Translate this page into:
Novel regimens and treatment strategies in neoadjuvant therapy for colorectal cancer: A systematic review
Address for correspondence: Muhammad Ali Muzammil, Dow University of Health Sciences, Karachi, Pakistan. E-mail: muzammil200077@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Qassim Uninversity and was migrated to Scientific Scholar after the change of Publisher.
Abstract
ABSTRACT
Objective:
The objective of this systematic review was to describe novel regimens and treatment strategies in neoadjuvant therapy for colorectal cancer (CRC). The aim was to summarize the current advancements in neoadjuvant chemotherapy (NACT) for CRC, including the use of cytotoxic drugs, targeted treatments, and immunotherapy. The analysis aimed to provide insights into the potential benefits and drawbacks of these novel approaches and highlight the need for further research to optimize NACT use in CRC and improve patient outcomes.
Methods:
From October 20, 2023, to December 10, 2023, a comprehensive literature search was conducted across multiple databases, including PubMed, Ovid, Web of Science, the Cochrane Library, Cumulative Index to Nursing and Allied Health Literature, Embase, and Scopus. Studies addressing the use of and treatment strategies for CRC and neoadjuvant therapies were included. Screening was conducted in two steps, initially by title and abstract and then by full-text articles. English-language articles were considered, while preprints, non-English publications, and articles published as grey literature were excluded from the study. A total of 85 studies were selected for further analysis after screening and filtering.
Results:
After filtering out duplicates and items that were irrelevant to our research query from the initial database search’s 510 results, 397 unique articles were found. Eighty-five studies were chosen for additional analysis after the articles underwent two rounds of screening.
Conclusion:
The review concluded that neoadjuvant therapy for CRC has evolved beyond conventional approaches and holds promise for improving patient outcomes. Future prospects for advancing neoadjuvant approaches are promising, with ongoing clinical trials investigating the refinement of strategies, identification of predictive biomarkers, and optimization of patient selection. The adoption of novel regimens, precision medicine, and immunotherapy offers opportunities to redefine treatment paradigms and enhance patient care in CRC.
Keywords
Chemotherapy
colorectal cancer
immunotherapy
neoadjuvant therapy
treatment strategies
Introduction
Colorectal cancer (CRC), which ranks as the second most lethal malignancy and the third most prevalent cancer, is a significant concern in the realm of global health. In the year 2020, it is projected that there will be a total of 1.9 million newly diagnosed cases of CRC worldwide, resulting in approximately 0.9 million fatalities. By 2040, 3.02 million more instances of CRC are anticipated, a significant increase. This enormous impact is anticipated to worsen over the ensuing decades, with 3.2 million new cases by 2040.[1] Different nations and areas display unique patterns of CRC prevalence. While rich countries traditionally have higher incidence rates, westernization and changes in lifestyle are placing an increasing burden on middle- and low-income countries.[2] Alarmingly, the development of early-onset CRC makes the situation worse. Over the next 20 years, China and the United States are anticipated to see the greatest number of new CRC cases. The projected rise in the number of cases in China, from 0.56 million in 2020 to 0.91 million in 2040, and in the US, from 0.16 million to 0.21 million during the same timeframe, serves as evidence of the growing global burden.[1,2]
To address this issue, heightened awareness, the promotion of healthy lifestyle choices, innovative management strategies, and the implementation of effective global screening programs are required. These interventions are crucial for preventing future CRC-related morbidity and mortality. The heterogeneity of CRC emphasizes the need for accurate subtype classification systems, which can contribute to research and clinical outcomes.[2] The CRC can be staged by a special staging called “Duke’s staging.” It can be seen below in Figure 1.
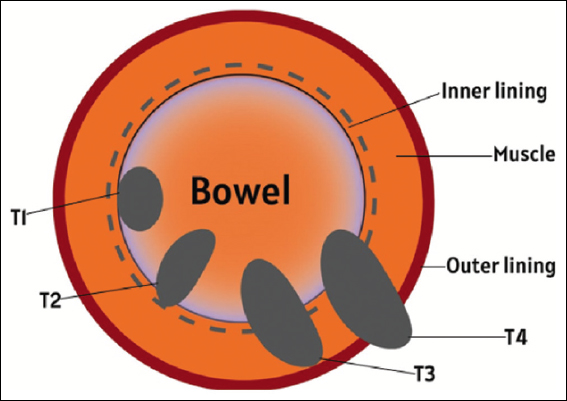
- Duke’s staging for colorectal cancer
Neoadjuvant therapy is crucial to the all-encompassing management of locally advanced CRC in this scenario. This strategy involves the administration of chemotherapy or chemoradiotherapy before surgery in an effort to enhance outcomes by shrinking tumors, facilitating surgical resection, and potentially reducing the risk of recurrence.[3-8] The significance of neoadjuvant therapy is highlighted by its potential to downstage tumors, improve the feasibility of conservative interventions, and ultimately contribute to higher survival rates.[9]
As the primary cause of cancer-related mortality, CRC requires a multidisciplinary approach for optimal management.[8] Neoadjuvant therapy has shown efficacy in reducing tumor size and increasing the likelihood of attaining complete resection, leading to improved disease control.[3,6] Neoadjuvant chemoradiotherapy has been widely regarded as the preferred treatment modality in various research studies that specifically investigate the management of locally advanced rectal cancer and has numerous benefits.[3-5] It permits early intervention, increases the likelihood of sphincter-preserving surgery, and decreases the risk of incomplete resections.[4,5] Moreover, this strategy has the potential to reduce the need for adjuvant therapy following surgery, potentially mitigating complications and enhancing patient outcomes overall.[3] The changing landscape of CRC treatment highlights the significance of neoadjuvant therapy in maximizing surgical outcomes and long-term survival. This approach has the ability to improve local tumor control, increase the feasibility of conservative interventions, and ultimately contribute to an improved patient prognosis.[3-9] The main pathway for colorectal carcinoma is depicted below in Figure 2.[10]
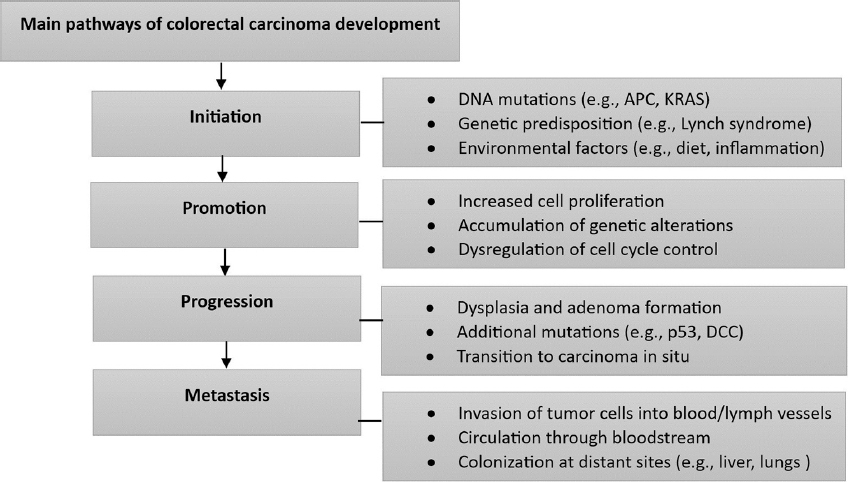
- Pathways of colorectal carcinoma development
Methods
This systemic review was carried to describe Novel regimens and treatment strategies in neoadjuvant therapy for CRC and that served as the basis for our investigation. From 20 October of 2023 until 10 December 2023, we searched PubMed, Ovid, Web of Science, the Cochrane Library, Cumulative Index to Nursing and Allied Health Literature, Embase, and Scopus. Filters were not included in the study. The PRISMA 2023 flow diagram in Figure 3 is used to offer information regarding research selection. We chose studies that addressed the use of and treatment strategies for CRC and neoadjyvant therapies. Case studies, case series, cross-sectional research, case-control research, cohort studies, and review papers were all considered. Only English-language articles were taken into consideration. Preprints, publications that were published in languages than English, and articles that were published as grey literature were not included in the study. The complete texts of records for which we had no access, letters to the editor, meeting reports, systematic reviews, cadaver, and animal studies were excluded from the study. Two steps of screening were carried out. We screened by title and abstract in the first step, and then we read the full-text articles in the second. All authors did the screening and tabulation, which they all recorded in an Excel sheet. The authors did not conduct a formal quality evaluation in accordance with the systemic review technique due to the topic’s heterogeneity and the wide variety of study types.
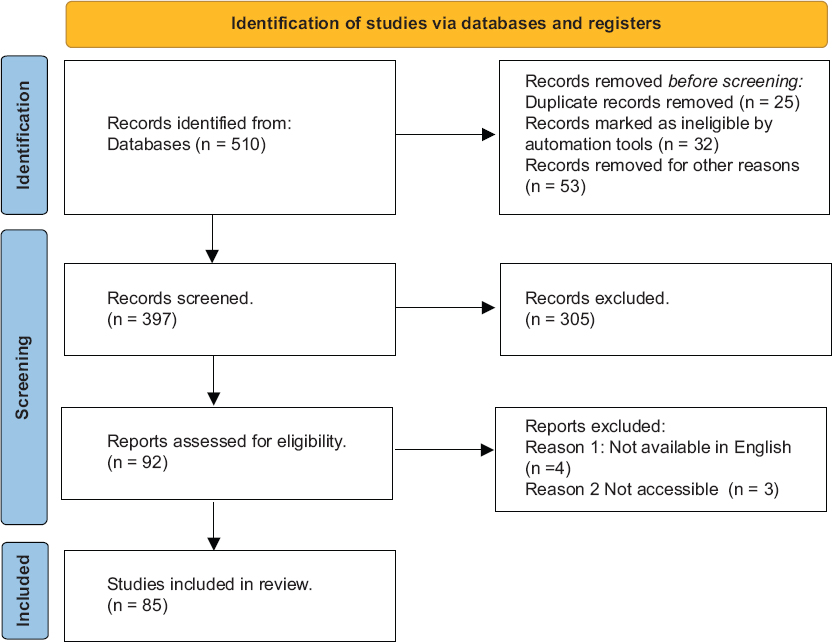
- Prisma flowchart of included studies
Results
After filtering out duplicates and items that were irrelevant to our research query from the initial database search’s 510 results, 397 unique articles were found. Eighty-five studies were chosen for additional analysis after the articles underwent two rounds of screening. The PRISMA 2023 flow chart [Figure 3] further explains the procedures and outcomes.
Current Normal Neoadjuvant Treatments
Surgical resection is considered the primary treatment option for CRC and is widely regarded as the cornerstone of therapy. This approach has the ability to cure localized stages (I-III) of the illness and, in some instances, even limited cases of metastatic disease (stage IV). The importance of surgery as a therapeutic modality for localized CRC cannot be emphasized. This therapy strategy is considered a basic therapeutic approach for early-stage CRC, offering a promising outlook for attaining complete tumor eradication and enhancing long-term survival rates.[11] Furthermore, it is worth noting that surgery continues to be a feasible alternative for patients who have limited metastatic disease. In such cases, the removal of the primary tumor alongside metastasectomy can significantly enhance the chances of extended survival.[12,13] Minimally invasive approaches and enhanced recovery protocols, both of which influence clinical outcomes and patient well-being, have influenced the evolution of surgical care. Even so, the decision-making process in Stage IV CRC remains intricate, encompassing not just professional expertise but also patient preferences and considerations for quality of life (QoL). The available information regarding the importance of initial surgery in asymptomatic patients is inconclusive, necessitating the use of clinical judgment in making decisions in these situations.[13]
Consequently, surgical resection remains the mainstay treatment for locally confined CRC, and it can even be beneficial in a subset of metastatic CRC cases. The changing landscape of CRC management highlights the significance of a multidisciplinary approach that takes into account not only the tumor stage but also patient preferences and QoL.[11,13] In recent years, the importance of chemotherapy in neoadjuvant settings for CRC has increased, particularly in locally advanced cases. Neoadjuvant chemotherapy (NACT) refers to the administration of chemotherapy before the primary surgical intervention with the goal of shrinking tumors, improving surgical outcomes, and possibly enhancing long-term survival.
NACT offers a number of prospective advantages in the treatment of CRC. It can target micro metastatic disease early on, potentially resulting in more effective resections and enhanced tolerability of treatment,[3] This strategy is crucial in locally advanced cases where complete resection may be difficult due to tumor size, location, and involvement of adjacent structures.[7] By reducing tumor burden and increasing the likelihood of attaining clear surgical margins, NACT may contribute to improved surgical outcomes and potentially higher R0 resection rates.
The efficacy of NACT in locally advanced colon cancer has been explored in clinical trials and systematic reviews.[7,14] The concept is now under investigation and subject to ongoing debate, with recommendations for its standardized implementation still in a state of development.[7] In general, the use of NACT in CRC shows potential as a beneficial addition to surgical intervention, aiming to improve the likelihood of successful curative resections and potentially enhance patient outcomes. Additional research and continued clinical trials are necessary to determine the most effective medication and further refine its role.
Radiation therapy plays a crucial role in the neoadjuvant treatment strategy for CRC, particularly in the context of rectal cancer. Neoadjuvant radiation is employed to decrease the size of tumors and improve the results of surgical procedures by minimizing the amount of cancerous tissue before curative surgery. The main aim is to attain a comprehensive resection with unambiguous margins, thereby reducing the likelihood of local recurrence and enhancing long-term survival.
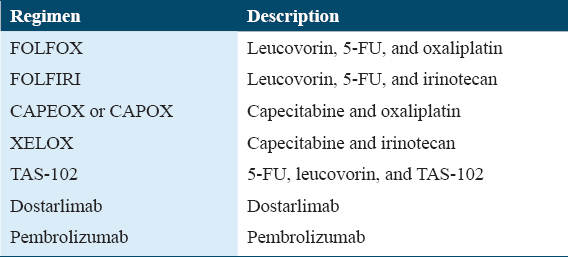
According to previous studies, neoadjuvant radiation therapy has demonstrated efficacy in reducing tumor size and improving the feasibility of surgical resection for patients diagnosed with rectal cancer.[15] The combined use of chemotherapy and radiation therapy, known as chemoradiation, has become the accepted treatment strategy for the treatment of rectal cancer that has advanced locally.[16] The use of a multimodal approach, encompassing chemotherapy as a potential component, aims to enhance the probability of sphincter preservation, diminish local relapse rates, and enhance overall therapeutic outcomes.[17,18] The treatment regimen commonly employed for CRC is presented in Table 1.[19] Nevertheless, the utilization of radiation therapy in neoadjuvant contexts for colon cancer is less common compared to its use in rectal cancer. In specific instances of colon cancer, the utilization of radiation therapy as a neoadjuvant intervention may be contemplated with the intention of reducing the size of tumors before surgical intervention, particularly in cases where the tumor is widespread or has infiltrated neighboring organs.[16] The efficacy of neoadjuvant radiotherapy in downstaging tumors and improving surgical outcomes in rectal cancer has been well documented. However, its role in colon cancer is not as well-established and may be influenced by patient and tumor characteristics. The evolving role of radiation therapy in CRC underscores the necessity for personalized treatment strategies that consider factors such as tumor stage, tumor location, and patient preferences. Figure 3 illustrates many non-adjuvant therapeutic routes in CRC.[20]
Total Neoadjuvant Therapy (TNT) Methodology
The TNT approach in the management of CRC involves the sequential administration of chemotherapy and radiation therapy before surgical intervention. This therapeutic strategy aims to enhance treatment efficacy and optimize outcomes for individuals diagnosed with locally advanced rectal cancer. The aforementioned comprehensive technique has gained significant traction in the field due to its demonstrated ability to raise the rate of pathological complete response (pCR), facilitate tumor shrinking, and enable sphincter preservation surgery.[19,21] The TNT strategy signifies a fundamental change in the management of locally advanced rectal cancer, as it allows for the integration of systemic chemotherapy at an earlier stage in the treatment process, with the ability to specifically target micro metastases and enhance disease control.[22,23]
The TNT approach addresses the challenge of local recurrence and distant metastases in CRC by administering NACT and radiation therapy before surgery. This approach is particularly applicable in cases of locally advanced rectal cancer, in which reducing tumor burden and enhancing surgical outcomes are essential objectives.[19,21] Utilizing TNT necessitates an individualized treatment strategy that takes into account the disease stage, tumor characteristics, and overall health status of the patient.[25] The ever-changing landscape of neoadjuvant therapies highlights the significance of refining treatment protocols and exploring novel therapeutic combinations to optimize patient outcomes.
The use of TNT might yield many advantages and disadvantages. According to meta-analytic studies, it has been found that the use of TNT results in a notably increased occurrence of enhanced pCR compared to traditional neoadjuvant chemoradiation. Polymerase chain reaction (PCR) has been found to be correlated with improved survival results. Furthermore, it has been observed that TNT has a higher level of disease-free survival (DFS) and overall survival in comparison to conventional therapy, as indicated by previous research.[28] The use of TNT has been shown to enhance the likelihood of sphincter-preserving surgery, hence maintaining the patient’s overall QoL.[4] The systemic chemotherapy component of the treatment known as TNT effectively targets and addresses micro metastases in their early stages, resulting in a decreased probability of the occurrence of distant metastases. The use of TNT has been found to have a positive impact on tumor downstaging, which in turn increases the feasibility of procedures and may even allow for observation instead of surgery in patients who have achieved a complete clinical response.[29]
Concerning disadvantages, the efficacy and safety of TNT remain debatable, with some trials demonstrating benefits and others not.[28] The increased toxicity that may result from TNT’s intensified treatment regimen may impact patient compliance and general health. Neoadjuvant therapy can result in postponed surgery, which may impact patient anxiety and tumor progression. The administration of TNT necessitates multidisciplinary teams that are well-coordinated and sophisticated infrastructure.[29] Personalized decisions are required to determine which patients will gain the greatest benefit from TNT and its optimal sequencing with surgery and other treatments.
Numerous clinical trials have been conducted to determine the efficacy of TNT in treating locally advanced rectal cancer. The study revealed a statistically significant correlation between the utilization of TNT and an increased probability of attaining a pathologic complete response (odds ratio [OR], 2.44; 95% confidence interval [CI], 1.99–2.98; I2 = 49%) as well as enhanced DFS (OR, 2.07; 95% CI, 1.03–3.56; I2 = 49%) in comparison to the conventional method of concurrent chemoradiotherapy followed by surgery and adjuvant chemotherapy plus A. PCR prevalence was 29.9% (range: 17.2%–38.5%) in the TNT group and 14.9% (range: 4.2%–21.3%) in the CRT plus A group. There were no statistically significant differences between the two approaches in terms of the frequency of sphincter-preserving surgery or the need for ileostomy.[4]
Comparing the efficacy of standard neoadjuvant chemoradiotherapy (CRT) with targeted neoadjuvant therapy (TNT) was the subject of a comprehensive evaluation and synthesis of 15 clinical trials from the existing literature. A twofold increase in the incidence of pCR was observed in the TNT arm compared to the CRT arm. Specifically, the TNT arm had a pCR rate of 25%, while the CRT arm had a rate of 13%. The calculated OR was 2.13, with a CI of 1.71–2.65 and a P > 0.001. At 47%, the heterogeneity statistic (I2) indicated a moderate amount of variation. 3-year rates of DFS were 74.2% in the arm receiving TNT and 69.0% in the arm receiving conventional radiotherapy (CRT) according to a meta-analysis of three studies. The calculated hazard ratio (HR) was 1.31, with a 95% CI ranging from 1.07 to 1.51. In addition, a P = 0.01 was determined. I2, a measurement of heterogeneity, revealed a value of 0%. The user has provided a reference to a number with no accompanying content or context.
The objective of this systematic review and meta-analysis was to compare the relative efficacy of TNT to that of routine neoadjuvant chemoradiotherapy. Eight phase II/III randomized controlled trials were included in the study. The delivery of TNT treatment led to a significant increase in the rate of pCR (OR, 1.77; 95% CI, 1.28–2.40; P = 0.0005). Compared to conventional chemoradiotherapy, the use of trinitrotoluene (TNT) has shown improvements in DFS and overall survival. The calculated HR for DFS was 0.83 (95% CI, 0.72–0.95; P = 0.03). Similarly, the overall survival HR was calculated to be 0.88 (95% CI, 0.74–1.05; P = 0.15). In addition, TNT therapy was associated with a significant reduction in the likelihood of distant metastases (HR = 0.81; 95% CI = 0.68–0.95; P = 0.012).[22] The results depicted in Figure 4 are presented in the following section.
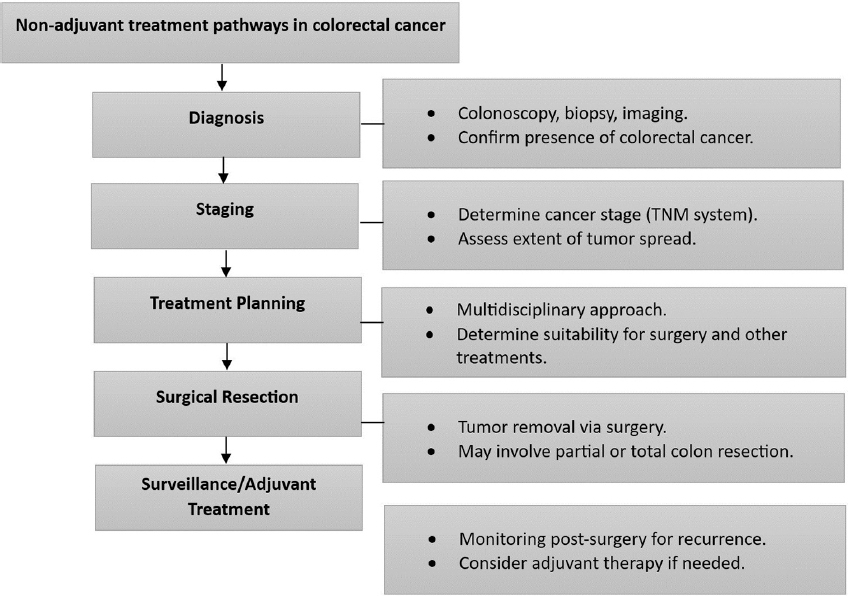
- Non-adjuvant treatment pathways in colorectal cancer
Novel-targeted Therapies in Neoadjuvant Settings
In the neoadjuvant setting for CRC, targeted therapies have acquired prominence as a valuable strategy. These treatments have the potential to target specific molecular alterations associated with CRC, thereby improving treatment efficacy and patient outcomes. CRC is a prevalent malignancy with differing stages of diagnosis, posing treatment challenges. Traditional methods such as surgery and chemotherapy have been employed, but targeted therapies are emerging as a promising alternative.[24,26,28]
Targeted therapies for CRC include a variety of agents designed to target specific molecular alterations fueling the progression of cancer. By focusing on actionable biomarkers expressed by CRC tumors, these therapies have revolutionized the treatment landscape. Prominent modes of action encompass the utilization of vascular endothelial growth factor (VEGF) Inhibitors, given that VEGF plays a pivotal role in promoting angiogenesis, hence enabling the growth of tumors. Bevacizumab, Ramucirumab, and Ziv-aflibercept are representative examples of targeted pharmacological agents that effectively suppress VEGF, hence impeding the angiogenesis process necessary for tumor sustenance.[28] Epidermal growth factor receptor (EGFR) inhibitors, including cetuximab and panitumumab, function by inhibiting the signaling of the EGFR, a crucial factor for the proliferation and viability of malignant cells. The aforementioned medicines have demonstrated effectiveness in combating cancers that exhibit mutations or overexpression of the EGFR.[29,30] BRAF inhibitors: The V600E mutation of the BRAF gene promotes the development of CRC. This mutated pathway is inhibited by targeted agents such as encorafenib and binimetinib, thereby limiting tumor progression. Trastuzumab and lapatinib are efficacious against CRC tumors with HER2 amplification or overexpression.[29,31] MEK inhibitors, such as trametinib, are utilized in BRAF-mutated CRC to inhibit tumor growth by blocking the downstream MEK/ERK signaling pathway.[29] The application of immune checkpoint inhibitors (ICIs), such as pembrolizumab and nivolumab, enhances the selective recognition and targeting of cancer cells by the immune system. The therapeutic efficacy of these treatments has been demonstrated in patients afflicted with microsatellite instability-high (MSI-H) or deficient mismatch repair (dMMR) malignancies.[27,31]
Evidence of efficacy in neoadjuvant settings
As highlighted by the American Society of Clinical Oncology guidelines for breast cancer, the efficacy of neoadjuvant therapies is evident in numerous cancer types. Although the specific reference material does not explicitly pertain to CRC, similar paradigms are emerging in CRC research.[1] However, neoadjuvant therapy principles are pertinent to all cancer types, including CRC.
With the advent of precision medicine, therapies that exploit the molecular characteristics of malignancies have become available. Recent studies concentrating on non-small cell lung cancer reveal the potential benefits of neoadjuvant targeted therapy. The trials in issue are predominately focused on biomarker-guided drugs, such as ICIs that are tailored to the genetic characteristics of the tumor.[2]
While there are no direct references to targeted therapy in neoadjuvant settings in the context of CRC, the effectiveness paradigm of neoadjuvant therapy is extensively applicable. Standardized definitions for efficacy endpoints in neoadjuvant clinical trials, such as NeoSTEEP for breast cancer,[4] assure consistent evaluation criteria to advance the field. The effective implementation of neoadjuvant targeted therapies in HER2-positive breast cancer demonstrates the potential for a comparable strategy in CRC.[6]
The efficacy of targeted therapies in the neoadjuvant setting is supported by evidence from a variety of cancer types. In the absence of direct CRC references, the principles of neoadjuvant therapy’s efficacy, guided by precision medicine, hold promise for improving CRC treatment outcomes.
Immunotherapy for Neoadjuvant Cancer Treatment
The application of immunotherapy has emerged as a potentially effective therapeutic strategy for CRC, especially in cases of metastatic disease. The CRC ranks as the third most prevalent type of cancer and is the second leading cause of cancer-related mortality on a global scale.[32] In instances of metastatic disease, standard therapeutic techniques, including chemotherapy, radiation therapy, and surgery, may produce less than optimum outcomes.[33] In recent years, there have been notable advancements in the field of immunotherapy, which have showcased its capacity to augment the immune system’s aptitude for discerning and eradicating cancerous cells in a targeted manner. In the realm of CRC, the utilization of ICIs, particularly those that selectively bind to programmed death-1 (PD-1) and programmed cell death ligand 1 (PD-L1), constitutes a noteworthy immunotherapeutic strategy. Various types of tumors, such as CRC, have demonstrated enduring responses to inhibitors,[34] particularly in cases marked by dMMR or MSI-H. The aforementioned molecular features are observed in around 5% of instances of CRC.[2] The challenge lies in the observation that a significant proportion, specifically 95%, of CRC instances demonstrate a proficient mismatch repair (pMMR) or microsatellite stable phenotype. The present phenotype is correlated with a reduced tumor mutation burden and less infiltration of tumor lymphocytes.[34]
The primary emphasis of ongoing research is directed at resolving the challenges associated with immune tolerance and evasion in the tumor microenvironment. At present, there is a concerted endeavor to ascertain biomarkers that might accurately predict the response to immunotherapy, as well as to formulate innovative therapeutic approaches. The prognostic and predictive relevance of the immunological tumor microenvironment (TME) in CRC is attributed to its intricate and heterogeneous nature. Although immune checkpoint drugs have demonstrated therapeutic effectiveness, individuals with pMMR or MSS CRC necessitate the implementation of efficacious immunotherapy approaches. This requires a deeper understanding of the immune system’s role in the tumor microenvironment and the need to design combination therapies that can augment the immune response against tumors.[35] The utilization of immunotherapy has significant promise in transforming the management of CRC, particularly in instances characterized by the presence of metastatic disease. The efficacy of ICIs has been found to be outstanding, particularly in cases characterized by the dMMR/MSI-H phenotype. However, current research endeavors to expand the advantages of immunotherapy to the majority of CRC cases characterized by the pMMR/MSS phenotype. This is being achieved by the identification of predictive biomarkers and the development of innovative therapeutic approaches.[36]
Investigating immunotherapy neoadjuvant combinations
The investigation of neoadjuvant immunotherapy combinations holds promise for enhancing the efficacy of cancer treatment through the utilization of the collaborative effects between different ICIs and other therapeutic agents. ICIs are administered before surgical resection in neoadjuvant immunotherapy to downstage tumors, enhance surgical outcomes, and elicit systemic antitumor immune responses. This strategy not only facilitates early evaluation of treatment efficacy but also affords the chance to detect undetected metastases and avoid superfluous surgical interventions.[4,5]
In the treatment of cutaneous squamous cell carcinoma, the potential efficacy of combining neoadjuvant immunotherapy with checkpoint inhibitors, particularly those targeting PD-1 and PD-L1, has been observed.[1,3] These studies have yielded significant findings regarding pathological responses and improved surgical outcomes. In addition, the use of neoadjuvant immunotherapy has broadened its application beyond melanoma and non-small cell lung cancer to include high-risk, operable cancers.[4,5] Nonetheless, it is essential to address the clinical obstacles associated with this strategy and to emphasize the significance of utilizing rigorous clinical trial methodologies to evaluate its efficacy and safety.[5]
The dynamic paradigm of neoadjuvant immunotherapy combinations offers a promising avenue for transforming cancer therapy by enhancing the antitumor immune response and possibly enhancing the efficacy of ICIs. To completely realize the potential of neoadjuvant immunotherapy combinations for improving patient outcomes, it is necessary to conduct additional research, employ innovative trial designs, and gain a deeper understanding of the immunological systems involved.
Biomarker-Informed Neoadjuvant Interventions
Emerging CRC biomarkers
The identification of emerging biomarkers for CRC is crucial to improve early detection, prognosis, and treatment approaches. Biomarkers play a crucial role in tailoring therapy to specific patients and enhancing patient outcomes. Advancements in genomics and molecular pathology have led to the identification of potential biomarkers.[34] These biomarkers have the potential to enhance the customization of therapy and facilitate the prediction of prognosis. It is important to highlight that the utilization of molecular testing to identify gene mutations, including KRAS, BRAF, and p53, together with the assessment of microsatellite instability and epigenetic modifications, holds significant significance in the advancement and advancement of CRC.[38]
Furthermore, scientists are also examining cell surface indicators as a means of identifying diverse colorectal tumors at an early stage. The purpose of these biomarkers is to enhance the sensitivity of colonoscopy, which is widely regarded as the most reliable method for detecting CRC.[36] The next figures, Figures 5 and 6, illustrate the mutation of BRAF and HER2, as well as their progression and signaling pathways in CRC.[39-43]
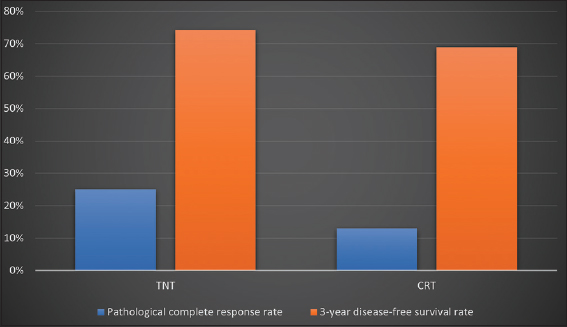
- Comparison of outcomes total neoadjuvant therapy versus CRT
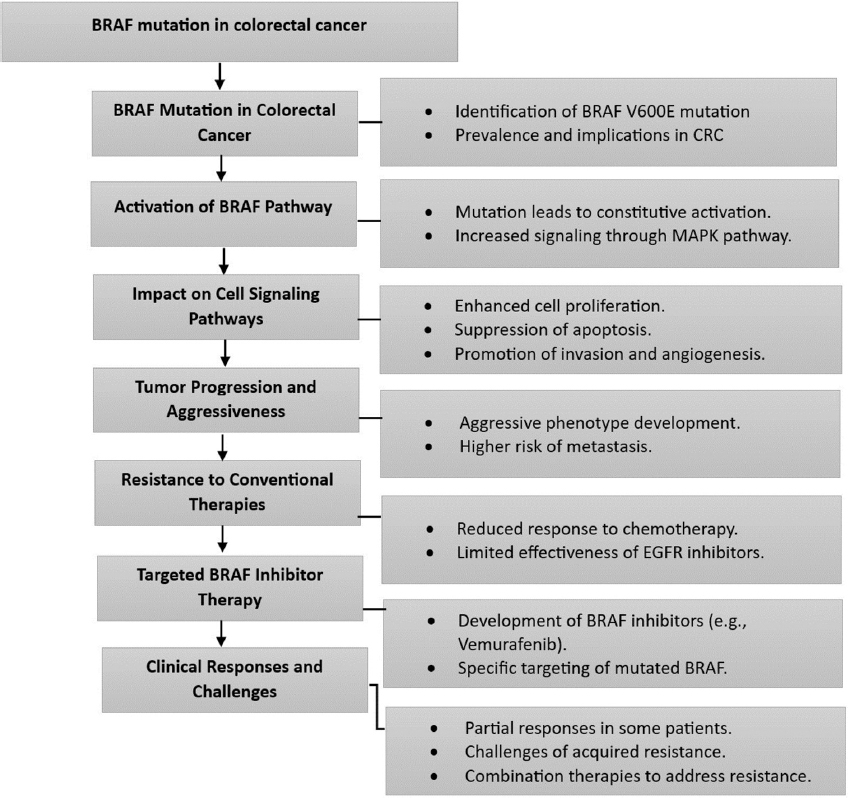
- BRAF mutation and pathway in colorectal cancer
Approaches to personalized treatment based on biomarkers
Biomarker-based personalized treatment approaches have acquired importance in the management of CRC, offering tailored therapies and improved patient outcomes. Molecular testing guides treatment decisions and facilitates the selection of targeted therapies. For instance, the identification of specific gene alterations or their absence can inform the choice of a specific adjuvant therapy, such as anti-EGFR antibodies.[35] Furthermore, the utilization of gene expression profiling has the potential to categorize CRC subtypes, consequently enhancing the accuracy of prognostic and predictive capabilities.
The prognostic significance of molecular biomarkers in individualized CRC therapy includes both treatment response and adverse effects. Biomarkers, such as the Oncobox medication effectiveness score, have been shown to reliably forecast therapy responses.[37] Furthermore, biomarkers play a crucial role in the early detection of patients who are at a higher risk of experiencing severe therapy-related toxicity. This, in turn, helps health-care professionals make informed decisions regarding the appropriate course of treatment.[41]
Obstacles and future paths for biomarker-guided therapy
Challenges and prospective directions in biomarker-guided therapy for CRC stem from the complexity of biomarker interactions, the ever-changing landscape of treatment options, and the requirement for comprehensive molecular profiling. As biomarkers become integral to prognosis and treatment decisions, it can be difficult to discern their combined impact due to their intricate interplay. Patient outcomes are affected by point mutations, gene amplifications, and global gene expression subtypes.[44]
Moreover, the expansion of available CRC treatment options, guided by biomarkers, introduces the difficulty of selecting the optimal therapy for individual patients. Even though precision oncology is promising, it requires careful consideration of clinical parameters and biomarker-drug interactions.[44,45] Future research will focus on refining biomarker-driven therapies, such as testing for BRAF mutations, MMR germline genes, and MLH1 promoter methylation, to distinguish between sporadic and hereditary CRC. In addition, investigating first-line ICIs for metastatic CRC and integrating liquid biopsies for rapid turnaround could revolutionize precision medicine in CRC.[45,46]
In Neoadjuvant Settings, Combination Therapies
Combinations of chemotherapy and targeted agents that are synergistic
In the field of CRC, the utilization of combined chemotherapy and targeted medicines has promise for improving therapeutic effectiveness and overcoming resistance. The logical sequence of these combinations entails the discernment of the precise pathways that are stimulated by chemotherapy, afterward accompanied by the judicious choice of targeted medications capable of augmenting the therapeutic impact. In the setting of CRC, the utilization of phosphokinome modifications induced by irinotecan was employed to inform the selection of specific treatment drugs. The utilization of this approach resulted in the identification of synergistic combinations, such as BKM120 (a phosphoinositide 3-kinase inhibitor) and MEK162 (a mitogen-activated protein kinase inhibitor), which demonstrated both cytostatic and cytotoxic effects in laboratory trials and animal models.[47]
Similarly, tumoroid models have helped identify synergistic drug combinations, such as the potential cytotoxicity of DDR1/BCR-ABL targeting in conjunction with EGFR-ERBB2 inhibitors for KRAS-driven chemoradioresistant CRC.[48] These methods emphasize the significance of targeting interconnected signaling pathways to improve treatment efficacy. While challenges persist in identifying effective synergistic combinations, these efforts advance personalized therapeutic strategies for CRC patients.
Combining immunotherapy with additional interventions
The potential enhancement of CRC treatment efficacy can be achieved through the integration of immunotherapy with other treatment techniques. Immunotherapy has demonstrated remarkable efficacy, particularly in mismatch repair-deficient mutations and tumors with elevated microsatellite instability.[49] Although CRC has been less responsive to immunotherapy than other types of cancer, initiatives to combine immunotherapy with conventional treatments have garnered increasing interest.
Researchers have investigated the integration of immunotherapy with chemotherapy, radiation therapy, and targeted medicines to augment the immune response and overcome treatment resistance.[50] The immunological tumor microenvironment of CRC plays a key role, rendering it a potential target for therapeutic interventions.[35] Combination immunotherapy approaches, such as checkpoint inhibitors combined with other immune modulators, have been evaluated in clinical trials.[51] Despite lingering obstacles, the ongoing investigation of combination strategies presents a novel method for transforming CRC treatment paradigms and enhancing patient outcomes.
Justifications and clinical studies of combination therapies
In the context of CRC, the primary objective of combination therapy is to optimize treatment efficacy by simultaneously targeting multiple signaling pathways. The complex molecular pathways of CRC and the need to surmount immune tolerance and resistance mechanisms present in the tumor microenvironment[34] provide justification for the integration of multiple therapeutic approaches. In patients with metastatic CRC, the use of ICIs, tyrosine kinase inhibitors, and mammalian target of rapamycin inhibitors has been associated with enhanced survival.[4]
Several clinical trials have been conducted to investigate the potential of integrating various treatment modalities, such as ICIs, targeted therapies, and immunomodulators, to augment immune responses and improve overall survival outcomes in CRC patients.[52] Immune resistance pathways within the tumor microenvironment have been the subject of a significant amount of research. Integration of PD-1 inhibitors with other therapeutic interventions, such as cetuximab, is a prominent strategy.[52] The combined tactics employed aim to produce long-lasting responses and improve overall survivability.[2] As the field of CRC treatment advances, the understanding of the disease’s molecular underpinnings and the interplay between pathways may provide valuable insights for the development of more effective and personalized combination therapies for patients with CRC.[53]
Reducing Treatment Toxicity and Managing Adverse Reactions
Methods to mitigate treatment-related toxicity
The attempt to reduce the incidence of therapy-induced toxicity in CRC involves implementing several approaches that seek to minimize adverse effects while maintaining the effectiveness of the treatment. The role of oncology nurses in the identification and management of toxicities related to chemotherapy and targeted therapies for CRC is of utmost importance. The implementation of these measures encompasses the prompt detection and management of unfavorable effects, hence mitigating the need for dosage reductions or discontinuation of medication.[54] Personalized treatment approaches that take into account patient characteristics, comorbidities, and performance status can maximize therapy selection and reduce toxicity.[50] Combining therapies with distinct mechanisms of action, such as cetuximab and bevacizumab, may decrease toxicity while increasing efficacy.[56] Moreover, it is anticipated that research on the tumor immune microenvironment (TME) and immunotherapy strategies will yield novel ways to reduce toxicity and improve outcomes.[55] Healthcare professionals aim to enhance the QoL for patients with CRC, promote adherence to treatment, and eventually enhance clinical outcomes through the implementation of these techniques.[57]
Supportive care during neoadjuvant treatment
The implementation of supportive care measures during neoadjuvant therapy for CRC is crucial in effectively controlling the adverse effects associated with treatment and enhancing the overall well-being of patients. The objective of neoadjuvant therapy, which includes chemotherapy, targeted therapy, and immunotherapy, is to reduce tumor size before surgery. Strategies for mitigating treatment-related toxicities include prompt monitoring and treatment of symptoms. The patient may experience fatigue, vertigo, diarrhea, and immune-related adverse events.
During neoadjuvant therapy for CRC, it is essential to integrate personalized approaches to patient management, including dose adjustments, symptom management, and nutritional support.[1] Furthermore, recent developments in neoadjuvant immunotherapy have exhibited potential in diminishing the occurrence of relapse and attaining notable outcomes in individuals diagnosed with localized CRC characterized by a dMMR.[2] Furthermore, the utilization of ICIs has exhibited effectiveness in both early-stage and advanced CRC, potentially modifying the therapy paradigm.[3] To optimize outcomes during neoadjuvant therapy for CRC, a multidisciplinary approach that takes into account the specific requirements of each patient and effectively manages treatment-related side effects is essential.
Addressing patients’ QoL
The assessment of QoL is essential in delivering complete care for persons diagnosed with CRC. Individuals who have survived CRC frequently experience prolonged decreases in health-related QoL due to the impacts of therapy, the management of symptoms, and various psychosocial factors. A multitude of research studies have been conducted to investigate different aspects of QoL in individuals who have survived CRC, with the aim of gaining a more comprehensive comprehension of their experiences and requirements.
According to research, CRC survivors face a variety of physical, psychological, social, and emotional challenges that can impact their QoL. These obstacles include symptoms, physical functioning, psychological well-being, relationship impacts, and financial toxicity.[53,54] Frequently, cancer survivors must deal with altered gastrointestinal function, stoma-related issues, fatigue, disturbed sleep, and anal pain.[53] Moreover, studies have emphasized the significance of addressing financial burden, particularly among survivors of early-onset CRC, as it can impact QoL.[55]
Understanding the determinants of HRQOL, such as sociodemographic factors, tumor characteristics, treatment, and stoma-related concerns, is crucial for customizing care plans and interventions for cancer survivors.[54] Patient-reported outcomes play an important role in evaluating QoL as they provide insight into the perspectives and experiences of cancer survivors. This data assists healthcare professionals in providing patient-centered care and support.[53,56]
In conclusion, addressing CRC patients’ QoL necessitates a multidimensional approach that considers the physical, psychological, social, and financial aspects of survivorship. By gaining an understanding of survivors’ experiences and obstacles, healthcare professionals can tailor interventions and support strategies to enhance their well-being and QoL.
Emerging Strategies and Future Paths
Novel neoadjuvant therapeutic agents in development
The development of ICIs that specifically target PD-1 and cytotoxic T cell-associated protein 4 is being explored for neoadjuvant therapy in CRC. This is particularly relevant in the context of MSI-H and dMMR CRC. These agents have the potential to revolutionize treatment strategies for both resectable primary CRC and metastatic CRC.[48,57-61]
Neoadjuvant immunotherapy with ICIs such as nivolumab and pembrolizumab has shown efficacy in early-stage and advanced CRC, especially in dMMR CRC. These therapies boost the immune system by ICIs, activating cytotoxic T cells, and initiating the destruction of cancer cells. Notably, the NICHE-1 study indicates the potential for immunotherapy in operable Stages I–III CRC, even in MMR-competent patients.[62]
The concept of neoadjuvant immunotherapy introduces new treatment strategies and possibilities for CRC. It addresses obstacles associated with local progression, distant metastases, and surgical complications, thereby increasing the rate of R0 resection and potentially attaining complete or partial pathological responses. MSI-H/dMMR CRC represents a promising candidate for neoadjuvant immunotherapy, a therapeutic approach that holds the potential to significantly transform the treatment paradigm for locally advanced CRC.[63] These novel agents offer prospective benefits for patient outcomes and pave the way for future advances in neoadjuvant therapy, making them a promising avenue for the treatment of CRC.
Advances in pre-operative imaging and selection of patients
In CRC, advances in pre-operative imaging and patient selection for neoadjuvant therapy have significantly improved treatment strategies. Utilizing enhanced pre-operative imaging techniques to precisely determine the tumor’s characteristics, stage, and extent is a crucial aspect. The techniques encompassed in this category are magnetic resonance imaging, computed tomography, and positron emission tomography. These imaging modalities offer a thorough assessment of tumor dimensions, spatial distribution, and the likelihood of metastatic spread.[64] The effectiveness of neoadjuvant therapy depends on the careful selection of patients. Adapting treatment to the unique characteristics of each patient facilitates the prediction of treatment responses and outcomes. The utilization of molecular profiling, biomarker analysis, and genetic testing has become essential in the identification of patients who are expected to exhibit positive responses to neoadjuvant therapy. Identifying MSI-H CRCs, for instance, can aid in selecting patients who may benefit from immunotherapies.[65,66]
Moreover, advances in radiomics and artificial intelligence have revolutionized image analysis, facilitating the identification of subtle characteristics that may not be visible to the unaided eye. These technologies facilitate the prediction of treatment response, thereby allowing for personalized treatment strategies and enhanced patient outcomes. Combining imaging data with clinical and molecular information in CRC generates a comprehensive patient profile that informs treatment decisions.[64,66]
Obstacles and Restrictions in Neoadjuvant Therapy
Challenges and limitations in neoadjuvant therapy for CRC involve a complex interplay of factors that influence treatment outcomes, patient compliance, and bridging the research-to-clinical practice gap. To maximize the efficacy of neoadjuvant approaches, these obstacles must be addressed.[66]
Heterogeneity and resistance in tumors
Intertumoral heterogeneity is a hallmark of CRC, in which distinct tumor cell populations manifest varying molecular profiles and treatment responses. This heterogeneity can result in insufficient treatment responses and treatment resistance. In addition, the influence of the microenvironment on treatment efficacy further complicates outcomes. It is crucial to develop strategies to target diverse tumor subpopulations and overcome resistance mechanisms.[65]
Compliance and adherence to treatment
It is difficult to ensure patient compliance with neoadjuvant therapy regimens. Long treatment durations, the possibility of adverse effects, and complex drug regimens may discourage patients from adhering to prescribed therapies. Incomplete adherence can compromise the efficacy of a treatment and affect its outcomes. To enhance patient understanding and compliance, health-care providers must implement effective communication strategies and support systems.[67]
Bridging the gap between clinical practice and research
Implementing optimistic research findings into standard clinical practice remains a formidable obstacle. The results of clinical trials may not always be directly applicable to the diverse patient populations encountered in the real world. In addition, the implementation of innovative therapies into clinical practice requires time, infrastructure, and resources. Effectively implementing cutting-edge therapies requires multidisciplinary collaboration, evidence-based guidelines, and implementation strategies.[66]
Utilization of molecular profiling
Although molecular profiling holds tremendous promise for identifying optimal treatment strategies, integrating these findings into clinical decision-making is difficult. Continual efforts are required to identify actionable targets, standardize testing methodologies, and interpret complex genetic data. Providing access to molecular profiling and improving the interpretation of results are crucial stages in the customization of neoadjuvant therapies.[65]
Individualized treatment strategies
CRC demonstrates considerable interpatient variability, necessitating individualized treatment approaches. However, individualized treatment based on patient characteristics can be difficult. To accurately predict treatment responses, take into account comorbidities, and balance the benefits and hazards of treatment, robust predictive models and biomarkers are required.[67]
Monitoring responses to treatment
It is essential to evaluate treatment responses during neoadjuvant therapy to make timely adjustments. Traditional techniques, such as radiological evaluations, may not always accurately reflect the short-term effects of treatment. Using advanced imaging techniques and liquid biopsies for real-time monitoring can provide more accurate insights into treatment responses and help direct modifications.[67]
Neoadjuvant therapy for CRC confronts numerous obstacles and limitations. Overcoming these obstacles requires a comprehensive strategy that combines molecular profiling, patient education, monitoring, and the collaborative efforts of scientists, clinicians, and policymakers. The resolution of these obstacles will result in enhanced treatment outcomes and a more seamless incorporation of innovative therapies into clinical practice.
Recent Clinical Trials
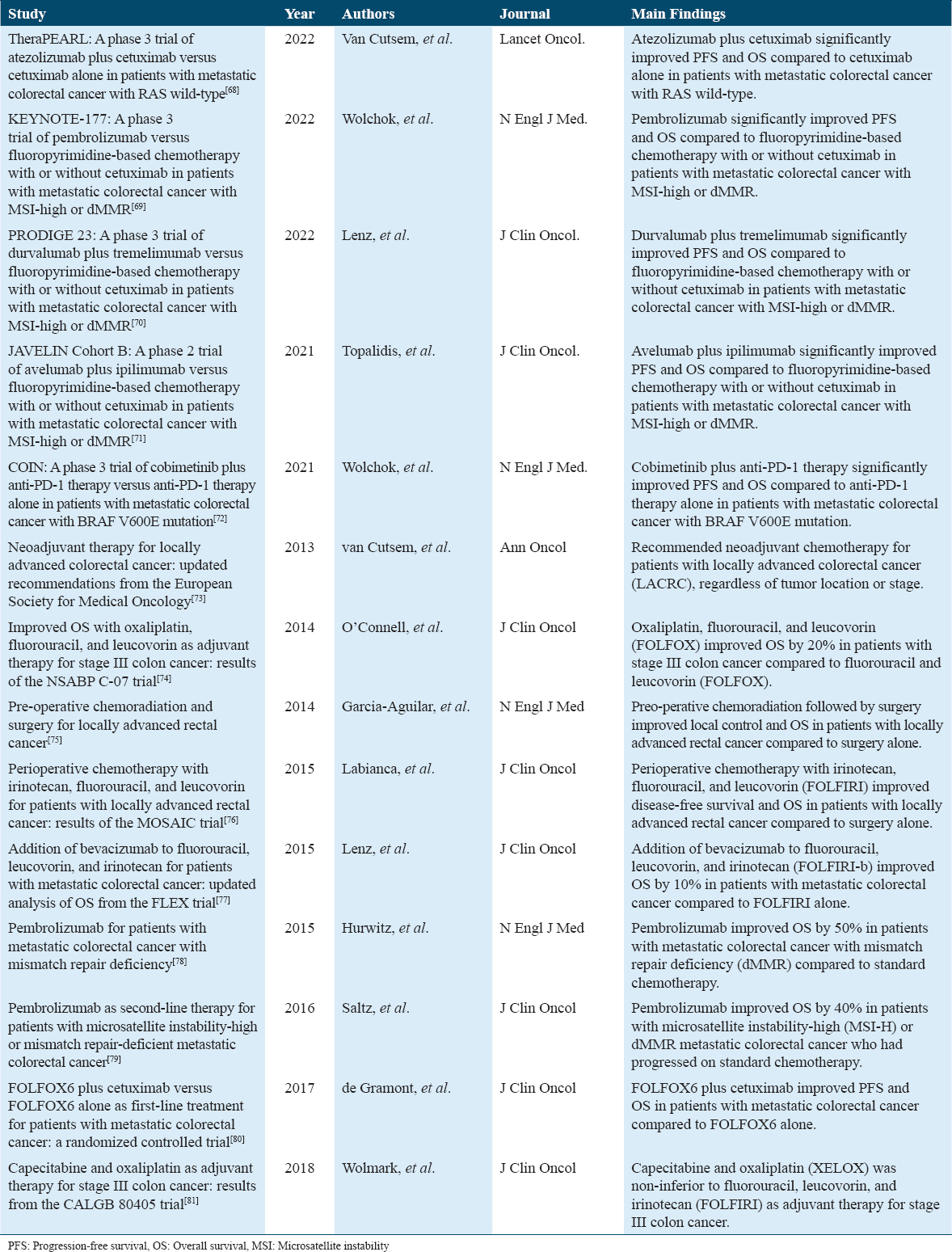
Following recent clinical trials done in the past 10 years of how different neoadjuvant therapies have worked for CRC are mentioned below in Table 2.
Conclusion
This review has provided a thorough examination of novel regimens and treatment strategies in CRC neoadjuvant therapy. The investigation of diverse approaches encompassing neoadjuvant radiotherapy, chemotherapy, immunotherapy, and combination therapies demonstrates the expanding potential to improve patient outcomes and transform the landscape of CRC treatment. Neoadjuvant therapy has evolved beyond conventional approaches, demonstrating the capacity to downstage locally advanced disease, facilitate more effective resections, and enhance treatment tolerability. Neoadjuvant radiotherapy, especially for rectal cancer, has demonstrated efficacy in reducing tumor burden before curative surgery, and its benefits are best realized through multidisciplinary collaboration and careful patient selection.[15] Similarly, NACT has demonstrated promise in the treatment of colon cancer, and ongoing research aims to uncover its complete potential and refine its administration.[82] The integration of ICIs into neoadjuvant therapy for MSI-H CRC has initiated a novel era and revolutionized the approach to managing this particular subtype of the disease. The emergence of precision medicine based on the unique biological characteristics of each individual’s CRC has paved the way for targeted therapies,[84] in which therapeutic approaches are tailored to the tumor’s specific characteristics. The prospective impact on patient outcomes is substantial as the field advances. The incorporation of innovative strategies can result in enhanced disease management, improved surgical outcomes, and increased organ preservation. Nevertheless, obstacles such as patient selection, treatment toxicity, and balancing the benefits and risks of neoadjuvant interventions require careful consideration and ongoing research.[83] Figure 7 illustrate HER 2 mutation, therapy, and signaling pathway in colorectal cancer.
- HER 2 mutation, therapy, and signaling pathway in colorectal cancer
Future prospects for the advancement of neoadjuvant approaches are promising. Current clinical trials investigate the duration, sequencing, and combination of various therapeutic modalities. The objective is to refine neoadjuvant strategies, identify predictive biomarkers, and optimize patient selection to achieve the best outcomes possible while minimizing treatment-related morbidity.[85] The rapidly changing landscape of neoadjuvant therapy for CRC offers numerous opportunities to enhance patient care. Adoption of novel regimens and strategies, implementation of precision medicine, and the emergence of immunotherapy all have the potential to improve outcomes, redefine treatment paradigms, and continue the trend toward more effective and individualized treatments for CRC. As research advances, it is crucial to maintain a patient-centered perspective, balancing innovation with careful consideration of patient requirements and QoL.
Patient’s Consent Statement
Not applicable.
Availability of Data and Material
The data presented in this article are available with the corresponding author and will be provided on reasonable request.
Competing Interests
None.
Funding Statement
None.
Authors’ Contributions
Yamama Al-Khazraji, Muhammad Ali Muzammil, Saman Javid,: Conceptualization, methodology, software, data curation, validation, writing-original draft preparation. Adarsh Vardhan Tangella, Namra Vinay Gohil, Hanya Saifullah, Sai Gautham Kanagala: Writing- reviewing and editing, project administration. Sai Gautham Kanagala, Ali Shariq: Writing- reviewing and editing, supervision.
References
- Global colorectal cancer burden in 2020 and projections to 2040. Transl Oncol. 2021;14:101174.
- [Google Scholar]
- Correlation study of the colorectal cancer statistics and economic indicators in selected Balkan Countries. Front Public Health. 2020;8:29.
- [Google Scholar]
- The role of neoadjuvant chemotherapy in patients with locally advanced colon cancer:A systematic review and meta-analysis. Front Oncol. 2022;12:1024345.
- [Google Scholar]
- Total neoadjuvant therapy vs standard therapy in locally advanced rectal cancer. JAMA Netw Open. 2020;3:e2030097.
- [Google Scholar]
- Importance of response to neoadjuvant chemotherapy in potentially curable colorectal cancer liver metastases. BMC Cancer. 2008;8:120.
- [Google Scholar]
- Neoadjuvant Oxaliplatin-Fluoropyrimidine Chemotherapy Can be Delivered Safely in Patients with Operable Colon Cancer, Producing Marked Histopatholo. Switzerland: ESMO; 2023.
- Current Data and Trends in Neoadjuvant Treatment in Colorectal Cancer. 2023. Daily News. Available from: https://dailynews.ascopubs.org/do/current-data-and-trends-neoadjuvant-treatment-colorectal-cancer
- [Google Scholar]
- Neoadjuvant treatment of rectal cancer:Where are we now? Gastroenterol Rep (Oxf). 2016;4:206-9.
- [Google Scholar]
- Colorectal cancer in the elderly patient:The role of neo-adjuvant therapy. Open Med (Wars). 2019;14:607-12.
- [Google Scholar]
- Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology. 2007;50:113-30.
- [Google Scholar]
- Colon Cancer Treatment and Management:Approach Considerations, Surgical Care, Ablation. Available from: https://emedicine.medscape.com/article/277496-treatment
- Surgical resection and survival outcomes in metastatic young adult colorectal cancer patients. Cancer Med. 2021;10:4269-81.
- [Google Scholar]
- Primary tumor resection in stage IV unresectable colorectal cancer:What has changed? Med Oncol. 2017;34:188.
- [Google Scholar]
- A pathological complete response after neoadjuvant triplet chemotherapy for locally advanced transverse colon cancer. Int J Surg Case Rep. 2020;72:127-32.
- [Google Scholar]
- Neoadjuvant radiotherapy for rectal cancer management. World J Gastroenterol. 2019;25:4850-69.
- [Google Scholar]
- Neoadjuvant Therapy, Transanal Excision. Available from: https://emedicine.medscape.com/article/281237-treatment?form=fpf
- The evolving role of radiotherapy in locally advanced rectal cancer and the potential for nonoperative management. Oncol Hematol Rev. 2020;16:43-51.
- [Google Scholar]
- Neoadjuvant chemoradiation for rectal cancer:Is more better? Cancer Network 2008:814-26. discussion 826, 828-31, 836
- [Google Scholar]
- CT Image-based biopsy to aid prediction of HOPX expression status and prognosis for non-small cell lung cancer patients. Cancers (Basel). 2023;15:2220.
- [Google Scholar]
- Management of stage II colon cancer - the use of molecular biomarkers for adjuvant therapy decision. BMC Gastroenterol. 2013;13:36.
- [Google Scholar]
- Total neoadjuvant treatment for locally advanced rectal cancer patients:Where do we stand? Int J Mol Sci. 2023;24:12159.
- [Google Scholar]
- Locally advanced rectal cancer receiving total neoadjuvant therapy combined with nivolumab:A case report and literature review. World J Surg Oncol. 2022;20:166.
- [Google Scholar]
- A contemporary assessment of total neoadjuvant therapy (TNT) protocols for locally advanced rectal cancer:Adoption and expert perspectives at German Cancer Society (DKG)-certified colorectal cancer centers. J Cancer Res Clin Oncol. 2023;149:12591-6.
- [Google Scholar]
- The role of neoadjuvant chemotherapy in locally advanced colon cancer. Cancer Manag Res. 2021;13:2567-79.
- [Google Scholar]
- Total neoadjuvant therapy (TNT) versus standard neoadjuvant chemoradiotherapy for locally advanced rectal cancer:A systematic review and meta-analysis. Oncologist. 2021;26:e1555-66.
- [Google Scholar]
- Total neoadjuvant therapy approach in rectal adenocarcinoma. Clin Adv Hematol Oncol. 2021;19:711-8.
- [Google Scholar]
- 54P Efficacy of total neoadjuvant therapy (TNT) in rectal cancer:A meta-analysis of randomized controlled trials. Ann Oncol. 2022;33:S1449-50.
- [Google Scholar]
- American Cancer Society. Available from: https://www.cancer.org/cancer/types/colon-rectal-cancer/treating/targeted-therapy.html
- Current targeted therapy for metastatic colorectal cancer. Int J Mol Sci. 2023;24:1702.
- [Google Scholar]
- Progress of research on molecular targeted therapies for colorectal cancer. Front Pharmacol. 2023;14:1160949.
- [Google Scholar]
- Targeted therapies in colorectal cancer:Recent advances in biomarkers, Landmark trials, and future perspectives. Cancers (Basel). 2023;15:3023.
- [Google Scholar]
- Immunotherapy in colorectal cancer:Current achievements and future perspective. Int J Biol Sci. 2021;17:3837-49.
- [Google Scholar]
- Emerging role of immunotherapy for colorectal cancer with liver metastasis. Onco Targets Ther. 2020;13:11645-58.
- [Google Scholar]
- Immunotherapy in colorectal cancer:Current and future strategies. J Anus Rectum Colon. 2021;5:11-24.
- [Google Scholar]
- Immunotherapy for colorectal cancer:Mechanisms and predictive biomarkers. Cancers (Basel). 2022;14:1028.
- [Google Scholar]
- Biomarkers in colorectal cancer:Current research and future prospects. Int J Mol Sci. 2020;21:5311.
- [Google Scholar]
- Biomarkers in colorectal cancer:Current clinical utility and future perspectives. World J Clin Cases. 2018;6:869-81.
- [Google Scholar]
- HER2 targeted therapy in colorectal cancer:New horizons. Cancer Treat Rev. 2022;105:102363.
- [Google Scholar]
- HER2-targeted therapy:An emerging strategy in advanced colorectal cancer. Expert Opin Investig Drugs. 2019;28:29-38.
- [Google Scholar]
- Beyond colonoscopy:Exploring new cell surface biomarkers for detection of early, heterogenous colorectal lesions. Front Oncol. 2021;11:657701.
- [Google Scholar]
- Personalized targeted therapy prescription in colorectal cancer using algorithmic analysis of RNA sequencing data. BMC Cancer. 2022;22:1113.
- [Google Scholar]
- Personalized treatment for patients with colorectal cancer:Role of biomarkers. Biomark Med. 2015;9:337-47.
- [Google Scholar]
- Biomarker-guided therapy for colorectal cancer:Strength in complexity. Nat Rev Clin Oncol. 2020;17:11-32.
- [Google Scholar]
- Using circulating tumor DNA in colorectal cancer:Current and evolving practices. J Clin Oncol. 2022;40:2846-57.
- [Google Scholar]
- Predictive and prognostic biomarkers with therapeutic targets in colorectal cancer:A 2021 update on current development, evidence, and recommendation. J Oncol Pharm Pract. 2022;28:850-69.
- [Google Scholar]
- Rational development of synergistic combinations of chemotherapy and molecular targeted agents for colorectal cancer treatment. BMC Cancer. 2018;18:812.
- [Google Scholar]
- Identification of synergistic drug combinations to target KRAS-driven chemoradioresistant cancers utilizing tumoroid models of colorectal adenocarcinoma and recurrent glioblastoma. Front Oncol. 2022;12:840241.
- [Google Scholar]
- Advances in immunotherapy for colorectal cancer:A review. Therap Adv Gastroenterol. 2020;13:1756284820917527.
- [Google Scholar]
- Combined treatment with immunotherapy-based strategies for MSS metastatic colorectal cancer. Cancers (Basel). 2021;13:6311.
- [Google Scholar]
- Rationale for combination of therapeutic antibodies targeting tumor cells and immune checkpoint receptors:Harnessing innate and adaptive immunity through IgG1 isotype immune effector stimulation. Cancer Treat Rev. 2018;63:48-60.
- [Google Scholar]
- Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct Target Ther. 2020;5:22.
- [Google Scholar]
- Managing toxicities associated with colorectal cancer chemotherapy and targeted therapy:A new guide for nurses. Clin J Oncol Nurs. 2009;13:285-96.
- [Google Scholar]
- A systematic review and meta-analysis of treatment-related toxicities of curative and palliative radiation therapy in non-small cell lung cancer. Sci Rep. 2021;11:5939.
- [Google Scholar]
- Reducing the toxicity of cancer therapy:Recognizing needs, taking action. Nat Rev Clin Oncol. 2012;9:471-8.
- [Google Scholar]
- Nursing care strategies for the management of side effects in patients treated for colorectal cancer. Semin Oncol. 1997;24:S18-S70.
- [Google Scholar]
- Patient-reported outcomes and experiences from the perspective of colorectal cancer survivors:Meta-synthesis of qualitative studies. J Patient Rep Outcomes. 2020;4:27.
- [Google Scholar]
- Health-related quality of life in long-term survivors of colorectal cancer and its association with all-cause mortality:A German cohort study. BMC Cancer. 2018;18:1156.
- [Google Scholar]
- Financial burden and quality of life among early-onset colorectal cancer survivors:A qualitative analysis. Health Expect. 2019;22:1050-7.
- [Google Scholar]
- Quality of life preferences in colorectal cancer patients aged 80 and over. ANZ J Surg. 2021;91:1859-65.
- [Google Scholar]
- Neoadjuvant immunotherapy in primary and metastatic colorectal cancer. Br J Surg. 2021;108:1417-25.
- [Google Scholar]
- Neoadjuvant immunotherapy for MSI-H/dMMR locally advanced colorectal cancer:New strategies and unveiled opportunities. Front Immunol. 2022;13:795972.
- [Google Scholar]
- The effect of outpatient preoperative evaluation of hospital inpatients on cancellation of surgery and length of hospital stay. Anesth Analg. 2002;94:644-9. table of contents
- [Google Scholar]
- Neoadjuvant immune checkpoint inhibitor therapy for patients with microsatellite instability-high colorectal cancer:Shedding light on the future. JCO Oncol Pract. 2023;19:251-9.
- [Google Scholar]
- Neoadjuvant immunotherapy:An evolving paradigm shift? J Natl Cancer Inst. 2021;113:799-800.
- [Google Scholar]
- J Clin Oncol. 2022;40:1151-61.
- J Clin Oncol. 2021;39:2045-54.
- J Clin Oncol. 2022;40:181-90.
- J Clin Oncol. 2021;39:2055-64.
- J Clin Oncol. 2022;40:191-9.
- Ann Oncol. 2022;33:388-98.
- J Clin Oncol. 2004;22:43-51.
- N Engl J Med. 2004;350:2240-8.
- J Clin Oncol. 2004;22:3405-14.
- J Clin Oncol. 2010;28:4096-104.
- N Engl J Med. 2017;377:1954-65.
- J Clin Oncol. 2018;36:2080-8.
- Short-course radiotherapy followed by chemotherapy before TME in locally advanced rectal cancer:The randomized RAPIDO trial. J Clin Oncol. 2020;38:4006.
- [Google Scholar]
- Single-arm, phase II study of intra-arterial chemotherapy plus total neoadjuvant therapy to optimise complete response in distal rectal cancer:A study protocol. BMJ Open. 2023;13:e075023.
- [Google Scholar]
- Neoadjuvant checkpoint blockade for cancer immunotherapy. Science. 2020;367:eaax0182.
- [Google Scholar]
- Total neoadjuvant therapy for locally advanced rectal cancer:Induction or consolidation chemotherapy? J Clin Oncol. 2022;40:2515-9.
- [Google Scholar]
- UpToDate. Available from: https://www.uptodate.com/contents/neoadjuvant-therapy-for-rectal-adenocarcinoma







