Translate this page into:
Evaluation of the relationship between H. Pylori-infected gastric mucosa and prognosis of gastrointestinal stromal tumor
Address for correspondence: Ola M. Omran, Department of Pathology, College of Medicine, Qassim University, Buridah, Qassim Region, Saudi Arabia/Department of Pathology, Faculty of Medicine Assiut University, Assiut, Egypt. E-mail: ola67oh@yahoo.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Qassim Uninversity and was migrated to Scientific Scholar after the change of Publisher.
Abstract
ABSTRACT
Objective:
Gastrointestinal stromal tumor (GIST) is the most common type of mesenchymal tumor accounting for 2.2% of all malignant gastric tumors. Mesenchymal stem cells (MSCs) play crucial roles in gastric carcinogenesis. In addition, Helicobacter pylori has been linked to GIST as it induces an epithelial response that can home MSCs to the stomach mucosa. This study aimed to investigate the relationship between H. pylori-infected gastric mucosa and the development of CD117-positive GIST and evaluate the prognosis of H. pylori-infected gastric mucosa of GIST patients that received anti-CD117 therapy.
Methods:
This is a retrospective study conducted on H. pylori-infected GIST patients diagnosed between 2015 and 2021. The follow-up period was performed for a minimum of 2 years. Clinicopathological factors for each patient were collected from cases selected from the Registry of Pathology and Surgery Departments at Assiut University Hospitals.
Results:
There was a statistically significant difference between our study population regarding the overall survival of studied patients, disease-free survival of studied patients, and the relationship between H. pylori-infected gastric mucosa and development, grading, therapy response, and overall survival of GIST except in status at last follow-up.
Conclusions:
Our study is the first to reveal that H. pylori infection is linked to a worse prognosis for GIST patients. H. pylori has the potential to be used as a strong predictive biomarker for GIST individuals in the future. Clinical research with large samples as well as prospective designs are needed to confirm this connection.
Keywords
Gastrointestinal stromal tumor (GIST)
Helicobacter pylori infected gastric mucosa
Overall survival
Introduction
Despite a growing body of research, little is known about the epidemiology of gastrointestinal stromal tumors (GISTs).[1,2] Formerly known as leiomyomas and leiomyosarcomas, GISTs have been reclassified as originating from interstitial cells of Cajal, setting them apart from other mesenchymal gastrointestinal (GI) cancers.[3] Diagnosis of GIST is aided by the expression of C-KIT (CD117) in 80–100% of cases and CD34 in 50–80% of cases.[4,5] DOG-1 is highly relevant in identifying c-KIT-negative stromal tumors, which are frequent in GIST.[1,6] While numerous studies have been conducted on GIST’s etiology, histology, and treatment, there is a dearth of information on the disease’s epidemiology.[7,8] Very few studies have investigated the possibility of racial disparities in GIST occurrence or frequency.[1,9]
More than half of the human population is colonized by Helicobacter pylori (H. pylori), a spiral-shaped Gram-negative bacterium that lives in the gastric epithelium.[10] Highly specific mechanisms help H. pylori bypass this 1st line of host defense, as well adaptive immunological processes also contribute to the infection’s persistence.[7] As a result, the host immune response fails to eradicate the infection, which continues to provoke chronic inflammation.[4] The resulting inflammation can promote ulcer development or tumor growth in susceptible individuals.[2]
Epithelial junction distribution, DNA damage, apoptosis, proliferation, cytokine production stimulation, in addition to cell transformation are only few of the many processes that H. pylori alters in gastric epithelial cells.[11] H. pylori infection has been linked to two other forms of stomach malignancies, adenocarcinoma, and MALT lymphoma, according to previous research.[8,12] H. pylori was classified as a carcinogen by the International Agency for Research on Cancer due to its relationship to the development of stomach adenocarcinoma.[11] There have been a few trials that have reported a link between GIST and infection with H. pylori.[4]
This work aimed to detect an association between H. pylori-infected gastric mucosa and the development of CD117-positive GIST and assess the prognosis of H. pylori-infected gastric mucosa of GIST patients that received anti-CD117 therapy.
Materials and Methods
This is retrospective trial done on individuals diagnosed with GIST at Assiut University Hospitals. Clinicopathological factors for each patient were collected from cases selected from the registry of Pathology and Surgery Departments at Assiut University Hospitals regarding the age, gender, site, and size of the lesions. The cases were evaluated with hematoxylin and eosin stain for pathological factors. H. pylori detection and tumor expression for H. pylori were assessed through immunohistochemistry on slides prepared from paraffin-embedded blocks. The response to therapy, disease-free survival, and overall survival were estimated and correlated to H. pylori expression.
Inclusion criteria
Patients aged over 18 years and Gastric c-KIT-positive GIST in patients who underwent radical or partial gastrectomy.
Exclusion criteria
-
Patients younger than 18 years
-
Endoscopic or fine-needle aspiration cytology specimens
-
C-KIT-negative GIST specimen and
-
Non-gastric GIST specimens.
Cases selection
This research included 48 individuals who underwent primary tumor resections for operable cases between the period of October 2017 and January 2018, with follow-up for a period of 5 years. The cases were chosen from the registry maintained by the Pathology Department of Assiut University Hospitals. All samples were first preserved and fixed in 10% buffered formalin, then processed along with embedded in paraffin according to standard procedure.[11] After cutting the sections to a thickness of 3–5 μm, they were placed on conventional slides and stained with hematoxylin along with eosin (H & E). At first, the slides that had been stained with H&E were scrutinized, and the tumors were categorized according to their level of low, middle, or high risk. The collection as well as recording of pathological and clinical data took place.
Immunohistochemistry
Deparaffinization and blocking were performed, along with positive and negative controls.
Examination methods
For evaluation of H. pylori immunoreactivity as positive or negative staining, the following procedures were adopted: (1) the slides were assessed by experienced pathologists and (2) in each case, the immune-stained sections were initially examined histologically at a lower magnification (×4 and ×10) to identify positive cells. The higher magnifications (×40 and ×100) were subsequently used to evaluate the positivity in five different areas.
Statistical analysis
Outcomes were subjected to statistical analysis using the Social Science Statistical Package (SPSS), version 16. The correlation among expression of anti-H. pylori antibody by the bacterium and tumor cell expression with other clinicopathological data, therapy response, disease-free survival, and overall survival were analyzed with a Mann–Whitney U test for quantitative data and a Chi-square test for qualitative data such as frequencies along with proportions. The results of these analyses were compared as well analyzed. Fisher’s exact test was used in situations, in which the assumptions of the Chi-square test were not satisfied. The Kaplan–Meier test (using the Log-rank test) in addition to the Cox regression model was utilized to evaluate the connections among H. pylori infection and the survival statistics, along with other factors. The threshold for significance was set at a P < 0.05.
Results
The study compared the characteristics of individuals with H. pylori-positive and H. pylori-negative conditions. As shown in Table 1 and Figures 1-11, the H. pylori-positive group had a significantly higher mean age (53.5 years) compared to the negative group (41.1 years). There was a significant alteration in sex distribution, with an increased proportion of males in the H. pylori-positive group and an equal distribution in the negative group. Every person in the H. pylori-positive group was classified as high risk, while none in the negative group had high risk. The positive group also had a significantly larger size of the condition compared to the negative group. There were significant variances in nuclear pleomorphism, necrosis, cell expression, and TILS (tumor-infiltrating lymphocytes) between the two groups. There was not a significant disparity in survival rates among both groups as per the most recent available data. These results suggest that H. pylori status may be related with age, sex, risk classification, size, and various pathological features, but not with survival outcomes.
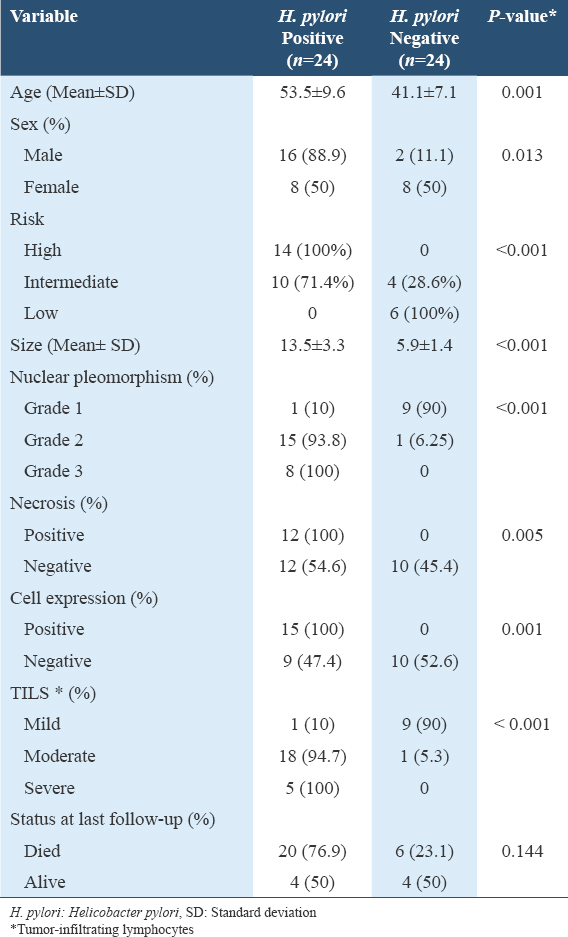
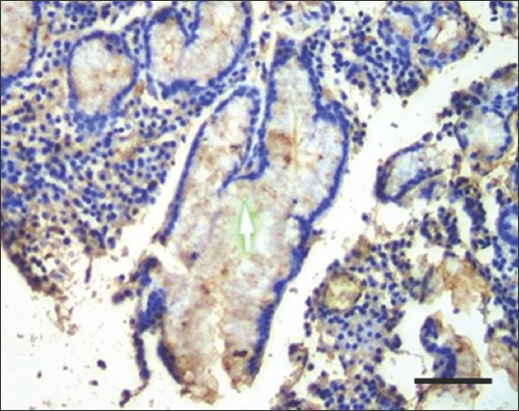
- Immunohistochemistry staining with anti-H. pylori antibody at ×100 magnification shows brownish stain of H. pylori (Green arrow) at the lumen of the gastric gland. Scale bar = 50 μm. H. pylori: Helicobacter pylori
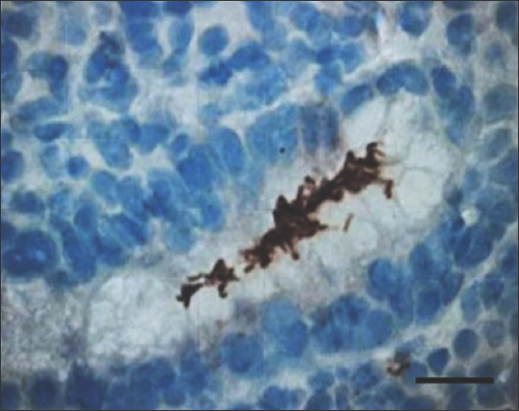
- Immunohistochemistry staining with anti-H. pylori antibody at ×400 magnification shows brownish stain of H. pylori at the gastric luminal gland. Scale bar = 200 μm. H. pylori: Helicobacter pylori
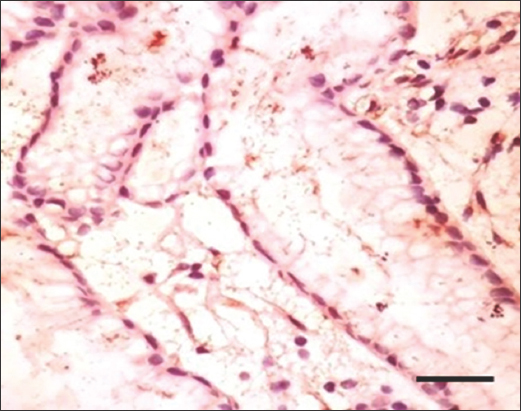
- Immunohistochemistry staining with anti-H. pylori antibody at ×200 magnification shows H. pylori at the gastric luminal gland. Scale bar = 100 μm. H. pylori: Helicobacter pylori
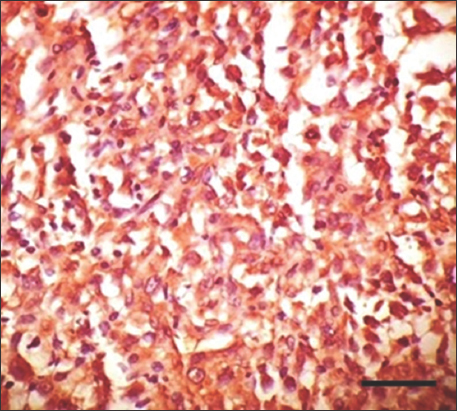
- Immunohistochemistry staining with anti-H. pylori antibody for gastrointestinal stromal tumor at ×400 magnification shows strong positive cytoplasmic staining of high-risk tumor cells. Scale bar = 200 μm. H. pylori: Helicobacter pylori
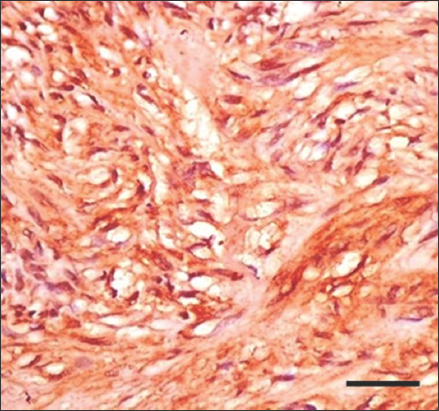
- Immunohistochemistry staining with anti-H. pylori antibody for gastrointestinal stromal tumor at ×200 magnification shows weak positive cytoplasmic staining of low-risk tumor cells. Scale bar = 100 μm. H. pylori: Helicobacter pylori
- Immunohistochemistry staining with anti-H. pylori antibody for gastrointestinal stromal tumor at ×400 magnification shows strong positive cytoplasmic staining of high-risk tumor cells. Scale bar = 200 μm. H. pylori: Helicobacter pylori
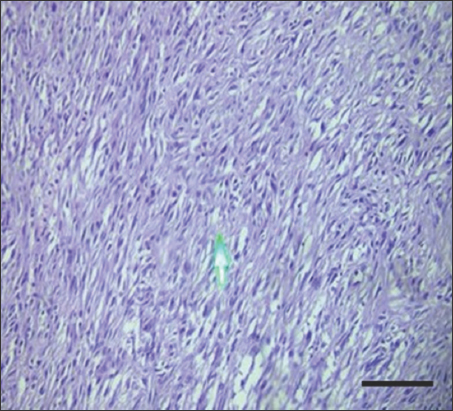
- Immunohistochemistry staining with anti-H. pylori antibody for gastrointestinal stromal tumor at ×200 magnification shows negative immunoreactivity. Scale bar = 100 μm. H. pylori: Helicobacter pylori

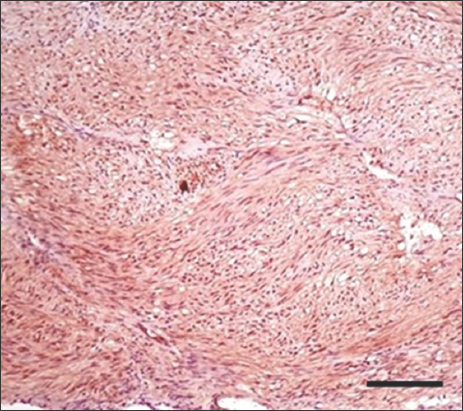
- H&E stain of gastrointestinal stromal tumor at ×100 magnification shows severe tumor-infiltrating lymphocytes. Scale bar = 50 μm
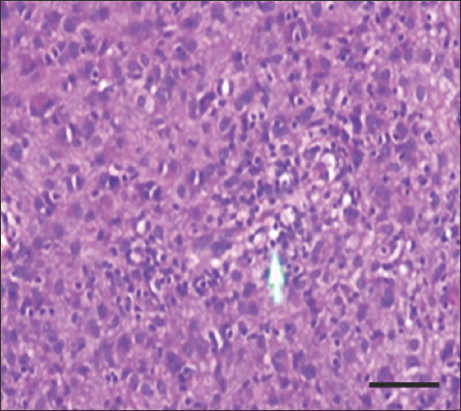
- H&E stain of high-risk gastrointestinal stromal tumor at ×400 magnification shows moderate tumor-infiltrating lymphocytes. Scale bar = 200 μm
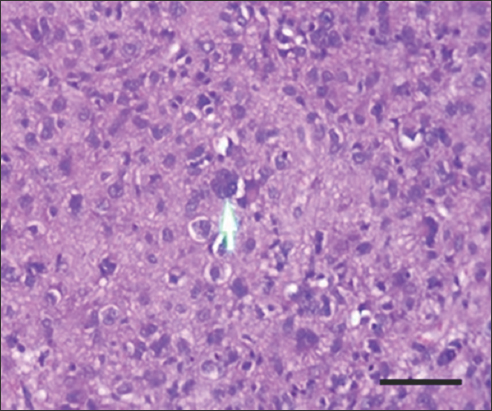
- H&E stain of high-risk gastrointestinal stromal tumor at ×400 magnification shows mild tumor-infiltrating lymphocytes. Scale bar = 200 μm
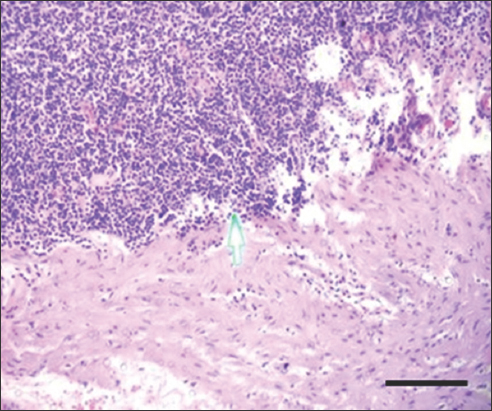
- H&E stain of gastrointestinal stromal tumor at ×200 magnification shows severe tumor-infiltrating lymphocytes. Scale bar = 100 μm
The median survival for the H. pylori-positive group is 53 months (with a 95% confidence interval [CI] of 48–64 months). In contrast, the H. pylori-negative group has a higher median survival of 98 months (with a 95% CI of 60–113 months). The P value of under 0.001 indicates a statistically significant change in overall survival among the two groups, as shown in Table 2 and Figure 12.

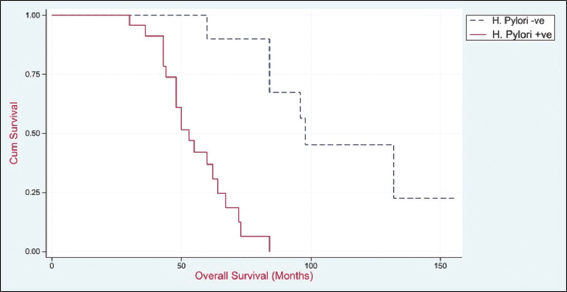
- Overall survival of studied patients
The median disease-free survival for the H. pylori-positive group was 39 months (with a 95% CI of 36–50 months), whereas for the H. pylori-negative group, it was 60 months (with a 95% CI of 36–92 months). The fact that the P < 0.001 suggests that there was a statistically significant distinction among both groups in terms of disease-free survival, as shown in Table 3 and Figure 13.

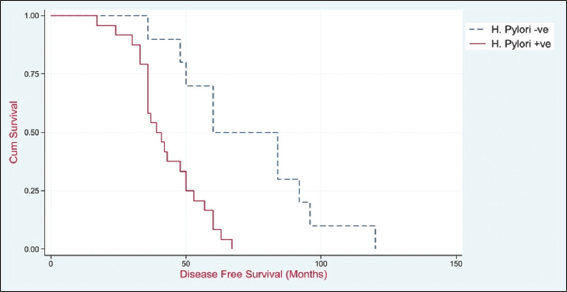
- Disease-free survival of studied patients
Discussion
The World Health Organization ranks gastric cancer (GC), as the third leading cause of death from cancer.[13] There are three degrees of differentiation that may be presented by adenocarcinoma of the GI tract: well differentiated, moderately differentiated, and poorly differentiated. The degree of differentiation is determined by glandular architecture, cellular pleomorphism, and mucosecretion.[14] The development of GC is often linked to environmental factors, particularly infection with H. pylori.[15,16]
On the other hand, GISTs, also known as gastric inflammatory stromal tumors, are neoplasms that originate in the mesenchymal tissue of the GI system.[17] There is no correlation between gender and the incidence of GISTs, which account for 2.2% of all malignant gastric tumors. Gastric inflammatory stromal tumors are the most common kind of mesenchymal tumor (mesenchymal stem cells [MSCs]).[18] It is because H. pylori induce an epithelial response that may lead to the homing of MSCs into the gastric mucosa; once there, MSCs play essential roles in the development and progression of GC. This is how H. pylori is linked to gastric inflammatory stromal tumors.[19,20]
This research analyzed the differences in personality traits between those who tested positive for H. pylori and those who tested negative. When compared to the negative group, the H. pylori-positive group had a substantially higher mean age (53.5 years) than the negative group (41.1 years). There was a notable difference in the gender distribution between the two groups, with an equal distribution of people in the H. pylori-negative group and a significantly greater number of men in the H. pylori-positive group. Everyone in the group who tested positive for H. pylori was considered to have a high risk, while none of the people in the group that tested negative did. In comparison to the negative group, the positive group had a much greater number of individuals affected by the condition. Both groups had statistically significant differences between them in terms of nuclear pleomorphism, necrosis, cell expression, and TILS. On the other hand, there was not a statistically significant alteration among the two groups in terms of survival status at the time of the most recent follow-up. According to these data, the existence of H. pylori may be connected with factors such as age, sex, risk categorization, size, and numerous clinical aspects; however, it is not associated with survival outcomes.
The odds ratio for the incidence of metaplasia linked with H. pylori infection was determined to be 1.40 by Rodrigues et al.,[21] and the 95% CI ranged from 1.18 to 1.66. It was discovered that 474 (10.3%) of persons had glandular atrophy. Similarly, the proportion of patients who had glandular atrophy was considerably greater among H. pylori (+) cases (17.6%) than it was among H. pylori (−) individuals (6.9%) (P = 0.01 for both comparisons). The odds of having glandular atrophy in H. pylori (+) patients were nearly 3 times greater than those in H. pylori (−) patients (Odds ratio: 2.88; 95% CI: 2.40–3.50).
In the research conducted by Krzysiek-Maczka et al.,[22] GI tissues were found to be positive for H. pylori infection by Giemsa stain in 62.9% of malignant GIST patients and 30% of non-GIST controls (P = 0.013). The study included 71 malignant GIST cases and 65 non-GIST controls.
Studies conducted on animal models of GC by Lee et al.[23] demonstrated that the elimination of H. pylori resulted in a regression of the stomach inflammation, which was accompanied by a decrease in the levels of pro-inflammatory cytokines, as well as a reduction in the proliferation of epithelial cells and restoration of the normal architecture. These characteristics contributed to a decreased rate of dysplasia as well as a reduction in the risk of GC, in particular when treatment was performed during the early stage of H. pylori infection.
According to the findings of Fang et al.,[24] patients who had an H. pylori infection had a significantly lower average age than those who did not have an H. pylori infection. We were able to show via this research that the average length of survival for those who tested positive for H. pylori is 53 months (with a 95% CI ranging from 48 to 64 months). On the other hand, those who tested negative for H. pylori had a significantly longer median survival time of 98 months (with a 95% CI ranging from 60 to 113 months). If the P < 0.001, it implies that there is a statistically significant alteration among the two groups in terms of overall survival. Furthermore, the findings of Li et al.[25] showed that those individuals who tested negative for H. pylori in both 1996 and 2003 had the lowest rates of GC incidence and death. However, risk reductions were also detected in those individuals who had signs of infection following treatment.
In research that was undertaken by Uemura et al.,[26] a nonrandomized H. pylori eradication trial was performed on participants whose stomach cancer was eliminated by endoscopic resection following a long-term clinical and endoscopic follow-up period. The conclusion reached that the elimination of H. pylori might lead to an improvement in neutrophil infiltration also intestinal metaplasia in the gastric mucosa, as well as an inhibition of the formation of new carcinomas.
Patients who did not have a history of H. pylori infection had substantially better overall survival rates after 5 years (87.7% vs. 73.9%, P = 0.012) than those who did have a history of H. pylori infection. For the H. pylori-positive group, we discovered that the median disease-free survival was 39 months (with a 95% CI of 36–50 months), but for the H. pylori-negative group, it was 60 months (with a 95% CI of 36–92 months). The fact that the P < 0.001 suggests that there is an alteration that can be considered statistically significant among the two groups. In terms of disease-free survival, the difference in DFS rates between patients with and without H. pylori infection was shown to be statistically significant (60.5% vs. 51.1%, P = 0.178) in the study by Fang et al.[24]
The difference in DFS rates between patients with and without H. pylori infection was shown to be statistically significant (60.5% vs. 51.1%, P = 0.178) in the study by Fang et al.[24] According to the findings of Chiba et al.,[27] patients who are infected with H. pylori have considerably lower DFS rates when compared to individuals who do not have H. pylori infection.
Conclusion
Our research is the first of its kind to demonstrate a connection among the presence of H. pylori infection as well as the final prognosis of GIST patients. It is possible that in the near future, H. pylori will emerge as a potent predictive biomarker for GIST individuals. However, prospective clinical trials involving multiple centers, large sample sizes, and long-term follow-up are required to substantiate the connection.
Ethics Approval and Consent to Participate
Institutional Review Board Approval for the conduction of this study was obtained from the Medical Ethics Committee, Assiut University, Egypt with an Approval number 04-2024-300346 dated on March 03, 2024. Written consents were obtained from all patients involved in this study.
Availability of Data and Material
All data generated or analyzed in this study are included in this published article. The data are available on request by the corresponding author.
Competing Interests
None.
Funding Statement
None.
Author Contributions
Rania A. Herdan: Pathology part, data collection, analysis, reviewing and editing. Mohamed Gamal Taher: Data collection, analysis of results, writing – reviewing and editing. Ahmed Mahran Shafiq: Data interpretation, coordination, manuscript drafting, and contributed to revisions of the manuscript. Ola M. Omran: Reviewing, editing of the original draft, pathology part, data extraction, and tabulation. Lobaina Abozaid: Pathology part, data analysis support, and data extraction. Nahla Babiker: Pathology part, data analysis, data extraction, and tabulation. Saeed A. Al-Qahtani: Data analysis, writing –reviewing, and editing. Nada M. Taha and Noha M. Taha: data collection, reviewing, and editing. Aisha Ahmed Y. Shubaili and Sumaya Ahmed A. Khubrani: analysis of results, contributed to revisions of the manuscript. Mahmoud Gamal Ameen: Pathology part writing and tabulation- reviewing and editing.
Conflicts of Interest
None.
References
- Novel association between Helicobacter pylori infection and gastrointestinal stromal tumors (GIST) in a multi-ethnic population. Gastrointest Stromal Tumor. 2020;3:1-6.
- [Google Scholar]
- Helicobacter pylori and gastric cancer:Pathogenetic mechanisms. Int J Mol Sci. 2023;24:2895-99.
- [Google Scholar]
- Laparoscopic distal gastrectomy for synchronous adenocarcinoma, diffuse large B cell lymphoma and gastrointestinal stromal tumor in the stomach:A case report. Surg Case Rep. 2022;8:89-92.
- [Google Scholar]
- Effects of Helicobacter pylori on tumor microenvironment and immunotherapy responses. Front Immunol. 2022;13:923477.
- [Google Scholar]
- Gastric cancer and gastrointestinal stromal tumors could be causes of non-Helicobacter pylori non-NSAIDs peptic ulcers in Thailand. Asian Pac J Cancer Prev. 2017;18:155-7.
- [Google Scholar]
- Clinicopathological and molecular characteristics of synchronous gastric adenocarcinoma and gastrointestinal stromal tumors. Sci Rep. 2017;7:12890.
- [Google Scholar]
- The interaction of Helicobacter pylori with cancer immunomodulatory stromal cells:New insight into gastric cancer pathogenesis. Semin Cancer Biol. 2022;86:951-9.
- [Google Scholar]
- Family history of gastric cancer and Helicobacter pylori treatment. N Engl J Med. 2020;382:427-36.
- [Google Scholar]
- Gastrointestinal stromal tumors:Association with Helicobacter pylori gastritis. Am J Clin Pathol. 2012;138:A351-2.
- [Google Scholar]
- A novel association of gastrointestinal stromal tumors (GIST) and Helicobacter pylori. Gastroenterology. 2015;148:S576.
- [Google Scholar]
- Cancer pain management in developing countries. Indian J Palliat Care. 2016;22:373-7.
- [Google Scholar]
- Helicobacter pylori and interleukin 1 genotyping:An opportunity to identify high-risk individuals for gastric carcinoma. J Natl Cancer Inst. 2002;94:1680-7.
- [Google Scholar]
- Salt intake and gastric cancer risk according to Helicobacter pylori infection, smoking, tumour site and histological type. Br J Cancer. 2011;104:198-207.
- [Google Scholar]
- Evaluation of biologic potential and risk stratification for predicting disease-free survival after resection of primary gastrointestinal stromal tumor:A multivariate clinicopathological study. Indian J Cancer. 2015;52:351-7.
- [Google Scholar]
- Gastric cancer originating from bone marrow-derived cells. Science. 2004;306:1568-71.
- [Google Scholar]
- Helicobacter pylori infection of gastrointestinal epithelial cells in vitro induces mesenchymal stem cell migration through an NF-kB-dependent pathway. PLoS One. 2011;6:e29007.
- [Google Scholar]
- Helicobacter pylori infection and gastric cancer precursor lesions:Prevalence and associated factors in a reference laboratory in Southeastern Brazil. Arq Gastroenterol. 2019;56:419-24.
- [Google Scholar]
- Time-extended exposure of gastric epithelial cells to secretome of Helicobacter pylori-activated fibroblasts induces reprogramming of gastric epithelium towards pre-cancerogenic and pro-invasive phenotype. Am J Cancer Res. 2022;12:1337-71.
- [Google Scholar]
- Helicobacter pylori eradication prevents progression of gastric cancer in hypergastrinemic INS-GAS mice. Cancer Res. 2008;68:3540-8.
- [Google Scholar]
- Comparison of the clinicopathological characteristics and genetic alterations between patients with gastric cancer with or without Helicobacter pylori infection. Oncologist. 2019;24:e845-53.
- [Google Scholar]
- Effects of Helicobacter pylori treatment on gastric cancer incidence and mortality in subgroups. J Natl Cancer Inst. 2014;106:116.
- [Google Scholar]
- Effect of Helicobacter pylori eradication on subsequent development of cancer after endoscopic resection of early gastric cancer. Cancer Epidemiol Biomarkers Prev. 1997;6:639-42.
- [Google Scholar]
- Mechanism for gastric cancer development by Helicobacter pylori infection. J Gastroenterol Hepatol. 2008;23:1175-81.
- [Google Scholar]







