Translate this page into:
A comparable risk of extensively drug-resistant typhoid fever in the pediatric cohort during the COVID-19 pandemic
Address for correspondence: Dr. Maria Khan, Microbiology Department, Pathology Laboratory, Rehman Medical Institute, Peshawar, Pakistan. Phone: +923319111986. E-mail: kmaria22@hotmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Qassim Uninversity and was migrated to Scientific Scholar after the change of Publisher.
Abstract
ABSTRACT
Objective:
The number of extremely drug-resistant (XDR) Salmonella typhi isolates is growing in the northwest of Pakistan, where health-care facilities are already under strain due to the coronavirus disease 2019 (COVID-19) issue. In Khyber Pakhtunkhwa, Pakistan, we currently describe the first widespread appearance of an XDR Salmonella typhi epidemic during the COVID-19 pandemic. This strain of Salmonella typhi is resistant to all first- and second-line drugs and even the third-generation cephalosporin.
Methods:
Salmonella species isolated from pediatric blood samples shown a high level of resistance to the various antibiotic classes evaluated between November and December 2020. Gender, age, address, and clinical symptoms were among the demographic information that was recorded. A total of 562 blood cultures from symptomatic patients have been collected by the Pathology Laboratory at a tertiary care institution of Rehman Medical Institute, Peshawar. All samples have been processed in accordance with regulatory requirements and incubated in BacT/ALERT 3D.
Results:
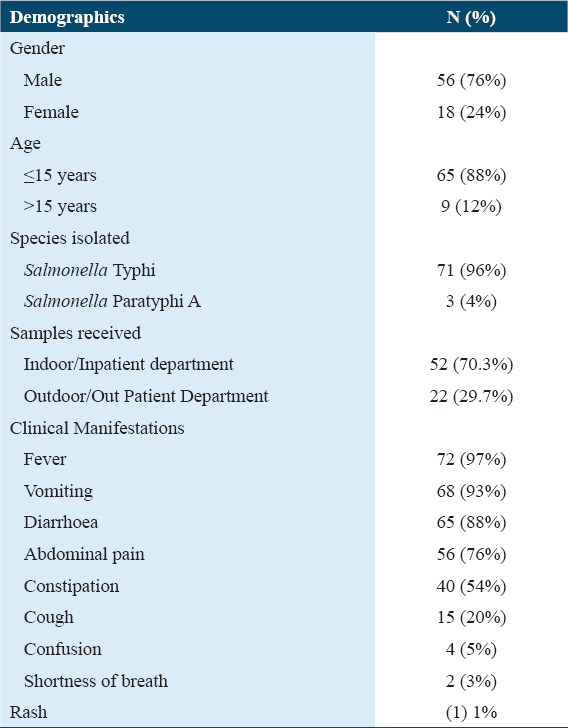
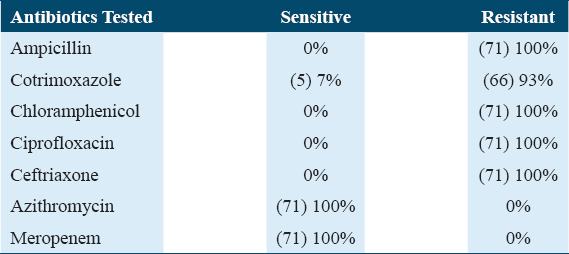
Of the 562 blood samples, 71 included Salmonella typhi, of which 66 (92.9%) and 5 (7%) were multidrug resistant (MDR) and XDR, respectively. Ciprofloxacin (100%), chloramphenicol (100%), ceftriaxone (100%), ampicillin (100%), and cotrimoxazole (93%) were completely resistant to all isolates. Azithromycin and carbapenems were effective against every Salmonella typhi isolate that was MDR or XDR. Males (76%) were more commonly affected than females (24%), and the frequency was substantially higher in children under 15 years of age (88%) than in adults (P = 0.0016).
Conclusion:
The emergence of XDR Salmonella typhi with a high level of resistance is in fact alarming. Due to the lack of viable treatment alternatives, the current situation necessitates the immediate implementation of efficient preventive measures, such as campaigns for typhoid vaccination and food and water safety.
Keywords
Antimicrobials
COVID-19
extensively drug resistant
Salmonella typhi
Introduction
In the course of time, Pakistan has been going through the third wave of coronavirus disease 2019 (COVID-19) since the start of March, 2021, as a consequence of low levels of compliance to preventive measures at community level and socioeconomic disparities. The potential link between COVID-19 and typhoid fever raises serious concerns but has not received much attention so far. Salmonella enterica serovar typhi (Salmonella typhi), which causes typhoid fever, is a systemic illness that is usually spread through tainted food and water.[1] Particularly, typhoid fever, responsible for an estimated 200,000 typhoid-related fatalities yearly, continues to pose an existential threat to public health in middle-low-income countries.[2] Typhoid fever resulting from Salmonella typhi, which is highly drug-resistant (XDR Salmonella typhi), has recently become a health issue in developing nations, particularly Pakistan.[3] Subsequently, due to the emergence of multidrug resistance (MDR) and now XDR, with an additional rise in international travel, the threat has become a substantial public health concern worldwide.[4] The impact of COVID-19 co-epidemic co-infections along with other infectious diseases on a previously overwhelmed health-care system has been depicted in recent papers.[5,6] Presently, the country is under the adversities of the COVID-19 cataclysm, as the morbidity and mortality toll is constantly on the rise and the situation is deteriorating, with around 637,042 COVID-19 cases nationwide and 82,067 in Khyber Pakhtunkhwa.[7] In June 2020, around 30, 000 typhoid cases have been reported along with COVID-19.[8] Clinically, patient with typhoid fever may present with fever, vomiting, abdominal pain, similar to SARS-CoV-2 symptoms, but microbiological culture of the pathogen from blood, bone marrow, or from a normally sterile site remains the “Goldstandard” for the definitive diagnosis.[9] To identify Salmonella typhi antigen or antibody, several serological assays, including the traditional Widal test, have been developed in the past; however, none of these tests have demonstrated efficacy (poor sensitivity and specificity) in clinical application.[10]
Typhoid fever causes 129,000 to 223,000 deaths annually, mostly in developing countries and among children, according to the World Health Organization (WHO).[11] An enduring outbreak of XDR Salmonella typhi has been reported in Sindh, Pakistan, since November 2016, for which the causative haplotype was H58 Salmonella typhi.[12] In Karachi, a total of 13,736 cases of extensive drug resistance typhoid fever were reported from January 2017 to February 2021 with an attack rate of 208/100,000 in 0–4 years followed by 5–9 year olds (attack rate 150/100,000).[13]
Historically, ampicillin (AMP), trimethoprim-sulfamethoxazole, and chloramphenicol have been used to treat typhoid.[14] According to the literature, during the early 1980s, MDR Salmonella typhi, shown resistance to chloramphenicol, trimethoprim-sulfamethoxazole, and AMP when they were initially noticed. Since Mycobacterium tuberculosis and other bacterial infections share a taxonomy, Salmonella typhi is referred to as “extensively XDR “ when it is resistant to five medications.[15] As a result, the macrolide antibiotic azithromycin (AZM), given in a dose of 1 gram once daily for 5 days, has been effective in treating typhoid fever.[16] On the other hand, individual cases of Salmonella typhi that is AZM-resistant have lately been documented. Since the typhoid strain is only susceptible to a handful of medications, such as AZM and meropenem, it is exceedingly expensive and difficult to cure.[17]
At the same time, the dearth of clinical microbiology services in endemic areas as well as culture-based diagnosis is the reasons for delay in management and thereupon fatal typhoid complications demanding hospital admission and fatalities from pneumonia, hepatitis, gastrointestinal hemorrhage, encephalopathy, shock, hepatitis, myocarditis, and intestinal perforation.[17,18]
This study emphasizes the mounting risk posed by Salmonella typhi’s antibiotic resistance and its significance of performing antibiotic susceptibility testing (AST) when there is a clinical suspicion of the pandemic due to SARS-CoV-2 (COVID-19). The objective was to assess Salmonella typhi’s antibiotic resistance trend and describe a local XDR salmonella outbreak.
Methods
The Rehman Medical Institute (RMI), a 442-bed private tertiary care teaching hospital in Peshawar, had its ethics council (institutional IRB) approve the study. Informed consent in writing from patients under the age of 18 was also acquired. The present study complies with the Declaration of Helsinki. In this prospective cross-sectional study, laboratory blood culture-proven XDR Salmonella typhi patients were enrolled and were treated as either inpatient or outpatient between periods of 1st November 2020 and 31st December 2020. Table 1 reports the baseline characteristics of the analyzed samples, i.e., at the time of admission, demographic and epidemiological data, including age, sex, address, and medical history, were collected. Patients with incomplete medical records were excluded from the study, particularly those with missing information on antimicrobial treatment, duration of treatment, and treatment failure. Additionally, those who had a positive blood culture but did not seek medical attention at either hospital or those who skipped their scheduled follow-up appointment at the clinic following the confirmation of the blood culture were excluded. The SARS-CoV-2 was detected by reverse transcriptase polymerase chain reaction (RT-PCR) nucleic acid amplification testing. Antimicrobial susceptibility pattern of 71 Salmonella species isolated from both inpatient and outpatient of pediatric age group in Peshawar was evaluated phenotypically using BacT/ALERT 3D automated system (bioMérieux, France). Then, sheep blood agar and MacConkey agar culture plates were injected with a positive blood culture. After an overnight incubation period, the isolate strains were identified as Salmonella typhi using the analytical profile index 20E (bioMérieux, France) and serological confirmation with antisera specific to the genus and serotype (Salmonella poly antiserum A-I [Difco], Salmonella O antiserum [Difco], and Salmonella Vi antiserum [Difco]). AST of the isolated bacteria was performed on Mueller–Hinton agar by modified Kirby-Bauer disk diffusion technique and minimum inhibitory concentration (MIC) testing. The suspension was compared to the 0.5 McFarland turbidity standard after being made up of three to four colonies of the causative organism and regular saline. The suspension was evenly dispersed over the Mueller–Hinton agar plates using the sterile swab, and antibiotic disks were applied to the plates aseptically. The plates were incubated for 18–24 h at 35°C, and the zones of inhibition were analyzed in accordance with CLSI 2020 recommendations. The isolates were tested against a panel of 7 antimicrobials, namely AMP10 μg, cotrimoxazole (SXT25 μg), chloramphenicol (C30 μg), ciprofloxacin (CIP5 μg), ceftriaxone (CRO 30 μg), meropenem (MEM 10μg), and MIC of AZM that was performed using E-strip (bioMérieux-USA). The phenotypic double-disk diffusion approach, which employed cefotaxime and ceftazidime alone and in combination with clavulanate, was used to identify the formation of extended-spectrum beta-lactamases. The data were analyzed using SPSS Statistics for Windows, version 23.0 (IBM Corp., Armonk, NY). The baseline parameters of the examined samples were presented as counts with percentages. A P < 0.05 was regarded as significant when using the Chi Square test to examine the relationship between blood CS and parent education and the length of the patient’s fever at admission. The mean and standard deviation of the patients’ ages were used to describe it. We used frequency and percentages to express the gender, patient department, frequency of positive blood cultures, and isolate antibiotic susceptibility pattern.
Results
Out of total 562 blood culture and susceptibility samples received from 1st October 2020 to 31st December 2020 at Pathology Laboratory of RMI, 189 (34%) showed bacterial growth, among which 71 (38%) were Salmonella typhi and 3 (2%) isolates were Salmonella Paratyphi A. Out of 71 culture-proven typhoidal Salmonella isolates, 66 (92.9%) were from pediatric age group while 5 (7%) were isolated from adult patients [Table 1].
The patient’s normal urine test and urine culture yielded unremarkable results. Direct gram staining of blood mostly showed the presence of Gram-negative rods. The antibiotic susceptibility pattern of majority of Salmonella typhi isolated, 66 (92.9%), was extensively XDR, i.e., resistant to all primary and secondary drugs but sensitive to investigational drugs such as MEM and AZM while only 5 (7%) were sensitive to only cotrimoxazole (SXT) [Table 2].
The SARS-CoV-2 RT-PCR (R-GENE® Real-time PCR assays) of all Salmonella typhi culture confirmed patients were negative. Moreover, majority (92%) of these XDR typhoid cases belong to same geographic location.
Discussion
According to the WHO, increasing prevalence of MDR Salmonella typhi from 20% to almost 80% has been observed over the past decade.[19] In the present study, we reported the antimicrobial resistance pattern of Salmonella typhi (XDR type) from a single, private tertiary care hospital in Peshawar, Pakistan. It is evident that XDR Salmonella typhi strains have caused significant public health concerns in Pakistan.[20] Additionally, a significant number of CRO-resistant cases have been discovered in the Pakistani province of Sindh since November 2016, mainly from the cities of Karachi and Hyderabad.[21] The majority of the youngsters in our study are between the ages of 5 and 15 (88%), due to the ease of access to street vendors and the impressionable brains’ lack of understanding of hygiene, this age group is especially vulnerable. Another comparable example involving a visitor from Pakistan who was returning to the United Kingdom was likewise found.[22] In Karachi, the age group 0–4 years was the most affected followed by age group 5–9 years.[13] Similarly, in our study, we reported 88% of XDR Salmonella cases in the pediatric age group. The majority of the participants in our study were men (76%), likely due to boys’ increased exposure to the outdoors, carefree demeanor, and risk-taking mindset. Our research revealed a significant link between the XDR cause and not drinking boiled or normal mineral water. The most frequent clinical symptom of enteric fever, particularly in pediatric age groups, is high-grade fever (97%) which increases parental concern. We noted a surge of XDR Salmonella typhi (92.9%) third-generation cephalosporins, AMP, trimethoprim-sulfamethoxazole, fluoroquinolones, and chloramphenicol resistance, during winter months that has already spread throughout the region. Effective management has become challenging due to the recent appearance of XDR Salmonella typhi strains (i.e., resistant to both first-line and second-line treatments).[23] This becomes extremely difficult when working with immunocompromised patient populations, such as children, the elderly, and pregnant women. Moreover, striving to manage such cases in developing countries amidst pandemic, lack of resources, and high medication cost is another colossal challenge.[4] Typhoid cases that are MDR or XDR are typically marked by high rates of complications, protracted hospital admissions, greater treatment costs, a higher risk of mortality, and subsequently protracted fecal shedding, which spreads the disease across communities.[23] Initially, during epidemics of multidrug-resistant (MDR) typhoid, the drug of choice for the treatment of typhoid in many countries till 1990s was CIP. With the widespread usage of fluoroquinolones in animal husbandry and over-the-counter availability of fluoroquinolone non-susceptibility in Salmonella typhi emerged, cumulating up to 96.5% globally till 2015.[24] In endemic nations, third-generation cephalosporins (CRO and cefixime) are the drug of choice for treating typhoid due to the progressive development of resistance against fluoroquinolones.[25]
An infection known as enteric fever exhibits multisystem involvement along with rapid illness development. The majority of our enrolled participants had various symptoms, including a protracted fever, stomach pain, anorexia, vomiting, diarrhea, myalgias, and headaches. These findings are in line with studies conducted in North India.[24]
Contrarily, since Salmonella typhi has developed CRO resistance, culture- and sensitivity-guided treatment is now essential because CRO empirical treatment is no longer effective in the area.[22] Moreover, our findings also reported 100% resistance to third-generation cephalosporins and other second-line antimicrobials with similar pattern observed in other studies as well.[26] In our example, AZM and MEM were used to successfully treat instances, and the majority of patients made a full recovery.
It should be emphasized that the only available treatments for typhoid are carbapenems and AZM as a result of the advent of XDR typhoid in our nation.[27] AZM is the sole oral antibiotic still used to treat individuals with XDR typhoid, which in our cases was 100% sensitive. Typhoid will become almost impossible to treat as an outpatient condition if the XDR strain of Salmonella typhi acquires the AZM non-susceptibility that is likely to arise. The battle against typhoid will be over quickly if antimicrobials continue to be employed as a substitute for inadequate water, sanitation, and a paralyzed health-care system.[28]
Despite being a reportable disease in Pakistan, typhoid fever’s abrupt emergence and rapid spread of resistant isolates over the past 3 years have drawn attention to the inadequacies of AMR surveillance as well as the lack of culture-based methods and reliance on non-culture-based methods (such as Widal and Typhidot tests), which do not yield results for susceptibility. Concern over antibiotic resistance has grown as the disease is being treated. Asia has produced isolated strains of Salmonella that are resistant to third-generation cephalosporins and first-line medicines. Salmonella typhi’s H58 MDR haplotype is the most prevalent and is becoming more and more widespread. There have been cases of XDR typhoid recorded in Pakistan, underlining the necessity for coordinated international actions for efficient continuous surveillance, upgrades to water and sanitation, the use of effective vaccines,[29,30] and antibiogram trends for empirical therapy. Therefore, unless the strain is listed on the antibiotic susceptibility report as resistant to first-line medications, CIP, and CRO, carbapenems, and AZM should not be routinely provided. Unfortunately, a widespread vaccination drive could not be launched right away to stop the development of this extremely resistant clone.[22] Even though the majority of control efforts are focused on water, sanitation, and hygiene (WaSH) measures, typhoid fever diagnosis and effective treatment may contribute to control by potentially lowering fecal carriers and shedders from the population.[28]
Conclusions
First- and second-line antibiotic resistance in Salmonella typhi has major consequences, and the likelihood of AZM resistance is a signal for the international community to intensify typhoid fever control efforts in the midst of the COVID-19 pandemic. To enhance sanitation, inform the public, immunize susceptible groups, and create good diagnostic and management methods, it is necessary for the government, stakeholders, and health-care professionals to cooperate together. Antibiotics can certainly save millions of lives, but due to the seeming simplicity and speed with which deadly bacteria like Salmonella typhi can acquire resistance, their efficacy will eventually run out. Our findings imply that more effective typhoid prevention measures, including early diagnosis, the introduction of vaccinations, and improved sanitation, are required.
Author’s Declaration Statement
Ethical approval and patient consent statement
The study was approved by the Ethics Committee of RMI, Peshawar. Written informed consent was obtained from all participants.
Consent for publication
The International Journal of Health Sciences has the permission of all authors to publish the work.
Availability of data and material
The authors claim that the publication contains the supporting data for the study’s conclusions.
Competing interest
The authors declare that they have no competing interests.
Funding statement
No funding body in the public, commercial, or non-profit sectors provided a particular grant for this research.
Data availability
The data used to support the findings of this study are included in the article.
Authors’ contributions
MK collected the clinical and laboratory data and revised the final manuscript. FZ and AG processed statistical analysis. MTK drafted the manuscript. MK and MR performed review.
References
- Salmonella Typhi, the causative agent of typhoid fever, is approximately 50,000 years old. Infect Genet Evol. 2002;2:39-45.
- [Google Scholar]
- Ceftriaxone-resistant Salmonella Typhi carries an IncI1-ST31 plasmid encoding CTX-M-15. J Med Microbiol. 2018;67:620-7.
- [Google Scholar]
- Multidrug resistant enteric fever in South Asia:Unmet medical needs and opportunities. BMJ. 2019;364:k5322.
- [Google Scholar]
- COVID-19 and arboviral diseases:Another challenge for Pakistan's dilapidated healthcare system. J Med Virol. 2021;93:4065-7.
- [Google Scholar]
- Influenza and COVID-19 co-infection:Report of six cases and review of the literature. J Med Virol. 2020;92:2657-65.
- [Google Scholar]
- Statistical analysis of forecasting COVID-19 for upcoming month in Pakistan. Chaos Solitons Fractals. 2020;138:109926.
- [Google Scholar]
- COVID-19 and Salmonella Typhi co-epidemics in Pakistan:A real problem. J Med Virol. 2021;93:184-6.
- [Google Scholar]
- Extensively drug-resistant Salmonella Typhi XDR infection at Rawalpindi medical university and allied hospitals. J Rawalpindi Med Coll. 2020;24:406-11.
- [Google Scholar]
- Comparative accuracy of typhoid diagnostic tools:A Bayesian latent-class network analysis. PLoS Negl Trop Dis. 2019;13:e0007303.
- [Google Scholar]
- Typhoid Fever -Islamic Republic of Pakistan. Available from: https://www.who.int/csr/don/27-december-2018-typhoid-pakistan/en/2018
- Typhoidal Salmonella strains in Pakistan:An impending threat of extensively drug-resistant Salmonella Typhi. Eur J Clin Microbiol Infect Dis. 2019;38:2145-9.
- [Google Scholar]
- Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of invasive Salmonella infections. Clin Microbiol Rev. 2015;28:901-37.
- [Google Scholar]
- Multidrug-resistant, extensively drug-resistant and pan drug-resistant bacteria:An international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268-81.
- [Google Scholar]
- Azithromycin versus ceftriaxone for the treatment of uncomplicated typhoid fever in children. Clin Infect Dis. 2000;31:1134-8.
- [Google Scholar]
- Typhoid fever infection-antibiotic resistance and vaccination strategies:A narrative review. Travel Med Infect Dis. 2021;40:101946.
- [Google Scholar]
- Unilateral breast abscess by an extremely drug resistant Salmonella enterica serovar Typhi:First case report from Pakistan. J Clin Diagn Res. 2019;13:DD01-2.
- [Google Scholar]
- World Health Organization. Weekly epidemiological Record. Wkly Epidemiol Rec. 2020;95:89-96.
- [Google Scholar]
- A three-year review of antimicrobial resistance of Salmonella enterica serovars Typhi and Paratyphi A in Pakistan. J Infect Dev Ctries. 2014;8:981-6.
- [Google Scholar]
- Ceftriaxone-resistant Salmonella Typhi outbreak in Hyderabad City of Sindh, Pakistan:High time for the introduction of typhoid conjugate vaccine. Clin Infect Dis. 2019;68((Supplement_1)):S16-21.
- [Google Scholar]
- Emergence of an extensively drug-resistant Salmonella enterica serovar Typhi clone harboring a promiscuous plasmid encoding resistance to fluoroquinolones and third-generation cephalosporins. MBio. 2018;9:e00105-18.
- [Google Scholar]
- Gastrointestinal and hepatobiliary complications of extensively drug-resistant typhoid at a tertiary care hospital in Pakistan. Cureus. 2020;12:e11055.
- [Google Scholar]
- Epidemiological profile and antimicrobial resistance pattern of enteric fever in a tertiary care hospital of North India-a seven year ambispective study. Acta Med. 2019;61:125-30.
- [Google Scholar]
- Typhoid fever:Issues in laboratory detection, treatment options and concerns in management in developing countries. Future Sci OA. 2018;4:FSO312.
- [Google Scholar]
- The clonal spread of multidrug-resistant non-typhi Salmonella serotypes. Microb Infect. 2006;8:1891-7.
- [Google Scholar]
- The implications of extensive drug-resistant typhoid fever:A case report. Cureus. 2019;11:e5032.
- [Google Scholar]
- Extensively drug-resistant (XDR) typhoid:Evolution, prevention, and its management. BioMed Res Int. 2020;2020:6432580.
- [Google Scholar]
- A Parallel Threat of Extensively Drug-resistant Typhoid Fever in Pediatric Cohort during Covid-19 Pandemic. 2023. Poster Session Presented Europeon Society for Pediatric Infectious Diseases, Lisbon, Portugal, PD0103/#544. Available from: https://espidmeeting.org/wp-content/uploads/sites/19/2023/05/espid23-abstracts-book.pdf
- [Google Scholar]







