glycosylated serine protease from the medicinal plant Euphorbia milii . Phytochemistry. 2006;67:1414-26.
- [Google Scholar]
- In vitro biological propensities and chemical profiling of Euphorbia milii Des Moul (Euphorbiaceae):A novel source for bioactive agents. Ind Crop Prod. 2019;130:9-15.
- [Google Scholar]
- The valuable impacts of halophytic genus Suaeda;nutritional, chemical, and biological values. Med Chem. 2020;16:1044-57.
- [Google Scholar]
- In vitro and in vivo synergistic wound healing and anti-methicillin-resistant Staphylococcus aureus (MRSA) evaluation of liquorice-decorated silver nanoparticles. J Antibiot (Tokyo). 2023;76:291-300.
- [Google Scholar]
- Green synthesis of silver nanoparticles using Rhazya stricta Decne extracts and their anti-microbial and anti-oxidant activities. Crystals. 2023;13:398.
- [Google Scholar]
- Antimicrobial and cytotoxic potential of Anemone tetrasepala Royle. Phytopharmacol Res J. 2023;2:41-8.
- [Google Scholar]
- Green synthesis of silver nanoparticles using Euphorbia wallichii Leaf extract:Its antibacterial action against citrus canker causal agent and antioxidant potential. Molecules. 2022;27:3525.
- [Google Scholar]
- Biogenic synthesis of iron oxide nanoparticles via Skimmia laureola and their antibacterial efficacy against bacterial wilt pathogen Ralstonia solanacearum. Mater Sci Eng C Mater Biol Appl. 2019;98:101-8.
- [Google Scholar]
- Green synthesis and characterizations of silver and gold nanoparticles using leaf extract of Rosa rugosa. Colloids Surf A Physicochem Eng Aspects. 2010;364:34-41.
- [Google Scholar]
- Green synthesis of silver nanoparticles using turmeric extracts and investigation of their antibacterial activities. Colloids Surf B Biointerfaces. 2018;171:398-405.
- [Google Scholar]
- Potent In vitro a-glucosidase and b-secretase inhibition of amyrin-type triterpenoid isolated from Datura metel Linnaeus (Angel's Trumpet) fruits. Biomed Res Int. 2020;2020:8530165.
- [Google Scholar]
- Xanthine oxidase inhibition of bioactive constituents isolated from Potentilla evestita. J Chem Soc Pak. 2016;38:429-32.
- [Google Scholar]
- Carbonic anhydrase and urease inhibitory potential of various plant phenolics using in vitro and in silico methods. Chem Biodivers. 2017;14:e1700024.
- [Google Scholar]
- Green synthesis, characterization, enzyme inhibition, antimicrobial potential, and cytotoxic activity of plant mediated silver nanoparticle using Ricinus communis leaf and root extracts. Biomolecules. 2021;11:206.
- [Google Scholar]
- Green synthesis of silver nanoparticles using medicinal plants:Characterization and application. J Radiat Res Appl Sci. 2022;15:109-24.
- [Google Scholar]
- Green synthesis of silver nanoparticles by Bacillus methylotrophicus, and their antimicrobial activity. Artif Cells Nanomed Biotechnol. 2016;44:1127-32.
- [Google Scholar]
- Green synthesis of silver nanoparticles from leaf extract of Mimusops elengi, Linnfor enhanced antibacterial activity against multi drug resistant clinical isolates. Colloids Surf B Biointerfaces. 2013;108:255-9.
- [Google Scholar]
- Biosynthesized silver nanoparticles using Polygonatum geminiflorum efficiently control fusarium wilt disease of tomato. Front Bioeng Biotechnol. 2022;10:988607.
- [Google Scholar]
- The potential of silver nanoparticles for antiviral and antibacterial applications:A mechanism of action. Nanomaterials (Basel). 2020;10:1566.
- [Google Scholar]
- In vitro antimicrobial activity of Rumex Dentatus L(Polygonaceae) plant extracts. Phytopharmacol Res J. 2022;1:32-42.
- [Google Scholar]
- Future prospects of antibacterial metal nanoparticles as enzyme inhibitor. Mater Sci Eng C Mater Biol Appl. 2016;68:939-47.
- [Google Scholar]






