Translate this page into:
Analysis of anxiolytic and anti-depressant potential of Bombyx mori in in vivo mouse mode
*Corresponding author: Abdul Hayee, University College of Conventional Medicine, Faculty of Pharmacy and Alternative Medicine, The Islamia University of Bahawalpur, Bahawalpur, Pakistan. khiljia.hayee@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Hayee A, Asif HM, Memoona, Khilji A, Khilji M, Nazar MS, et al. Analysis of anxiolytic and anti-depressant potential of Bombyx mori in in vivo mouse mode. Inter J Health Sci. 2025;19:11-9. doi: 10.25259/OA02_8751
Abstract
Objectives
Anxiety and depression are common mental health problems that affect people’s quality of life all around the world, and medicinal plants have been utilized for centuries. The anxiolytic and depressive activities of a hydro-alcoholic extract of Bombyx mori L. (Silkworm) were investigated using animal models in this study.
Methods
Anxiolytic activity was measured using an elevated plus maze model (EPMM) and open field test (OFT). The antidepressant activity was evaluated using the tail suspension test and forced swim test (FST). The EPMM recorded the amount of time spent in both closed and open arms. A period of unchanging status was detected for every animal for around 300 s in FST. One-way analysis of variance was used to analyze results, and values were considered significant where P < 0.05.
Results
Anxiolytic action of B. mori through OFT revealed dose-dependent increases in the frequency of line crossings and rearings at the dose of 200 mg/kg b.w. comparable with that of Diazepam. In EPMM, extract-treated groups spent more time in open arms and less time in closed arms compared to the control group. B. mori increased mobility time while decreasing immobility time in both experimental animals in a dose-dependent manner. B. mori was well-tolerated and showed no signs of toxicity or mortality up to a dose of 5 g/kg.
Conclusion
B. mori displayed anxiolytic and antidepressant effects, indicating its promise as a natural treatment. The extract was well tolerated at tested levels, suggesting its safety for further research and possible medicinal applications.
Keywords
Antidepressant action
Anxiolytic activity
Bombyx mori
Elevated plus maze model
Forced swim model
Open field test
Tail suspension model
INTRODUCTION
Anxiety and depression are among the most prevalent psychiatric disorders worldwide, with lifetime prevalence rates of 14% and 12%, respectively.[1] Such conditions present a significant financial burden due to the high cost of medical care. Among the most prevalent mental illnesses are anxiety and depression. Due to their strong comorbidity, these conditions are collectively referred to as internalizing mental disorders.[2] Psychological health is a crucial aspect of overall health, and it is just as vital in later life as it is in any other age.[3] The exact etiology of both disorders is not clearly known; however, some genetic, biological, environmental, and psychological factors are considered to be the major factors. Both anxiety and depression are characterized by increased concentrations of corticotropin-releasing factors in the cerebrospinal fluid. However, the release of other hormones or peptides of the hypothalamic-pituitary-adrenal (HPA) axis is regulated in the opposite manner in both disorders. Hypocortisolemia in anxiety leads to supersuppression after dexamethasone and increased numbers of glucocorticoid receptors, while hypercortisolemia non-suppression after dexamethasone leads to decreased numbers of glucocorticoid receptors in depression has been reported.[4] Brain-derived neurotrophic factors, inflammatory cytokines, endogenous metabolites, and some gut microbiomes have been considered to exhibit significant roles in the pathogenesis of these depressive disorders.[5,6] The major clinical features of these depressive disorders are sadness, loss of concentration and interest, loss of happiness, feelings of guilt or low self-worth, disturbed sleep, loss of appetite, and feelings of tiredness.[7] Targeting specific neurotransmitters in the brain is frequently used in psychiatric disorders that are successful in treating anxiety and depression.[8] Key neurotransmitters associated with these conditions include serotonin, gamma-aminobutyric acid (GABA), noradrenaline (norepinephrine), and dopamine.[9] Serotonin is a primary focus in various medications like selective serotonin reuptake inhibitors, which elevate serotonin levels to alleviate mood disorders.[10] GABA, an inhibitory neurotransmitter, is crucial for reducing anxiety, and drugs that enhance GABA function are used in anxiety management.[11] Noradrenaline and dopamine are associated with motivation and pleasure and are targeted by some medications to address symptoms of depression.[12] Efficient drug therapy for depression will mainly decrease anxiety disorders too. For various disorders of anxiety, such as obsessive compulsive disorder, increased quantities of antidepressants are needed than for depression.[13] Small quantities of unusual antipsychotic agents can decrease anxiety. Anti-depressants are the most important choices for enduring pharmacological management of anxiety and its related disorders.[14] The naturally occurring silk cocoon scientifically termed Bombyx mori L. (Lepidoptera, Bombycidae) silkworms is one of the most well-known medicinal substances with a variety of therapeutic uses.[15] Due to the presence of chemo-factors (morin and β-sitosterol) and antioxidants (flavonoids, anthocyanin, and alkaloids), mulberry leaves (Morus alba L.) are considered useful plants rich in nutrients and nutraceuticals. These leaves are also used as a traditional diet for silkworm larvae.[16] Due to its comparatively low resilience to illness and stress, B. mori is a potential biological model system for research on the toxicity of environmental pollutants, antibiotic resistance, and drug screening.[17] Due to the widespread perception of herbal products’ safety, their usage for medicinal and therapeutic evidence has increased worldwide.[18] When given orally to healthy individuals, silk fibroin hydrolysate had a notable neuroprotective effect as well as enhanced verbal and visual memory. When patients with mixed anxiety-depressive conditions were given a therapeutic syrup containing an aqueous extract of a silkworm cocoon, the mean levels of both anxiety and depression were dramatically decreased.[19] B. mori has been reported as a source of protein fibroin and sericin, flavonoids, quercetin and kaempferol, alkaloids, coumarin derivatives, and phenolic acids. Several pharmacological investigations of B. mori and its phytochemical constituents have revealed antioxidant, antihyperlipidemic, cardioprotective, gastroprotective, anticancer, and antidiabetic properties.[20] The present study aimed to evaluate the dose-dependent effects of hydro-alcoholic extract of B. mori for the management of depression and anxiety at various doses in well-established animal models.
MATERIALS AND METHODS
Insect material and preparation of crude extract
B. mori was purchased from the local market of Bahawalpur, Pakistan. It was identified and authenticated by Prof. Dr. Ghazala H. Rizwani, Director Research, Hamdard University Karachi, Pakistan and was given the reference number A141 for use in the future. After cleaning the fabric in which it was contained, the insect was dried in the shade. The crushed material was soaked in a watery ethanol solvent (30:70), which was stirred periodically at room temperature. Next, the immersed material was separated using Whatman #1 filter paper and muslin cloth. There were two more times that the soaking and filtering procedure was repeated. After discarding the remaining insect material, the collected filtrate was concentrated by first dissolving it under a rotating evaporator and then, after a little oven time, achieving a thick, semisolid extract structure akin to glue. After that, the botanical crude extract was given a name and an estimate of its yield in percentage, and it was placed in a cooler for later use.[21]
Drugs
One hour before the activity, hydro-alcoholic extract of B. mori at doses of 50, 100, and 200 mg/kg was administered separately. Diazepam (DZP; 1 mg/kg) was used as a standard anxiolytic. Fluoxetine (FXT) (10 mg/kg, Sigma-Aldrich, USA) was used as a conventional antidepressant medication. The control groups were managed using normal saline (NS) (10 mL/kg).[22]
Experimental animals
For this investigation, male Swiss albino mice weighing between 22 and 25 g were purchased from the Faculty of Pharmacy and Alternative Medicine’s animal house. Every animal was kept in reserve at the pharmacology research laboratory’s animal stock experimental area at the Islamia University of Bahawalpur’s Faculty of Pharmacy and Alternative Medicine. The critters were housed in conventional research center settings, with 12-h light/dim cycles at 22 + 2℃, and were fed standard food and drink on an as-needed basis for one week before the start of the experiment.
Experimental groups
All animals were divided into five different groups for management, with five animals in each group. The first group, which was labeled as control, had only normal saline with a concentration of 0.9% medium sodium chloride. The second group was called the standard group and was given DZP 1 mg/kg for anxiolytic effects and 10 mg of FXT for antidepressant effects. B. mori extract was administered in different quantities (50, 100, and 200 mg/kg b.w.) to the third to fifth groups. The oral intake of each medication was done 1 h before the experiment.
Evaluation of anxiolytic activity of B.mori
Open field test (OFT)
The OFT model was made of white compacted wood and had a length of (32 cm × 32 cm) with partitions 18 cm apart. Animals could be seen in the model since one wall was constructed of plain Plexiglas. Using an indication, blue stripes were painted on the base that was visible from the first to the last of the visible Plexiglas floor. The foundation was split into 16 (12 cm × 12 cm) figures with four sides each by the stripes. A (12 cm × 12 cm) focus square was positioned in the direction of the open field’s center. The center square is used because some mouse strains have fine motor abilities and travel over the stripes of the assessment compartment several times during a test session. In a similar vein, the core square has enough room all around it to emphasize the focal point as being unique before the exterior sections. The maze was lighted with a 60-watt red light meant for foundation lighting in a test room measuring (1.84.6 m). Between mice, the open field maze was cleaned with 70% ethyl alcohol. The critters’ exploratory mobility was evaluated in the open field. The number of rearings and the number of crossing lines (with four paws) were the monitored metrics.[23]
Elevated plus maze test
When it comes to measuring anxiety in animal models, the elevated plus maze test (EPMT) is the most widely used and widely acknowledged approach. Four arms made up the mechanical connection; two of them remained open while the other two were closed. Open arms (35 cm × 5 cm) were intersected with closed arms (35 cm × 5 cm × 20 cm) at an inside point (5 cm × 5 cm). From the first stage, EPM was raised to a height of 50 cm and put in a room with dim lighting. After a ½ h, the creatures were separated into management groups and placed in different groups to handle one of the closed arms on elevated plus maze devices at the center. Three hundred seconds were recorded as the amount of time that each animal spent with its arms open and closed.[24]
Assessment of antidepressant activity of B. mori
Forced swimming test
It is the most widely accepted and utilized in vivo method for evaluating the antidepressant effect. The apparatus was a simple Plexiglass box measuring 20 cm × 12 cm. It was filled with water (24°C + 1°C) to a depth of 15 cm, and all the animals were given a 15-min swim each day leading up to the particular test session as a pretest. Animals were treated with a specific collection of medicines after the pretest session, 6 h before the exact session of investigation, and ½ h before the final session of investigation. Half an hour after a specific section or one day after the pretest session, every experimental animal was separately put in a Plexiglass chamber for the final session of swimming (5 min each). A period of unchanging status was detected for every animal for around 300 s. Creatures were observed as unchanging when no attempts were made to escape, apart from developments significant to keep their heads out of water.[25]
Tail suspension test (TST)
Mechanical assembly was built consisting of a 70-cm-high wooden chamber for the TST. Between the load’s side separators, a bar was placed 60 cm above the floor or 10 cm above the top of the mechanical assembly. One inch of sticky tape was put one inch from the tip of the creature’s tail to hang it from the pole. Before their daily test session, animals had a 15-min pretest each day. After the pretest, 3 h before the final test, and 30 min before the test itself, the animals were given distinct collection medications. Every creature was individually hung from a pole for 300 s after a specific segment or one day after the pretest session. Each organism’s stability was measured for 300 s. The mouse was regarded steady when it clung still to the bar with no move to flee.[26]
Acute toxicity study of B. mori
To conduct acute lethality tests, the Organization for Economic Cooperation and Development’s test guideline 425 was followed. All experimental animals were divided into five groups and every animal’s typical behavioral characteristics were recorded. While fasting through the night, the experimental animals had unlimited access to water. B. mori extract was given to four groups of mice at dosages of 1, 3, 5, and 10 g/kg orally, whereas normal saline (10 mL/kg) was given to one group. After 30 min, 1, 2, 4, 6, 12, 24, and 48 h, behavioral changes were observed and recorded. Hyperactivity, alertness, convulsions, grooming, lacrimation, sweating, urine, corneal reflex, righting reflex, pain response, touch reaction, gripping quality, and mortality are examples of reaction parameters.[27]
Ethical consideration
The research was conducted under guidelines approved by the Islamia University of Bahawalpur’s Institutional Animal Ethics Committee. The Ethics Board of the University College of Conventional Medicine of the Faculty of Pharmacy and Alternative Medicine, Islamia University of Bahawalpur provided funding for the study, wide study/project no. 7117/Pharm.
Statistical analysis
Descriptive statistics was applied to calculate the mean and standard deviation. The study of the results was totaled by revealing the information to quantifiable investigation, making use of one-way analysis of variance. All of the results were examined using GraphPad Prism programming version 5. Impacts were found to be significant, where P < 0.05.
RESULTS
Anxiolytic activity of B. mori
Findings of the anxiolytic effect of B. mori using an OFT
The OFT was used to investigate the botanical extract’s effect on impulsive motor activity. In general, no line crossings or rearings into OFTs were seen by the experimental animals from the control group throughout a 5-min exercise. The inspection was done 1 h after the medicine was administered and the results were recorded. B. mori showed a dose-dependent increase in the number of line crossings and the number of rearings [Table 1]. The consequences of OFTs certainly illustrate the anxiolytic action of B. mori as it increased the number of line crossings and rearings in a dosage-dependent design. The number of line crossings and rearings by B. mori at the dose 50 mg/kg was 90.01 ± 3.9 and 35 ± 9, by 100 mg/kg was 98.23 ± 4.5 and 44.50 ± 8.8, and by 200 mg/kg was 119.90 ± 8. 7 and 48 ± 4.2, respectively [Table 1 and Figure 1]. The results revealed that a dose of 200 mg/kg of B. mori extract had a comparable effect to DZP.
| Group | Open field test | Elevated plus maze model | ||
|---|---|---|---|---|
| No of line crossing | No of rearing | Time spent in open arms (S) | Time spent in closed arm (S) | |
| Control (N/S 10 mL/kg) | 87.16±7.0ns | 34.12±13ns | 32.14±3.05ns | 262.19±3.11ns |
| Standard (DZP 1 mg/kg) | 133.83±9.10*** | 59.02±10*** | 104±8.14*** | 191.5±8.99*** |
| B. mori (50 mg/kg) | 90.01±3.9ns | 35±9ns | 49.5±3.76ns | 245.14±3.92ns |
| B. mori (100 mg/kg) | 98.23±4.5* | 44.50±8.8* | 76.44±4.41* | 218.0±4.01* |
| B. mori (200 mg/kg) | 119±8.7** | 48±4.2** | 94.05±5.11** | 200.45±4.92** |
N/S: Normal saline, DZP: Diazepam, SEM: Standard error of the mean, ns: Not significant, (*) Progressively significant (**) if P<0.01, and Highly significant (***) if P<0.001
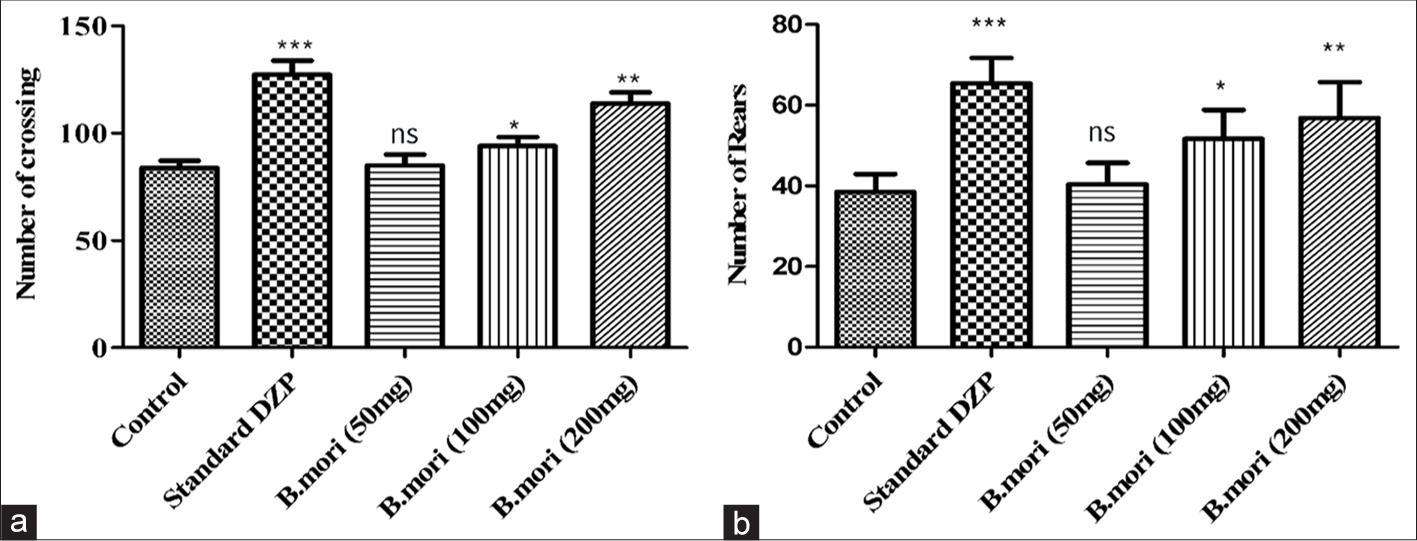
- Open field test data. (a) The figures show the groups’ means and standard deviations based on how many crossings occurred over 5 min. (b) The figures show the groups’ means and standard deviations concerning the total number of rears during the 5-min duration. *P < 0.05, **P < 0.01, ***P < 0.001, ns: Non-significant. DZP: Diazepam
Results of the anxiolytic effect of B. mori utilizing elevated plus maze model (EPMM)
The time invested in open and shut arms by animals from the standard and management groups in a 5-min interval was compared with that of control group. After 1 h of management, an inspection was conducted and the results were recorded. Time used up in open arms by animals managed with B. mori 50, 100, and 200 mg/kg was examined to be 50.49 ± 5, 74.5 ± 5.43, and 91.5 ± 6.41 s individually. Time spent in close arms by B. mori 50, 100, and 200 mg/kg was 243.8 ± 8.1, 220.0 ± 5.79, and 203.5 ± 6.24 s independently [Figure 2].
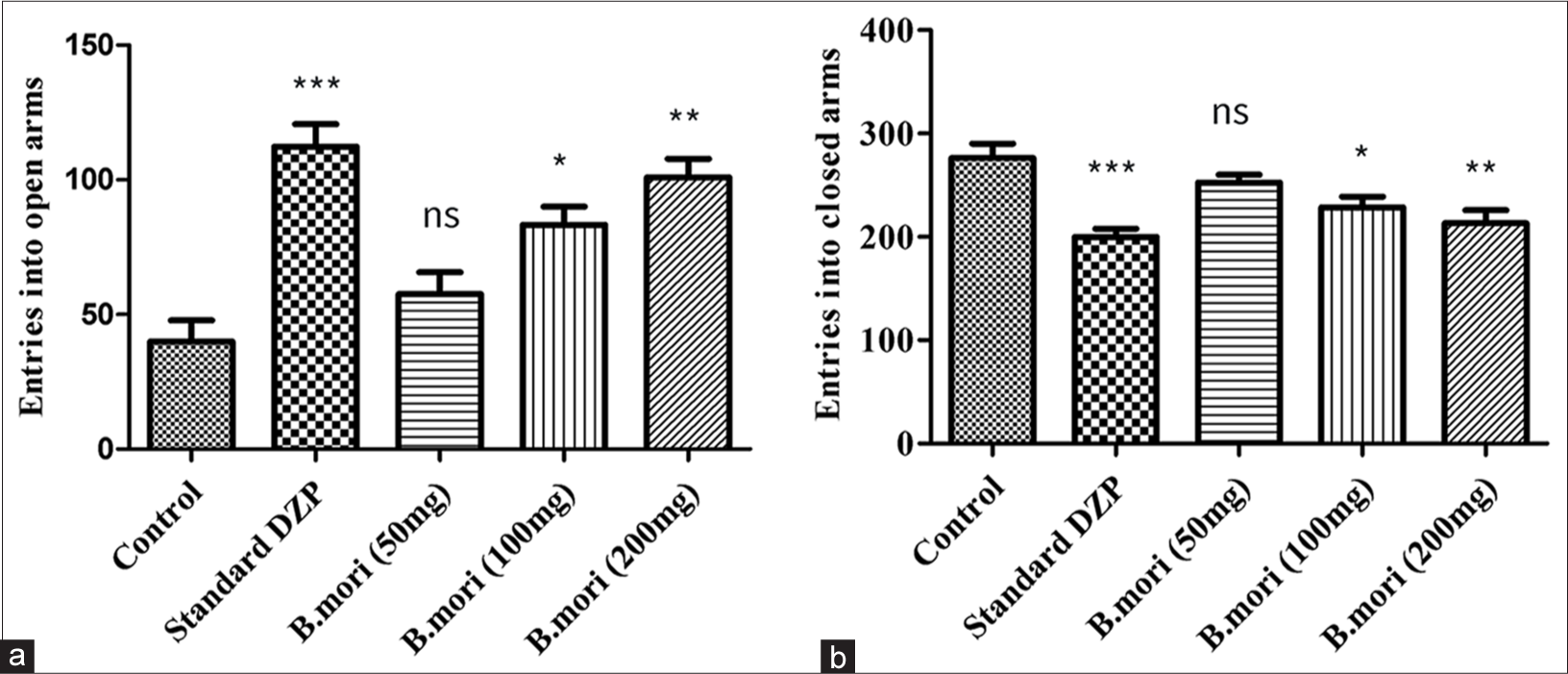
- Anxiety-like behavior in the elevated plus maze (EPM). Bar graphs show the results of the control (normal saline) and standard DZP with B.mori at different doses. (a) Entries into the open arms. (b) Entries into the closed arms. *P < 0.05, **P < 0.01, ***P < 0.001, ns means non-significant. DZP: Diazepam
Antidepressant activity of the Crude Extracts of B. mori
Results of antidepressant activity of B. mori using forced swim model
The animals in the standard and experimental groups were observed to exhibit varying degrees of mobility and immobility during three hundred-second swimming sessions. The results were compared to those of the standard control group. In a dosage-dependent manner, B. mori shortened the period of immobility and lengthened the period of movement [Figure 3 and Table 2].
| Group | Forced swim model | Tail suspension model | ||
|---|---|---|---|---|
| Duration of mobility (S) | Duration of immobility (S) | Duration of mobility (S) | Duration of immobility (S) | |
| Control (N/S 10 mL/kg) | 138.4±8.6ns | 161.6±8.6ns | 66.50±7.14ns | 233.5±7.14ns |
| FXT (1 mg/kg) | 247.1±5.90*** | 52.9±5.90*** | 223.4±3.45*** | 76.6±3.45*** |
| B. mori (50 mg/kg) | 170.2±4.11* | 129.8±4.11* | 101.2±8.0ns | 198.8±8.0ns |
| B. mori (100 mg/kg) | 199.3±4.32** | 100.7±4.32** | 149.1±6.73* | 150.9±6.73* |
| B. mori (200 mg/kg) | 231.4±3.92*** | 68.6±3.92*** | 165.0±5.99** | 135.0±5.99** |
B. mori: Bombyx mori, FXT: Fluoxetine, N/S: Normal saline, ns: Not significant. If the results are P<0.05, they are considered notable (*), P<0.01 indicates more significant (**), and P<0.001 indicates extraordinarily significant (***)
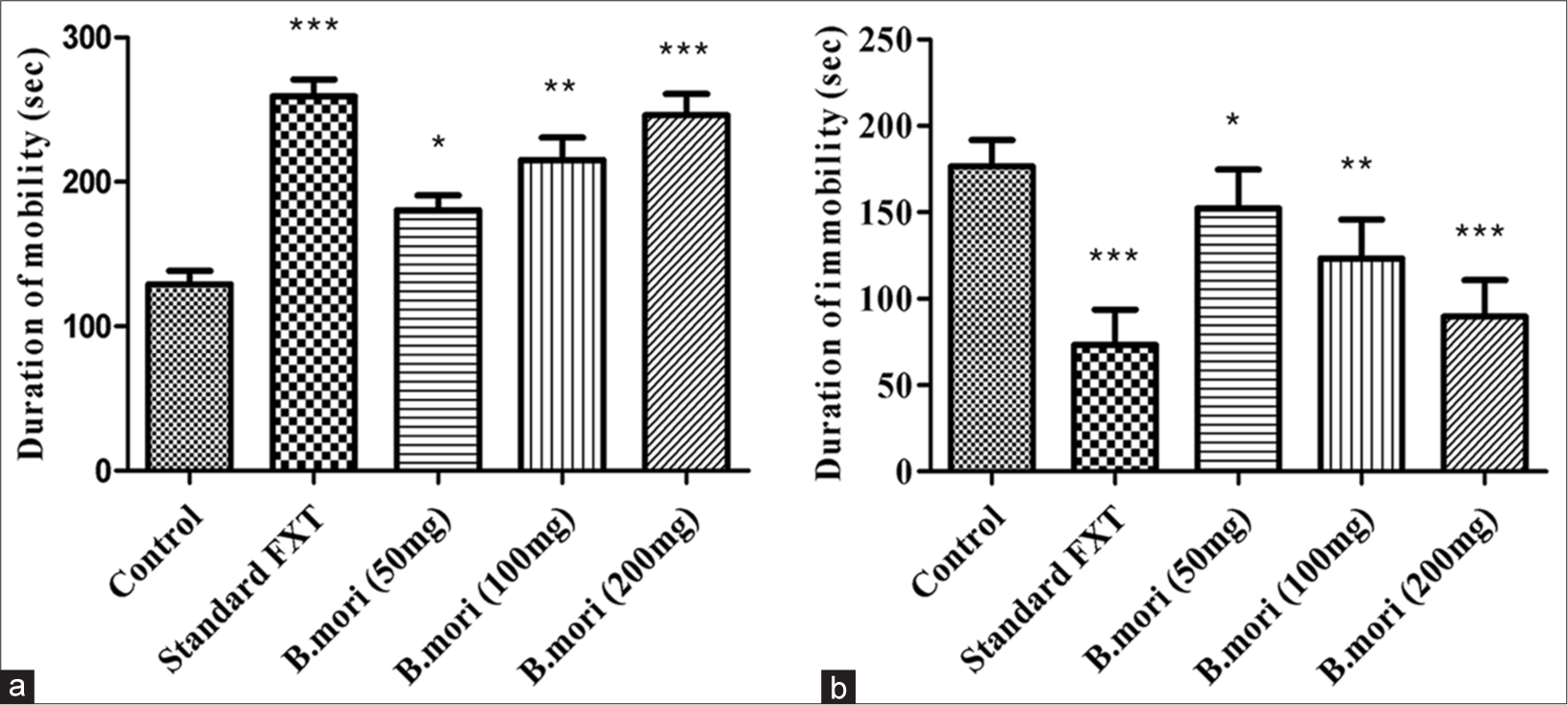
- Depression-like behavior in the forced-swim test. (a) Duration of mobility in (sec) compared with control and standard FXT with different doses of B. mori. (b) Duration of immobility in (sec) with control and standard FXT with different doses of B. mori. *P < 0.05, **P < 0.01, ***P < 0.001. B. mori: Bombyx mori, FXT: Fluoxetine.
Result of antidepressant activity of B. mori using tail suspension model
The length of the animals’ mobility and immobility in the conventional control group and the cure groups were compared. In a similar manner, and a dosage-dependent manner, B. mori increased the duration of mobility and decreased immobility [Figure 4 and Table 2].
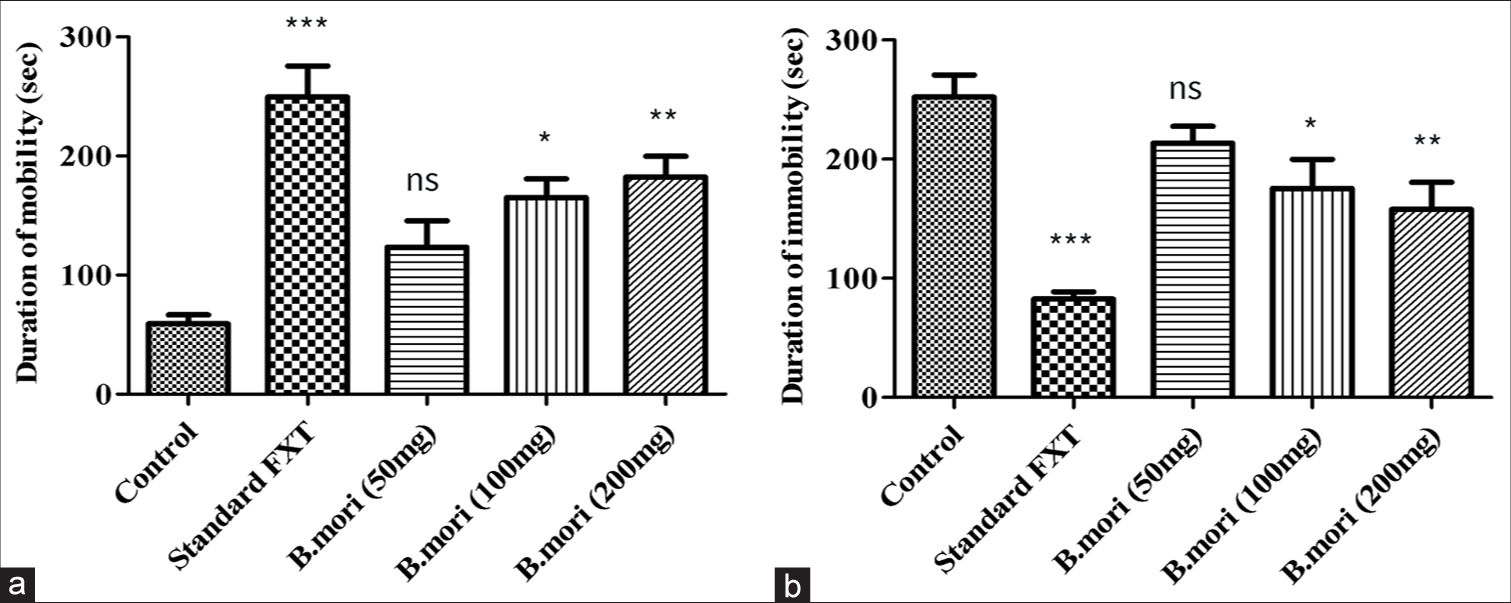
- Tail suspension model. (a) Duration of mobility in (sec) compared with control and standard FXT with different doses of B. mori. (b) Duration of immobility in (sec) with control and standard FXT with different doses of B. mori. *P < 0.05, **P < 0.01, ***P < 0.001, ns means non-significant. B. mori: Bombyx mori, FXT: Fluoxetine.
Acute toxicity studies of B. mori
The mice were given the unprocessed concentrate of B. mori intraperitoneal at dosage rates of 1, 3, and 5 mg/kg. No signs of toxic quality and fatality were seen up to 5 mg/kg dosage.
DISCUSSION
The preliminary phytochemical constituents that have been reported in B. mori are carbohydrates, flavonoids, phenols, proteins, etc. Furthermore, flavonoids contained in B. mori have been proven to reduce anxiety and depression.[28] Studies of plant chemicals have revealed that the Silk Shelter consists of 12-16% total protein, 1.2-1.8% carbohydrate, and 11-20% fat. Alanine, glycine, and serine represent about 82% of the amino acids found in the cocoon. The remaining amino acids present in the cocoon are cystine, histidine, aspartic acid, lysine, arginine, threonine, valine, proline, glutamic acid, tyrosine, methionine, tryptophan, phenylalanine, leucine, and isoleucine. As stated in several researches, the amino acids phenylalanine, tryptophan, methionine, and tyrosine are a lot useful in managing numerous mood disorders, including depression.[29] Studies have specified that everyday supplements of essential nutrients are very helpful in minimizing the symptoms of anxiety and depression in patients. Supplements holding amino acids have also been identified to minimize the symptoms, as they are changed to neurotransmitters, which in turn, ease depression and other mental health issues. These may be suitable for managing and to some point, preventing anxiety, depression, and a lot of other mental disorders,[30] while the content of fat comprises alpha-linolenic acid (ALA) - the indispensable fatty acids (FAs) together with palmitic acid. There are five flavanol glycosides recognized in a cocoon of silk that are responsible for the powerful anti-oxidant activity of B. mori.[31]
These in vivo assays signify that this hydro-alcoholic extract of B. mori is a considerable source of natural antioxidants that can reduce anxiety. In the present research, hydro-alcoholic extract of B. mori was shown to have an anxiolytic effect in the EPMT and OFT. Several plants that are utilized in folk medicine to reduce anxiety are accounted to create a rise in the investigation of the open-arms in the EPMT, while the locomotor activity of the mice was examined using the OFT.[32] Raised T maze pharmacological research verifies that the avoidance of open-arm and escape behaviors are expressions of emotional situations similar to the comprehensive panic and anxiety disorder, correspondingly.[33] In the OFT, B. mori amplifies the number of lines crossing in addition to several rearing in dose-reliant design. The effects of a 200 mg/kg dose of B. mori extract were comparable to those of DZP. In the elevated plus maze test, B. mori enhanced the time spent in open arms while decreasing the time spent in closed arms in a dosage-dependent way.
According to the EPM Model, B. mori (200 mg/kg) exhibited anxiolytic properties similar to DZP. DZP as a standard considerably augmented the number of line crossings in open fields along with the number of rearing (P < 0.001). The researched plant was ascertained to be comparably competent and better than placebo in reducing the symptoms. Statistically, considerable improvement was detected in the experimental group (P < 0.001) in anxiety. However, little considerable progress was identified in the anxiety of the placebo group. In this study, the potentiated effect of B. mori extract on anxiety and depression was studied using the forced swim model and tail suspension models. The results showed that B. mori reduced immobility time and increased mobility time in a dose-dependent manner. A dose of 200 mg/kg of B. mori had a strong antidepressant effect (P < 0.001) equivalent to that of FXT. The forced swim and tail suspension model results showed B. mori possess considerable antidepressant potential. The antidepressant effect of the extracts may be due to an inhibitory action on monoamine oxidase-A. As a result of selective serotonin reuptake inhibition, FXT demonstrated substantial antidepressant potential. In the tail suspension model, 200mg/kg of B. mori extract was significantly more significant (P < 0.01) than FXT. Our findings are comparable with a previous study reported by Xu et al., in which antidepressant potential of curcumin supplement (1.25-10 mg/kg, po for 14 days) exhibited significant improvement in the open field essays. In addition, immobility time was decreased by curcumin treatment in the forced swimming test by improving the noradrenaline, bilateral olfactory bulbectomy-induced alteration of 5-HT, and dopamine in the hippocampus.[34] Hyperactivation of the HPA axis in mice model reported by Bhutada et al., in a pre-clinical study by the induction of quercetin (20-40 mg/kg, po), resulting the prevention of depression-like behaviors comparing with FXT (10-20 mg/kg, ip) also demonstrated the significant importance of polyphenolic flavonoids for the management of depressive disorders.[35] Our results are comparable with another previous report in which fruit of Nelumbo nucifera (Nelumbinis semen) was prescribed in a rat model exhibiting antidepressant potential through reversing a decrease in 5-HT1A receptor binding. Rearing number, start latency, and grooming time was significantly improved while visiting counts were decreased by chronic mild stress.[36]
In a previous report, the neuroprotective activity was restored by silk fibroin in a traumatic brain injury model of rats by reverting the neurological status up to 25% after 4 days of injection indicating the growth of primary neuronal cells and astrocytes.[37] Tyrosinases inhibition has been reported by silk sericin which may leads to protection of several psychological disorders such as in Parkinson’s disease, hydroxylation is caused by overexpression of tyrosinases, resulting in the reduction in dopamine content. It further causes the neuronal cell death by increasing the oxidative stress.[38] Dopaminergic neurons have also been reported to be preserved by silk fibroin peptides in a Parkinson’s disease model by the effects of 6-hydroxydopamine neurotoxicity.[39] Aqueous extract of silkworm cocoon syrup was prescribed in a previous trial to the patients suffering from anxiety and depressive disorder exhibiting a reduction in anxiety and depression scores in weeks 6 and week 12 comparable with standard group (P < 0.001).[40] In a nutshell, our current research revealed that B. mori possess significant anxiolytic and anti-depressant activities. Further pharmacological and chemical investigations of the extract are required to segregate and differentiate the active constituents responsible for these effects of B. mori.
Limitations and future directions
Hydroalcoholic extract of B. mori showed possible anxiolytic and depressive effects using the EPMM and the OFT but it is needed to be further validate these effects by more diverse methods to analyze the anxiolytic and depressive activities. Furthermore, the mechanism of action of B. mori and biochemical estimations of specific neurotransmitters related to anxiety and depressant effects are needed to be evaluated in future studies to utilize it in the management of anxiety and depression.
CONCLUSION
The hydroalcoholic extract of B. mori showed promising anxiolytic activity using the EPMT and the OFT, while the antidepressant activity was confirmed using the TST and the forced swim test. Overall, this study demonstrates the therapeutic potential of B. mori extract in the management of anxiety and depression, providing a promising route for further research and potential clinical applications.
Authors’ contributions
AH and MRA: Designed the study, performed experiments, and prepared initial draft of manuscript; M, AK, and MK: Organized and interpreted data; SAZ, MSN, and WA: Helped performed data analysis; HMA: Supervised, reviewed, and approved final manuscript.
Ethical approval
The research was conducted under guidelines approved by the Islamia University of Bahawalpur’’s Institutional Animal Ethics Committee.
Declaration of patient consent
Patient’s consent is not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
The Ethics Board of the University College of Conventional Medicine of the Faculty of Pharmacy and Alternative Medicine, Islamia University of Bahawalpur provided funding for the study, wide study/project no. 7117/Pharm.
Conflicts of interest
There are no conflicts of interest.
Availability of data and material
All data and material are presented in this article and nothing submitted to any other directory and will be provided on request to corresponding author.
References
- National screening for anxiety and depression in saudi arabia 2022. Front Public Health. 2023;11:1213851.
- [CrossRef] [PubMed] [Google Scholar]
- The critical relationship between anxiety and depression. Am J Psychiatry. 2020;177:365-7.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence and factors associated with anxiety and depression among community-dwelling older adults in hunan, China: A cross-sectional study. BMC Psychiatry. 2023;23:107.
- [CrossRef] [PubMed] [Google Scholar]
- The link between childhood trauma and depression: Insights from HPA Axis Studies in Humans. Psychoneuroendocrinology. 2008;33:693-710.
- [CrossRef] [PubMed] [Google Scholar]
- Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience. 2013;246:199-229.
- [CrossRef] [PubMed] [Google Scholar]
- The role of microbiome in insomnia, circadian disturbance and depression. Front Psychiatry. 2018;9:669.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of treatment on serum brain-derived neurotrophic factor levels in depressed patients. Eur Arch Psychiatry Clin Neurosci. 2005;255:381-6.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular targets in the treatment of anxiety. Biol Psychiatry. 2002;52:1008-30.
- [CrossRef] [PubMed] [Google Scholar]
- Neurotransmitters and neurotransmission. Introduction to basics of pharmacology and toxicology In: Essentials of systemic pharmacology: From principles to practice. Vol 2. Singapore: Springer Verlag; 2021. p. :69-95.
- [CrossRef] [Google Scholar]
- Selective serotonin reuptake inhibitors and suicidal behaviour: A population-based cohort study. Neuropsychopharmacology. 2022;47:817-23.
- [CrossRef] [PubMed] [Google Scholar]
- Modulators of GABAA receptor-mediated inhibition in the treatment of neuropsychiatric disorders: Past, present, and future. Neuropsychopharmacology. 2024;49:83-95.
- [CrossRef] [PubMed] [Google Scholar]
- Anhedonia as a central factor in depression: Neural mechanisms revealed from preclinical to clinical evidence. Prog Neuropsychopharmacol Biol Psychiatry. 2021;110:110289.
- [CrossRef] [PubMed] [Google Scholar]
- Therapeutic implications of microRNAs in depressive disorders: A review. Int J Mol Sci. 2022;23:13530.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical advances in obsessive-compulsive disorder: A position statement by the international college of obsessive-compulsive spectrum disorders. Int Clin Psychopharmacol. 2020;35:173-93.
- [CrossRef] [PubMed] [Google Scholar]
- Bombyx mori cocoon as a promising pharmacological agent: A review of ethnopharmacology, chemistry, and biological activities. Heliyon. 2022;8:e10496.
- [CrossRef] [PubMed] [Google Scholar]
- Artificial diet as an alternative in silkworm (Bombyx mori L.) Feeding-a review. Sci Papers Ser D Anim Sci. 2023;66:76-86.
- [Google Scholar]
- Short-term triphenyltin exposure alters microbial homeostasis in the silkworm (Bombyx mori) Midgut. Sci Rep. 2023;13:15183.
- [CrossRef] [PubMed] [Google Scholar]
- Unpredictable Adverse Effects of Herbal Products. Food Chem Toxicol. 2022;159:112762.
- [CrossRef] [PubMed] [Google Scholar]
- Bombyx mori cocoon as a promising pharmacological agent: A review of ethnopharmacology, chemistry, and biological activities. Heliyon. 2022;8:e10496.
- [CrossRef] [PubMed] [Google Scholar]
- The effects of Bombyx mori silk strain and extraction time on the molecular and biological characteristics of sericin. Biosci Biotechnol Biochem. 2016;80:241-9.
- [CrossRef] [PubMed] [Google Scholar]
- Antidepressant and sedative-hypnotic activities of methanolic extract of Grewia asiatica Linn Leaves in mice. Bangladesh Pharm J. 2019;22:185-91.
- [CrossRef] [Google Scholar]
- Effect of crude extract of Bombyx mori coccoons in hyperlipidemia and atherosclerosis. J Ayurveda Integr Med. 2011;2:72-8.
- [CrossRef] [PubMed] [Google Scholar]
- Anxiolytic, antidepressant, and antistress activities of the aqueous extract of Cinnamomum tamala nees and eberm in rats. Indian J Pharmacol. 2016;48:555.
- [CrossRef] [PubMed] [Google Scholar]
- Banana fruit pulp and peel involved in antianxiety and antidepressant effects while invigorate memory performance in male mice: Possible role of potential antioxidants. Pak J Pharm Sci. 2017;30:989-95.
- [Google Scholar]
- Phytochemical screening, acute toxicity, anxiolytic and antidepressant activities of the Nelumbo nucifera fruit. Metab Brain Dis. 2017;32:743-9.
- [CrossRef] [PubMed] [Google Scholar]
- A Novel monobactam lacking antimicrobial activity, MC-100093, Reduces sex-specific ethanol preference and depressive-like behaviors in mice. Neuropharmacology. 2023;232:109515.
- [CrossRef] [PubMed] [Google Scholar]
- Acute and sub-acute toxicities of thai silkworm powder (Bombyx mori Linn.) from three races in male wistar rats and in vitro antioxidant activities. Pharmacogn J. 2017;9:541-5.
- [CrossRef] [Google Scholar]
- Silkworm, Bombyx mori as an alternative model organism in toxicological research. Environ Sci Pollut Res. 2018;25:35048-54.
- [CrossRef] [PubMed] [Google Scholar]
- Depression and Lifestyle: Focusing on nutrition, exercise, and their possible relevance to molecular mechanisms. Psychiatry Clin Neurosci. 2023;77:420-33.
- [CrossRef] [PubMed] [Google Scholar]
- Nutritional aspects of depression in adolescents-A systematic review. Int J Prev Med. 2019;10:42.
- [CrossRef] [PubMed] [Google Scholar]
- Protective effect of aabresham (Bombyx mori) against atherosclerosis. Int J Ayurv Herb Med. 2018;8:1.
- [CrossRef] [Google Scholar]
- Anxiolytic activity of Nymphaea alba linn. In mice as experimental models of anxiety. Indian J Pharmacol. 2011;43:50-5.
- [CrossRef] [PubMed] [Google Scholar]
- The elevated t-maze as an experimental model of anxiety. Neurosci Biobehav Rev. 1998;23:237-46.
- [CrossRef] [PubMed] [Google Scholar]
- Antidepressant effects of curcumin in the forced swim test and olfactory bulbectomy models of depression in rats. Pharmacol Biochem Behav. 2005;82:200-6.
- [CrossRef] [PubMed] [Google Scholar]
- Reversal by quercetin of corticotrophin releasing factor induced anxiety-and depression-like effect in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:955-60.
- [CrossRef] [PubMed] [Google Scholar]
- Nelumbinis semen reverses a decrease in 5-HT1A receptor binding induced by chronic mild stress, a depression-like symptom. Arch Pharm Res. 2004;27:1065-72.
- [CrossRef] [Google Scholar]
- Effect of silk fibroin on neuroregeneration after traumatic brain injury. Neurochem Res. 2019;44:2261-72.
- [CrossRef] [PubMed] [Google Scholar]
- Phytochemicals as future drugs for parkinson's disease: A comprehensive review. Rev Neurosci. 2016;27:651-68.
- [CrossRef] [PubMed] [Google Scholar]
- Tyrosine-fortified silk amino acids improve physical function of parkinson's disease rats. Food Sci Biotechnol. 2011;20:79-84.
- [CrossRef] [Google Scholar]
- The effect of medicinal syrup made from silkworm cocoon on mixed anxiety-depression disorder: A triple-blind randomized clinical trial. 2021. Iran Red Crescent Med J. Available from: https://www.ircmj.com/article_188935.html
- [Google Scholar]








