Translate this page into:
Anticoagulative activity of Commiphora gileadensis, aspirin, and heparin on blood coagulation profiles in naïve mice
Address for correspondence: Ayman Saeed Alhazmi, Department of Clinical Biochemistry, Applied Medical Sciences, Taif University, Saudi Arabia. E-mail: s.ayman@tu.edu.sa
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Qassim Uninversity and was migrated to Scientific Scholar after the change of Publisher.
Abstract
ABSTRACT
Objective:
Commiphora gileadensis is a small tree under the genus Commiphora. Previous studies showed medical applications, such as antibacterial and antihypertensive, for C. gileadensis.
Methods:
Sixty naïve mice were classified into six groups: control, C. gileadensis sap-treated group, C. gileadensis methanol extract-treated group, C. gileadensis acetone extract-treated group, heparin-treated group, and aspirin-treated group. Blood samples from each mouse in the six groups were collected in EDTA, sodium citrate, and heparin tubes. The body weight of each mouse was measured at the beginning and end of the experiment. Furthermore, complete blood count, kidney and renal function tests, coagulation profiles, prothrombin time (PT), activated partial thromboplastin time (aPTT), international normalized ratio (INR), D-dimer, and fibrinogen concentrations were estimated for each mouse.
Results:
The sodium, potassium, chloride, blood urea nitrogen, creatinine, alanine transaminase, and aspartate transaminase levels did not show statistical differences between all groups. Moreover, PT, aPTT, and INR were prolonged in the C. gileadensis sap, methanol, and acetone extracts-treated mice compared with those in the heparin and aspirin-treated groups (P < 0.01). D-dimer and fibrinogen concentrations did not show significant statistical differences between all groups.
Conclusion:
The current study concludes that the C. gileadensis sap, methanol, and acetone extracts prolonged PT, aPTT, and bleeding time in naïve mice more than heparin and aspirin. This means that the C. gileadensis extracts may have antithrombotic activity and may be used in the future to resolve intravascular thrombosis in patients having prosthetic valves.
Keywords
Activated thromboplastin time
Commiphora gileadensis
fibrinogen
prothrombin time
Introduction
Thrombosis has the potential to occur in either arteries or veins. The occurrence of atherothrombosis is initiated by the disruption of atherosclerotic plaque inside arteries. This disruption leads to the aggregation of platelets and activation of coagulation, ultimately resulting in the production of thrombi that are rich in platelets. These thrombi restrict the flow of blood within the affected arteries. This mechanism serves as the fundamental etiology for myocardial infarction, ischemic stroke, and acute limb ischemia. The velocity of blood flow in veins is comparatively lower than that observed in arteries, resulting in venous thrombi exhibiting a reduced platelet count and an increased fibrin content when compared to arterial thrombi.[1] The occurrence of thrombosis within veins gives rise to two distinct conditions: deep-vein thrombosis and pulmonary embolism. These conditions, when considered together, are usually described as venous thromboembolism (VTE). Arterial and venous thrombosis collectively contribute to approximately 25% of global mortality, resulting in an estimated annual death total of 18 million individuals.[2,3] Antiplatelet therapy has been widely recognized as the fundamental approach for both the prevention and treatment of atherothrombosis.[4,5] On the other hand, the primary approach for the prevention and treatment of VTE is anticoagulant therapy, owing to the prevalence of fibrin and scarcity of platelets in venous thrombi.[6] The primary adverse event associated with antithrombotic medication is bleeding, which is more prevalent when dual antiplatelet therapy (DAPT) is administered. DAPT involves the concurrent use of aspirin and a P2Y12 (a chemoreceptor for adenosine diphosphate that belongs to the Gi class of a group of G protein-coupled receptors) inhibitor, such as clopidogrel, as opposed to the use of aspirin alone. The addition of anticoagulants to single antiplatelet therapy or DAPT is associated with an elevated risk of bleeding. Consequently, the likelihood of significant bleeding is approximately 1.8 times greater when DAPT is used compared to when aspirin is used alone. Furthermore, a 2.5-fold increase in the risk of severe bleeding from aspirin occurs when coupled with a therapeutic dosage of a Vitamin K antagonist (VKA) such as warfarin.[7,8] A potentially fourfold increase in significant bleeding and a fivefold increase in mortality occurs in ischemia events, which can be attributed, at least in part, to the discontinuation of antithrombotic treatment.[9,10] The utilization of a standard dosage of anticoagulant medication is based on the knowledge gained through the administration of VKAs. To optimize effectiveness, it is necessary to make dose adjustments for VKAs to attain an international normalized ratio (INR) value >2.[11,12] Due to the side effects of anticoagulants and aspirin, new studies are focused on the use of natural products for the prevention and treatment of arterial and venous thrombi. Commiphora gileadensis, sometimes described as the Arabian balsam tree, belongs to the genus Commiphora and is native to the Arabian Peninsula and southern Egypt. The sap, wood, bark, and seeds of the tree possess noteworthy therapeutic characteristics.[13] Applications of the tree in traditional Arabian medicine include the treatment of many ailments, such as inflammatory diseases, constipation, stomachaches, joint discomfort, and headaches. Several studies have documented the antibacterial properties of the tree, although there is a dearth of research investigating its impact on infertility and erectile dysfunction in rats.[14] Furthermore, the sap of C. gileadensis has been employed as an antibacterial agent in many experiments conducted both in vivo and in vitro.[15] Moreover, a recent investigation showed that the methanolic extract derived from C. gileadensis exhibits antibacterial properties and facilitates the process of wound healing.[16]
Materials and Methods
The present analytical investigation was conducted at the Faculty of Applied Medical Sciences at Taif University from December 2022 to April 2023.
C. gileadensis collection
C. gileadensis was obtained from a high-altitude location known as the Alaab Valley, situated in the western part of Saudi Arabia’s Al-Madinah region. In December 2022, the leaves and fallen branches of the tree were gathered.
Preparation of C. gileadensis sap
The growing tips of C. gileadensis branches were lopped, leaving a 5-mm distance from the tips. Subsequently, the exuding sap was promptly collected following the incision. After being mixed with an equal volume of ethanol, the sap was subjected to centrifugation at a speed of 10,000 revolutions per minute for 10 min, following agitation for 15 min at ambient temperature. Next, the liquid portion of the mixture was stored at a temperature of −20°C until it was ready for examination.[17]
Preparation of C. gileadensis methanolic extract
Before drying, the leaves and branches of C. gileadensis were subjected to a cleaning process using tap water and subsequently dried in a hot-air oven maintained at a temperature of 40°C. After undergoing the drying process, the substance was further transformed into a finely ground powder and then subjected to sieving to eliminate any significant impurities. Thereafter, 10 g of the aforementioned powder were subjected to maceration within a sterile funnel for 24 h, utilizing a solution consisting of 100 mL of methanol with a purity of 100%. The funnel underwent strong agitation before the filtration of the extract, which was accomplished with the use of sterile filter paper. The obtained C. gileadensis extract was subjected to drying in a water bath at a temperature of 40°C to produce a concentrated extract. The sample was then refrigerated at a temperature of 4°C for 2 weeks and subsequently stored at a temperature of − 20°C for the purpose of subsequent analysis.[18]
Preparation of C. gileadensis acetone extract
The leaves and branches of C. gileadensis were subjected to a drying process at a temperature of 60°C for 6 h in a vacuum oven. A razor blade was then employed to chop the dried plant material into minute fragments, resulting in a powdered form. Subsequently, 10 g of the unprocessed C. gileadensis fragments were submerged in a solution consisting of 200 mL of acetone for 3 days, which maintained the ambient temperature. Throughout this time frame, the acetone solution, which was homogenized with a magnetic stirrer, was renewed on a daily basis. The sample obtained from the acetone extract was dried with a rotary evaporator to eliminate any remaining traces of acetone. The specimen was subsequently preserved at a temperature of − 20°C until the analysis.[19]
Experiment design
Sixty male BALB/c naïve mice, with an average age of 2 months and a weight range of 20–25 g, were obtained from the animal house at Umm Al-Qura University. The mice were accommodated in a conventional rodent cage with woodchip bedding. The cage was positioned within a well-ventilated room with a light and a dark cycle of 12 h each. The room temperature was consistently maintained at 25°C. The mice were supplied with conventional rodent meals and tap water throughout the experiment. Following a 2-week acclimation period, 60 mice were subjected to random assignment, resulting in the formation of six groups, each consisting of 10 mice.
-
The first group served as the negative control and received no treatment.
-
The second group was the C. gileadensis, methanolic extract-treated group. The mice in this group received 200 mg/kg of body weight per day of C. gileadensis methanolic extract for 8 weeks through intragastric gavage.[20]
-
The third group was designated as the C. gileadensis acetone extract-treated group. All the mice in this group were treated with C. gileadensis acetone extract as the second group.
-
The fourth group was the C. gileadensis sap-treated group. As the second group, the mice in this group were orally administered C. gileadensis sap.
-
The fifth group was the heparin-treated group. The mice, in this group, received one subcutaneous heparin dose of 40 U/kg of body weight, and after four hours, blood samples were collected from the mice.[21]
-
The sixth group was assigned as an aspirin-treated group. The mice, in this group, were administered aspirin orally in the amount of 5 mg/kg of body weight by intragastric gavage for 5 days. Then, blood samples were collected from each mouse in this group.[22]
Measurement of body weight
The initial body weight of each mouse was recorded at the commencement of the experiment, and thereafter, every 2 weeks for the course of the 8-week period of the study using a digital balance manufactured by OHAUS (model: Scout Pro SPU601, China).
Blood samples collection
Blood samples were obtained from all 60 mice through the retro-orbital venous plexus in EDTA, sodium citrate, and plain tubes. Blood samples collected in sodium citrate and plain tubes were immediately centrifuged at 2500 rpm for 15 min, and the resulting blood sera were stored at 80°C for further analysis. The blood collected in EDTA was used immediately for the estimation of complete blood count (CBC).
Estimation of CBC
The Beckman Coulter UniCel® D×H 500 was used to estimate CBC for all mice included in the current study.[23]
Estimation of coagulation parameters
The sera estimated from blood collected in the sodium citrate tube were used for measurements —obtained with a Sysmex CS5100 automatic coagulation analyzer (Japan) and proprietary reagents — of prothrombin time (PT), activated partial thromboplastin time (aPTT), INR, fibrinogen, and D-dimer. The bleeding time was estimated manually.[24]
Estimation of biochemical parameters
The sodium (Na+), potassium (K+), chloride (Cl−), carbon dioxide (CO2), blood urea nitrogen (BUN), anion gap, and glucose were estimated with Beckman Coulter AU 480.[25]
Statistical analysis
Statistical analysis was performed with SPSS software version 16 (SPSS Inc., Chicago, IL, USA). All data were expressed as mean ± SD, and all comparisons of total chemical parameters between different groups were performed through one-way analysis of variance (ANOVA). The level of significance was set at P < 0.05.
Results
Body weight
Table 1 represents the body weight of the mice in all six groups. There was no significant difference found among the groups, but the mice who were administered the different C. gileadensis extracts experienced a greater increase in weight than those in the control, heparin, and aspirin-treated groups.
Biochemical parameters
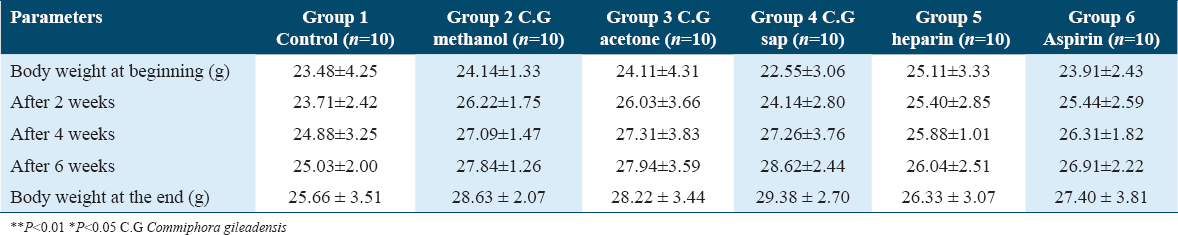
The sodium, potassium, chloride, BUN, creatinine, alanine transaminase (ALT), and aspartate transaminase (AST) levels in the six groups are represented in Table 2. There were no significant differences in any of the biochemical parameters among all six groups. However, the results showed that renal and hepatic function were unaffected in the mice who were administered the different extracts of C. gileadensis.
CBC
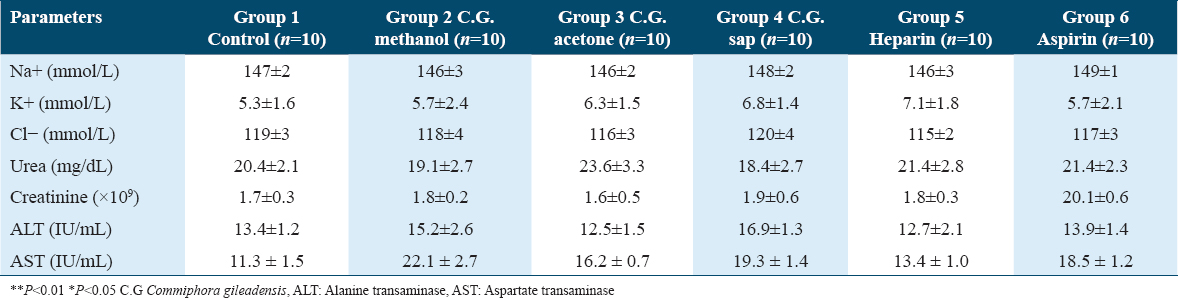
Table 3 represents the CBC of the six groups. There were no significant differences noted in the CBC of the mice in all groups. The C. gileadensis extracts did not affect white blood cell (WBC), red blood cell (RBC), or platelet count. Furthermore, hemoglobin and hematocrit results were normal in the mice to whom different extracts of C. gileadensis were administered.
Coagulation profiles
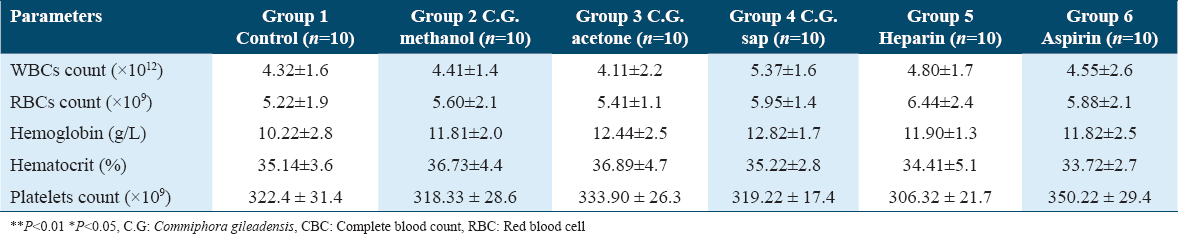
PT
Represented in Table 4 are the coagulation profiles of all mice in the six groups. The PT of the C. gileadensis methanol-treated group (58.8 ± 2.6) was higher than in the heparin and aspirin-treated groups (32.2 ± 3.3 and 25.5 ± 3.1, respectively) (P < 0.01). The PT of the C. gileadensis acetone extract-treated group (53.6 ± 2.8) was also higher than in the heparin and aspirin-treated groups (P < 0.01). Moreover, the PT of the C. gileadensis sap-treated group (89.9 ± 6.3) was higher than in the heparin and aspirin-treated groups (P < 0.01).
aPTT
The aPTT of the C. gileadensis methanol extract-treated group (81.4 ± 3.5) was significantly higher than in the heparin (62.5 ± 9.4) and aspirin-treated (52.6 ± 1.4) groups (P < 0.01). In addition, the aPTT of the C. gileadensis acetone (83.4 ± 4.6) and sap-treated (154.3 ± 14.9) groups was significantly higher than in the heparin and aspirin-treated groups (P < 0.01).
INR
The INR of the C. gileadensis methanol-treated group was significantly higher than in the heparin and aspirin-treated groups (P < 0.01). Furthermore, the INR of the C. gileadensis acetone-treated group showed a significant difference from that of the heparin and aspirin groups (P < 0.01). Furthermore, the INR of the C. gileadensis sap-treated group was significantly higher than that of the heparin and aspirin-treated groups (P < 0.01).
Bleeding time
The bleeding time of the C. gileadensis methanol (248.4 ± 17.2), acetone (223.3 ± 15.7), and sap-treated (321.5 ± 17.7) groups showed significant statistical differences from the heparin (165.4 ± 22.2) and aspirin-treated (98.3 ± 25.6) groups (P < 0.01).
D-dimer
No significant statistical difference was shown in the D-dimer levels between all groups.
Fibrinogen
Fibrinogen levels did not show any significant statistical difference between the six groups.
Discussion
C. gileadensis is a small tree that grows in the Arabian Peninsula. Previous studies demonstrated the therapeutic properties of this tree, proving its antibacterial effects in vivo and in vitro. Moreover, the studies showed the effectiveness of C. gileadensis in lowering blood pressure and as an antidote for the venom of scorpions and snakes. One study by Alhazmi, which was conducted to determine the lethal dose of C. gileadensis, proved that the tree is commensal and did not induce toxicity in mice at high doses. In the current study, the groups treated with C. gileadensis methanol, acetone, and sap extracts induce the body weight of mice, albeit insignificantly, and compared with other groups. Furthermore, these extracts did not produce hepatic or renal toxicity, which was proved by the normal hepatic (ALT and AST) and renal (urea and creatinine) function tests. The CBC of mice treated with different C. gileadensis extracts did not show any abnormality in WBC, RBC, and platelet count. Furthermore, these mice had normal hemoglobin and hematocrit values. Aspirin is the most commonly used antiplatelet agent. The enzyme cyclooxygenase-1 is effectively inhibited by aspirin, leading to the disruption of the initial stage in the synthesis pathway of prostaglandin and thromboxane A2. Consequently, the process of platelet aggregation is inhibited. The maximum antiplatelet action of aspirin is achieved within minutes and lasts throughout the life of the platelets, which typically ranges from 5–7 days.[26,27] Heparin is widely utilized as a parenteral anticoagulant in clinical practice. The use of this medication is recommended for both primary and secondary prevention of venous thromboembolism, acute coronary syndrome, mechanical heart valves, atrial fibrillation, and for transitioning patients from and to long-acting oral anticoagulants. The binding of heparin to antithrombin induces conformational alterations, resulting in the conversion of antithrombin from a sluggish inhibitor to a fast inhibitor of thrombin. Furthermore, heparin hinders the activity of activated coagulation factors IX, X, XI, and XII and plasmin, along with impeding the conversion of fibrinogen into fibrin.[28-31] During vascular injury, fibrinogen is converted to thrombin to initiate blood clotting. D-dimer is a fibrin degradation product that is present in the blood after a clot. In the current study, the PT was increased in mice treated with C. gileadensis, methanol, and acetone extracts. The PT of mice treated with C. gileadensis sap was more than 2 times greater than in those treated with heparin and aspirin. The aPTT of the groups treated with C. gileadensis methanol and acetone was greater than in the heparin and aspirin-treated groups. Moreover, the aPTT in the C. gileadensis sap-treated group was 3 times greater than in the heparin and aspirin-treated groups. The INR of the groups treated with C. gileadensis methanol and acetone extracts was higher than that of the heparin and aspirin-treated groups. Furthermore, the INR of the C. gileadensis sap-treated group was 6 times greater than that of the heparin and aspirin-treated groups. The bleeding time of the C. gileadensis sap, methanol, and acetone extracts-treated groups was increased and higher than in the groups treated with heparin and aspirin. A previous study done by Alhazmi et al. made an ultra-performance liquid chromatography coupled with mass spectroscopy for extracts of C. gileadensis and found that these extracts had high levels of glycosaminoglycans such as chondroitin sulfate and dermatan sulfate.[32] These molecules have an anticoagulative property and may be responsible for the prolongation of PT, aPTT, and bleeding time and may be used in the future to prevent intravascular thrombosis in patients having prosthetic valves. A comparison of coagulation profiles showed that PT, aPTT, INR, and bleeding time were higher in the C. gileadensis sap-treated group than in the methanol and acetone-treated groups. These may be due to that the sap is more viscous than the methanol and acetone extracts, so the glycosaminoglycans such as chondroitin sulfate and heparan sulfate were higher in the sap than those in methanol and acetone extracts.
Conclusion
The current study concludes that the C. gileadensis sap, methanol, and acetone extracts prolonged PT, aPTT, and bleeding time more than heparin and aspirin. This means that the C. gileadensis sap, methanol, and acetone extracts may have antithrombotic activity and may be used as an antithrombotic agent in patients with prosthetic heart valves. A future study is recommended to discover the manner in which C. gileadensis prevents intravascular thrombosis.
Limitation of the study
The limitation of the present study is the use of naïve mice. A future study will be conducted on rodents, specifically rats, as they possess larger blood arteries than mice. The study aims to induce intravascular thrombosis in these rats and discover if C. gileadensis can relieve the thrombus.
Acknowledgment
The author would like to thank Dr. Abdullah Dairi and Dr. MumdouhAllehyani, who facilitated everything for the author.
Authors’ Declaration Statements
Ethical approval
The animal study protocol was accredited by the National Committee for Bioethics at Taif University (protocol code HAO-02-T-105) and the Committee considered that the proposal fulfills the requirements.
Consent for publication
Not applicable.
Availability of data and material
The datasets utilized and/or examined in the present work can be obtained from the relevant authors upon a reasonable request.
Competing interests
The author declares that they have no competing interests.
Funding statement
The study was not funded.
Authors’ contributions
All experiments, data presentation, and writing were done by the author.
Conflicts of interest
The authors declare that they have no conflicts of interest.
References
- Thrombosis:A major contributor to global disease burden. Arterioscler Thromb Vasc Biol. 2014;34:2363-71.
- [Google Scholar]
- Demographic and epidemiologic drivers of global cardiovascular mortality. N Engl J Med. 2015;372:1333-41.
- [Google Scholar]
- Antiplatelet drugs:Antithrombotic therapy and prevention of thrombosis, 9th ed:American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:S89-119.
- [Google Scholar]
- Antithrombotic therapy for VTE disease:CHEST guideline and expert panel report. Chest. 2016;149:315-52.
- [Google Scholar]
- Meta-analysis of the efficacy and safety of clopidogrel plus aspirin as compared to antiplatelet monotherapy for the prevention of vascular events. Am J Cardiol. 2008;101:960-6.
- [Google Scholar]
- Warfarin plus aspirin after myocardial infarction or the acute coronary syndrome:Meta-analysis with estimates of risk and benefit. Ann Intern Med. 2005;143:241-50.
- [Google Scholar]
- Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation. 2006;114:774-82.
- [Google Scholar]
- Optimal INR for prevention of stroke and death in atrial fibrillation:A critical appraisal. Thromb Res. 2006;117:493-9.
- [Google Scholar]
- Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-51.
- [Google Scholar]
- Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883-91.
- [Google Scholar]
- The antibacterial activity of traditionally used Salvadora persica L (miswak) and Commiphora gileadensis (palsam) in Saudi Arabia. Afr J Tradit Complement Altern Med. 2014;11:23-7.
- [Google Scholar]
- Virucidal effect of guggulsterone isolated from Commiphora gileadensis . Planta Med. 2019;85:1225-32.
- [Google Scholar]
- In vitro and in vivo antibacterial effect of Commiphora gileadensis methanolic extract against methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa. Pak J Biol Sci. 2020;23:1676-80.
- [Google Scholar]
- Antibacterial effects of Commiphora gileadensis methanolic extract on wound healing. Molecules. 2022;27:3320.
- [Google Scholar]
- Medicinal properties of Commiphora gileadensis. Afr J Pharm Pharmacol. 2010;4:516-20.
- [Google Scholar]
- Commiphora gileadensis sap extract induces cell cycle-dependent death in immortalized keratinocytes and human dermoid carcinoma cells. J Herb Med. 2015;5:199-206.
- [Google Scholar]
- Prenylated flavonoids from the stem wood of Commiphora opobalsamum (L.) Engl. (Burseraceae) J King Saud Univ Sci. 2015;27:71-75.
- [Google Scholar]
- Protective effect of Commiphora gileadensis methanolic extract in mice against acetaminophen, gentamycin, and Nerium oleanderethanolic extract toxicity. Fresenius Environ Bulletin. 2022;31:381-9.
- [Google Scholar]
- The effect of heparin administration in animal models of sepsis:A prospective study in Escherichia coli-challenged mice and a systematic review and metaregression analysis of published studies. Crit Care Med. 2011;39:1104-12.
- [Google Scholar]
- Effect of different doses of acetylsalicylic acid on the antithrombotic activity of clopidogrel in a mouse arterial thrombosis model. Arterioscler Thromb Vasc Biol. 2018;38:2338-44.
- [Google Scholar]
- Chabot-Richards. White blood cell counts:Reference methodology. Clin Lab Med. 2015;35:11-24.
- [Google Scholar]
- A pilot study to assess kidney functions and toxic dimethyl-arginines as risk biomarkers in women with low vitamin d levels. J Med Biochem. 2019;38:145-52.
- [Google Scholar]
- Measurement of prothrombin time (PT) and activated partial thromboplastin time (APTT), fibrinogen level, D-dimer in sudanese infants and children with sepsis Khartoum State. Int J Med Sci Clin Invent. 2022;9:6217-22.
- [Google Scholar]
- Beyond COX-1:The effects of aspirin on platelet biology and potential mechanisms of chemoprevention. Cancer Metastasis Rev. 2017;36:289-303.
- [Google Scholar]
- The role of thromboxane in the course and treatment of ischemic stroke:Review. Int J Mol Sci. 2021;22:11644.
- [Google Scholar]
- Antiplatelet drugs:A review of their pharmacology and management in the perioperative period. Anesth Analg. 2011;112:292-318.
- [Google Scholar]
- Coagulation pathways in neurological disease:Multiple sclerosis. Front Neurol. 2019;10:409.
- [Google Scholar]
- Antibacterial effects of Commiphora gileadensis methanolic extract on wound healing. Molecules. 2022;27:3320.
- [Google Scholar]







