Translate this page into:
Antioxidative and ameliorative properties of probiotic-enriched fermented and unfermented turmeric-camel milk in streptozotocin-induced diabetes and oxidative stress in rats
Address for correspondence: Hassan Barakat, Department of Food Science and Human Nutrition, College of Agriculture and Food, Qassim University, Buraydah, Saudi Arabia; Department of Food Technology, Faculty of Agriculture, Benha University, Moshtohor, Egypt. Tel.: +966-547141277. E-mail: haa.mohamed@qu.edu.sa
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Qassim Uninversity and was migrated to Scientific Scholar after the change of Publisher.
Abstract
ABSTRACT
Objectives:
Probiotic-enriched fermented turmeric-camel milk (FTCM) and unfermented (TCM) were examined on streptozotocin (STZ)-induced type 2 diabetes and oxidative stress in rats.
Methods:
High phenolics and antioxidant activity were found in TCM and FTCM. FTCM and TCM at 10 or 20 mL/kg were investigated for antidiabetic and hypolipidemic effects in an animal model. The positive group diabetic rats (DR) received 45 mg/kg STZ intraperitoneally; G3 DR (DR+Met) received 50 mg metformin kg−1. G4 (DR+TCM10), G5 (DR+TCM20), G6 (DR+FTCM10), and G7 (DR+FTCM20) received 10 or 20 mL/kg from either TCM or FTCM orally daily, respectively. Blood glucose, liver and kidney functions, antioxidant biomarkers, and histopathology were investigated.
Results:
TCM and FTCM had strong phenolics and antioxidant potential. High-performance liquid chromatography analysis quantified ten phenolic acids and four flavonoids, with ferulic acid and resveratrol dominating. TCM and FTCM dramatically decreased random blood glucose and fasting blood glucose. The hypolipidemic effects of TCM and FTCM were more significant at 20 mL/kg than at 10 mL/kg, with substantial reductions in triglycerides, total cholesterol, high- and low-density lipoproteins cholesterol, and very-low-density lipoproteins cholesterol. TCM and FTCM at 20 mL/kg improved liver and kidney functions more than metformin or 10 mL/kg. FTCM and TCM dose-dependently increased antioxidant enzyme activity, glutathione, catalase, and superoxide dismutase, and decreased malondialdehyde. Histopathologically, TCM and FTCM showed typical Langerhans cell and acini structure, outperforming metformin.
Conclusion:
TCM and FTCM may help profitably manage diabetes complications and oxidative stress.
Keywords
Antidiabetes
antioxidants
diabetes mellitus
oxidative stress
turmeric-camel milk
Introduction
The International Diabetes Federation reports 463 million 20–79-year olds with diabetes. It represents 9.3% of global adults and will reach 10.2% by 2030.[1] Diabetes is a condition marked by insulin’s insufficient or improper action.[2] Type 2 diabetes is more complex than Type 1 diabetes, with many pathophysiological pathways affecting the pancreas and metabolic organs, making successful therapy difficult.[3,4] The gut microbiota (prebiotic) in camel milk (CM) may contribute to its health advantages.[5,6] Antiviral, antibacterial, antitumor, antifungal, antioxidant, hypoglycemic, anticancer, antiaging, and anti-autoimmune activities of CM have been scientifically proven.[7,8] CM impacts digestion, absorption, maturation, and defenses.[9] CM proteins promote immune responses, including antibacterial and antiviral activities.[10] Lactoferrin, immunoglobulin (Ig) IgGs, lactoperoxidase, lysozyme, peptidoglycan recognition protein-1, and other enzymes are abundant in CM and contribute to its antibacterial properties.[10-12]
According to the studies, yogurt and cheese affect food intake, satiety, and obesity-related metabolic issues.[13-15] Yet, fermented dairy products like fermented camel milk (FCM) remain controversial. It would help if you considered gut microbiota makeup. Postbiotics and other bacteria contribute to obesity and metabolic syndrome. Fewer short-chain fatty acid (SCFA)-producing bacteria in type 2 diabetics.[16] and some SCFAs (e.g., butyrate) facilitate improved insulin sensitivity, muscle fatty acid oxidation, and increased satiety.[17] FCM by Lactococcus lactis subsp. cremoris increased superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx) activity, oxidative stress, and decreased cardiac damage induced by acute exposure of mouse heart tissues to CCl4.[18] Fermented CM protects heart tissue due to its antioxidant chemical composition. The substances include oligosaccharides, vitamins, bioactive peptides, and conjugated linoleic acid.[19] Fermented CM also contains magnesium, which is essential for glutathione (GSH) biosynthesis,[20] reducing oxidative stress. Vitamin C also fights free radicals and protects cells.[21]
Based on scientific research on the low prevalence of diabetes, the population regularly drinks CM.[22] Every experiment decreased glucose and glycosylated hemoglobin. Curcumin improves diabetes, obesity, inflammation, arthritis, cancer, and digestion.[23] Cinnamon helps reduce pain and increase blood circulation. Anti-inflammatory helps with colds, flu, sore throats, and brain and intestinal health. Cinnamon reduces blood sugar and digestion. Lowering blood sugar with cinnamon helps type 2 diabetics. Some proteins and substances promote insulin hormone intake and activate blood proteins to absorb insulin.[24] Clove improves digestion, sexual health, blood sugar balance, liver function, immunity, and tooth and gum discomfort.[25] Cardamom’s nutrition contributes to health. It helps with digestion, cholesterol reduction, and cancer treatment. Fiber-rich cardamom may aid weight loss. It reduces blood sugar, protects the liver, relieves sleepiness, avoids depression, and promotes blood flow.[26] Ginger reduces cardiovascular disease risk, increases circulation, and lowers blood sugar. Antioxidants and active compounds in ginger may combat cancer.[27]
Turmeric has many biological properties, including the potential for antidiabetes.[28] In streptozotocin (STZ)-induced diabetic rats (DR), Sivaranjani et al.[29] found that a cinnamon-turmeric bi-herbal extract had hypoglycemic, hypolipidemic, and organ-protective effects. Golden milk may be used as it contains turmeric, cinnamon, cloves, ginger, cardamom, and honey. Thus, this work prepared unfermented (TCM) and probiotic-enriched fermented turmeric-camel milk (FTCM) and tested its antioxidant and ameliorative effects in rats with STZ-induced diabetes and oxidative stress. This evaluation included random and fasting glucose levels, lipid profiles, liver and kidney functions, oxidative stress indicators, and pancreatic histopathology.
Materials and Methods
STZ
Male albino rats were injected with 45 mg/kg STZ to induce type 2 diabetes. STZ was purchased from Sigma–Aldrich. Metformin (Glucophage) was purchased from the local pharmacy (Buraydah, Saudi Arabia).
Ingredients
The Fresh CM was sourced from the Qassim University College of Agriculture and Veterinary Medicine Farm. The standard culture contained a mix of Streptococcus thermophiles, Lactobacillus acidophilus, and Bifidobacterium bifidum strains in freeze-dried direct-to-vat set form kept at −18±1°C was obtained from Christian Hansen (Copenhagen, Denmark). Dry turmeric powder (Curcuma longa), ginger rhizomes powder (Zingiber officinale L.), cinnamon bark powder (Cinnamomum burmannii L.), clove pods (Syzygium aromaticum), cardamom (Elettaria cardamomum), and pepper (Piper nigrum), honey was obtained from spices local markets (Aba Al-khail, https://jiadalqassim.com/en/. All spices were kept at 4 ± 1°C in a dry place until further use.
Preparation of turmeric-camel milk (TCM) and FTCM
The fresh CM was chemically analyzed before being subjected to manufacturing, and the procedure has been adapted following Aljutaily.[11] CM was heated at 50°C; 2 g turmeric powder, 5 g honey, 1 g cinnamon powder, 1 g ginger powder, 0.1 g cardamom powder, 0.05 g clove powder, and 0.05 g white pepper powder per 100 mL−1 fresh milk were added and vigorously mixed. The prepared TCM was filtered through cheesecloth to remove sediments, divided into two equal portions (2 L each), filled in scraw-capped glass bottles, pasteurized at 85 °C for 15 min, and cooled to 42°C. FTCM was made by adding 1 g/1 L ABT-5 starter to TCM under aseptic conditions, incubated at 42°C for 4–5 h to reach a pH of 4.6–4.7, and cooling for 12–18 h. The second TCM batch was stored at 4 ± 1°C without fermentation. For microbiological examination regarding viable bacterial count, 50 mL FTCM samples were collected aseptically in sterile bags.
Determination of total phenolic content (TPC), total carotenoids (TC), total flavonoids (TF), and total flavonols (TFL) in TCM and FTCM
The TPC in TCM was assayed using the Folin–Ciocalteu reagent, according to Nsimba et al.[30] with slight modification. In Eppendorf tubes, 150 μL of the sample was mixed with 300 μL of Folin–Ciocalteu reagent for 5 min. Then, 300 μL of an alkali solution (7.5% sodium carbonate solution) was added. The mixture was incubated in the dark for 60 min at 23℃, then centrifuged at 10,000 g for 10 min at 4℃, and 200 μL of supernatant from each Eppendorf was transferred to a new plate. The absorbance was then measured at 765 nm using a microplate reader (BioTek, Winooski, USA). A standard curve using Gallic acid (GA) solution was prepared to compare the measurements. Gallic acid equivalents (GAE) per g represented TPC content (mg of GAE g−1 dw).
According to Yuan et al., to determine the total carotenoids (TC), one g of the freeze-dried sample was repeatedly extracted with a mixture of acetone and petroleum ether (1:1, v/v).[31] TC concentration was measured at 450 nm and reported as mg/g dw. The TF content of TCM using the same extract was determined. Briefly, 1 mL of clear extract aliquots were combined with 1 mL of 2% aluminum chloride (AlCl3) and monitored for 60 min at 420 nm, as Mohdaly et al.[32] described. The TFL concentrations in TCM were determined by reacting methanolic extract aliquots with sodium acetate (5 %). AlCl3 (2%) was added after 5 min, and the optical density (OD) was measured after 150 min at 440 nm, as described by Kumaran and Karunakaran.[33] The TF and TFL concentrations were reported in milligrams of Quercetin-Equivalent (mg QE) per gram of dry weight (mg QE/g).
Antioxidant capacity determination
According to Nsimba et al.,[30] DPPH radical bleaching in a purple solution assessed radical scavenging activity spectrophotometrically with slight modifications. Briefly, 600 μL of DPPH solution was added to 120 μL of the sample in Eppendorf tubes. After incubating in the dark for 60 min at 23°C, tubes were centrifuged at 10,000 g for 10 min at 4°C then measured at 517 nm using a microplate reader (BioTek, Winooski, USA). The antiradical activity was measured in Trolox Equivalents (TE) per gram (μmol TE/g).
Using the Lu et al.[34] adapted protocol, TCM’s radical scavenging activity (RSA) against the stable ABTS radical (2,2′-casino-bis (3-ethylbenzothiazoline-6-sulphonic acid)) cation was determined. The antiradical activity was measured in TE per gram (μmol TE/g). Koleva et al.[35] were used to compare the antioxidant capacity of TCM to butylated hydroxyanisole (BHA) using a β-carotene bleaching assay. The results were given as BHA-related percentages. The chelating activity of TCM was measured as protocoled by Zhao et al.[36] The inhibition % of ferrozine-Fe2+ complex creation as metal chelating action was calculated and presented as (mg/mL) when ethylenediaminetetraacetic acid as a positive control was used.
High-performance liquid chromatography (HPLC) - Phenolic compounds quantification in TCM and FTCM
The phenolic compounds TCM and FTCM were determined by the HPLC system HP1100 (Agilent Technologies, Palo Alto, CA, USA) equipped with an autosampler, quaternary pump, and diode array detector DAD (Hewlett Packard 1050), using a column (Altima C18, 5 × 150 mm, 4.6 mm ID) and a guard column Altima C18, 5 mm (Alltech) according to Kim et al.[37] The solvent system contained a gradient of A (Acetic acid 2.5%), B (Acetic acid 8%), and C (Acetonitrile). The 10 μL of solvent was injected at a flow rate of 1 mL/min, and separation was performed at 25°C. Phenolic compounds (μg/g) were identified by comparing each peak’s retention time and mass spectra with library and external standards.
Experimental animal design
The animals were rendered diabetic by a single intraperitoneal injection of STZ after the injection of Nicotinamide. The induction of diabetes was performed according to Shiju et al.[38] method. STZ was freshly prepared in 0.1 M citrate buffer (pH 4.5) at a dose of 45 mg/kg intraperitoneally injected for one single dose; the STZ-injected animals were then given 5% glucose solution for 24 h following STZ injection to prevent initial drug-induced hypoglycemic mortality. After 72 h of STZ injection, blood was drawn from the retro-orbital plexus of the rats, and fasting blood sugar was estimated using a glucometer (Accu-Chek, Roche, Germany). The animals with fasting blood glucose (FBG) above 200–250 mg/kg dL were included in the treatment below. Treatment was started 4 days after the induction of diabetes.
Animal model
Male albino Wistar rats weighing 180–200 g (6–8 weeks old) were obtained from the Animal House of the Pharmacy College, King Saud University (Riyadh, Saudi Arabia). The rats were housed at the Department of Food Science and Human Nutrition, College of Agriculture and Food, Qassim University, Saudi Arabia, in polypropylene cages under standard laboratory conditions (22±3°C, 40–60% humidity, 12-h light/dark cycle) supplied with a basal diet and water ad libitum. The Qassim University, Saudi Arabia Committee of Research Ethics (IRB) approved the experiment on Monday, October 10, 2022 (Approval No. 22-06-03). The rats were acclimated for 1 week before starting the experiment. Forty-nine rats were randomly divided into two groups; the first group (n = 7) was the negative control (NC) group, and the second group (n = 42) was the STZ induced DR.
Experimental design
Experimental groups were divided into two parts. The first group (G1, NR) consisted of seven rats treated as a negative control. In contrast, the rest of the rats were injected with STZ intraperitoneally at 45 mg/kg body weight. After 24 h, the blood glucose level was measured to determine the extent of disease development. A rate of more than 200 mg/dL was a limit to classify rats for induction. After confirming that all rats were diabetics, they were divided into six groups and treated as follows: the second group (G2, STZ) (was dosed with a saline solution) and was left without any treatments (positive control), the third group (G3, Metformin) was treated with oral doses daily using metformin solution at a rate (50 mg/kg). The fourth (G4, TCM10) and fifth (G5, TCM20) groups were administered orally at 10 mL and 20 mL/kg of body weight with Unfermented Turmeric-CM. At the same time, the sixth (G6, FTCM10) and seventh (G7, FTCM20) groups were administered orally at a rate of 10 mL and 20 mL/kg of body weight with fermented and turmeric-CM, respectively.
Blood and organs collection
After completing the experiment, blood samples were collected from each mouse by fasting rats, sacrificed under anesthesia, and then blood was taken directly from the jugular vein. On blood sample collection in red screw-capped tubes (Jiangsu Kangjian Medical Apparatus Co., P.R. China), a portion of the blood samples was centrifuged at 3000 rpm for 15 min for serum extraction. Afterward, a portion of the pancreatic tail from each rat was collected and fixed in 10% neutral-buffered formalin and kept for histopathological examination.
Determination of FBG
FBG (mg/dL) was determined using the glucose oxidase peroxidase-4-aminoantipyrine method’s enzymatic colorimetric test kit, according to Barham and Trinder.[39]
Determination of lipid profile
The lipid profile includes triglycerides (TG, mg/dL) and total cholesterol (CHO, mg/dL) using an enzymatic colorimetric test kit and applying the glycerol phosphate oxidase peroxidase-4-aminoantipyrine method.[40] High-density lipoproteins (HDL, mg/dL) were determined using an enzymatic colorimetric direct homogenous test kit following company protocols.[41] Low-density lipoproteins (LDL, mg/dL1) and very-low-density lipoproteins (VLDL, mg/dL) were mathematically calculated according to Friedewald et al.[42] The atherogenic index (AI) is an essential predictor of atherosclerosis and reflects the ratio of TG and HDL.[43]
Determination of livers and kidneys’ functions
The liver’s functions, such as alanine aminotransferase (ALT, UL−1), aspartate Aminotransferase (AST, UL−1), alkaline phosphatase (ALP, UL−1), and total bilirubin (T. bili, mg/dL) in blood serum using alanine aminotransferase kit (EC 2.6.1.2), aspartate aminotransferase kit (EC 2.6.1.1), optimum alkaline kit (EC 3.1.3.1), and photometric test kits for T. bili were examined, respectively. Total protein (T. protein, g/dL) and albumin (g/dL), creatinine (mg/dL), and urea (mg/dL) concentrations using photometric, colorimetric test kits were determined applying the Biuret method, photometric, colorimetric test kits applying BCG method, photometric, colorimetric test kits, and fully enzymatic test kit using GLDH method was, respectively, determined according to the instructions of the manufacturer. Globulin (g/dL) was calculated by subtracting albumin from T. protein concentrations. Blood urea nitrogen (BUN, mg/dL) was calculated by multiplying urea concentration by 0.47. All biochemical examination kits were purchased from Human Co., Wiesbaden, Germany.
Oxidative stress biomarkers
Reduced GSH, μg/dL was estimated using a GSH colorimetric assay kit according to the method described by Beutler et al.[44] Lipid peroxidation was assessed using malondialdehyde (MDA, nmol/mL) colorimetric assay kit by measuring thiobarbituric acid reactive substance and expressed in terms of MDA content according to Ohkawa et al.[45] MDA, a product of fatty acid peroxidation, forms a colored complex reacting with thiobarbituric acid. The absorbance of the supernatant was measured at 532 nm, and the results were calculated as nmol/mL. Superoxide dismutase (SOD, U/L) activity using SOD typed activity assay kit was determined according to Giannopolitis and Ries.[46] The color reaction was measured at 550 nm and expressed as U/L. According to Aebi’s method, a CAT activity assay kit determined catalase (CAT, U/L) activity activity.[47] All oxidative stress markers were determined in the blood plasma using a blood chemistry analyzer (HumaLyzer 4000, Germany).
Histopathology
Autopsy samples were taken from rats’ pancreas in different groups and fixed in 10% neutral-buffered formalin for 48 h. Pancreas tissues were prepared, flushed in xylene, and embedded in paraffin at 56°C in a hot air oven for 24 h, then prepared in paraffin beeswax and slid by microtome. Tissue sections were deparaffinized and stained with hematoxylin-eosin after microtome sectioning (H&E). According to Bancroft et al.,[48] stained sections were diagnosed for histological pancreatic architectural changes and photomicrographed. Subsequently, two pathologists re-diagnosed the undefined experimental groups to verify the results.
Statistical analysis
Statistics were performed with the Statistical Package for the Social Sciences (Ver. 22.0 for Windows, IBM, Houston, Texas, USA). Experimental results are presented as mean±standard error. Statistical significance was assessed using one-way analysis of variance and post hoc tests, with P < 0.05, according to Steel et al.[49]
Results
Phytochemicals and antioxidant capacities of TCM and FTCM
The phytochemicals and antioxidant activities of TCM and FTCM were measured using DPPH, ABTS radical scavenging, and chelating capacity (CA) assays. As shown in Table 1, TPC content in TCM and FTCM was 175.56 and 179.39 mg GAE 100 mL−1, respectively. The TC content was 12.19 and 12.98 μg 100 mL−1. The TF and TFL contents were 16.98 and 16.29 mg QE 100 mL−1 and 10.22 and 11.07 mg QE 100 mL−1 in TCM and FTCM, respectively. The antioxidant capacities indicated 226.46 and 313.18 μmol TE 100 mL−1 in TCM and 230.12 and 319.24 μmol TE 100 mL−1 in FTCM for DPPH-RSA and ABTS-RSA, respectively. In addition, the antioxidant activity (AOA) of TCM and FTCM is presented in Table 1. Evaluation of the metal-chelating activity revealed 93.83 and 95.01 mg 100 mL−1 for TCM and FTCM, respectively, which seem proficient in interfering with Fe2+–ferrozine complex formation, indicating their capability to chelate oxidation metals.
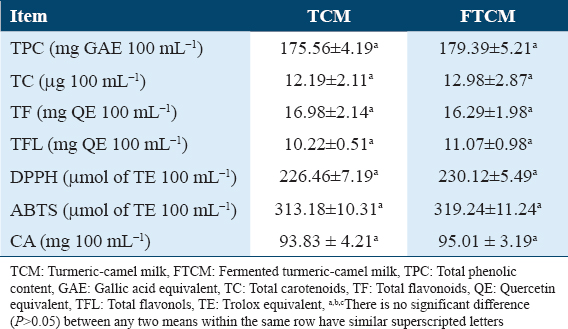
Quantification of phenolic compounds in TCM and FTCM
The quantitative analysis of TCM and FTCM phenolic components is reported in Table 2. In detectable amounts, ten phenolic acids and four flavonoids were identified in TCM and FTCM. The most abundant hydroxy-benzoic acid was ellagic acid (182.54 μg/g) in TCM and (194.04 μg/g) in FTCM, followed by GA with 108.54 and 133.58 μg/g in TCM and FTCM, respectively. Pyrocatechol presented higher content in TCM than FTCM, as it presented 108.34 μg/g in TCM and 35.33 μg/g in FTCM. A moderate amount of chlorogenic acid was observed by 65.49 and 34.34 μg/g in TCM and FTCM, respectively. In addition, cinnamic acid exhibited 45.74 and 35.88 μg/g in TCM and FTCM, respectively. Vanillic acid was higher in FTCM than TCM, as 14.19 and 12.75 μg/g were recorded, respectively. Coumaric acid was more elevated in TCM than FTCM, as 19.13 and 16.63 μg/g were recorded, respectively. Caffeic acid was detected in TCM with a low amount while not in FTCM, as shown in Table 2. The TCM and FTCM were abundant in flavonoid content, as indicated in Table 2. Catechin, quercetin, daidzein, and hesperidin were found in increased levels. TCM has higher catechin, quercetin, daidzein, and hesperidin than FTCM. Table 2 shows that FTCM did not contain daidzein or hesperidin.

The hypoglycemic efficiency of TCM and FTCM
The hypoglycemic efficiency of TCM and FTCM at 10 and 20 mL/kg and metformin at 50 mg/kg on STZ-induced diabetes in rats were followed by both random blood glucose (RBG) and FBG; data are tabulated in Table 3. Using 20 mL/kg of TCM or FTCM or 50 mg/kg of metformin was more effective in lowering RBG than using 10 mL/kg. After 6 weeks of treatment, TCM and FTCM significantly suppressed FBG to levels similar to normal rats, as measured by FBG levels. Interestingly, compared to the normal rats (NR) and metformin groups, the treated group with FTCM was far better than the TCM group. Two weeks after treatment began, those in the TCM10, TCM20, FTCM10, and FTCM20 groups saw significantly more significant improvements than those in the metformin group. Table 3 shows that after 4 weeks, TCM and FTCM at 20 mL/kg were more effective at lowering RBG than metformin at 50 mg/kg.
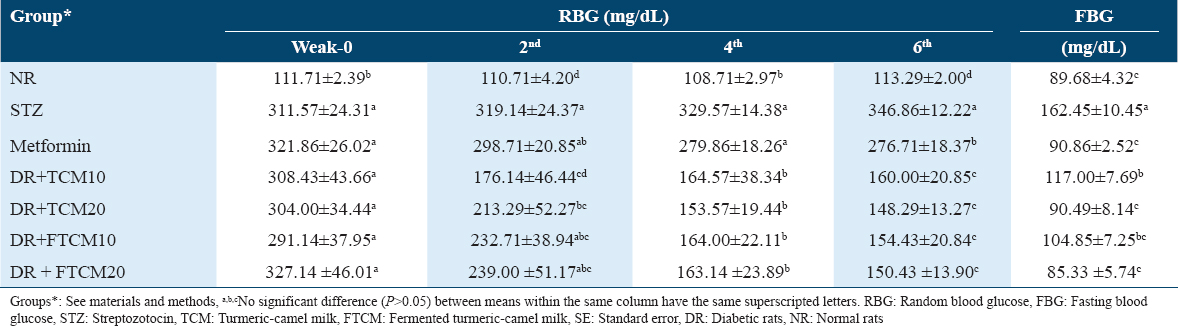
The hypolipidemic efficiency of TCM and FTCM
The hypolipidemic efficiency of TCM and FTCM at 10 and 20 mL/kg and metformin at 50 mg/kg on STZ-induced diabetes in rats were examined; data are shown in Table 4. Injecting STZ into DR caused a dramatic rise in TG, CHO, LDL, and VLDL levels, as well as many other complications. HDL levels were significantly lower in STZ-injected rats compared to the NR group. Compared to NA and metformin groups, the TG, CHO, LDL-C, and VLDL-C levels were considerably reduced when TCM and FTCM were administered at 10 and 20 mL/kg, respectively. The most efficient treatment for improving the blood profile was the TCM and FTCM at 20 mL/kg; even FTCM was much better than the TCM. TG levels were attenuated by 25.63, 36.70, 27.31, and 39.86% when rats were treated with TCM and FTCM at 10 and 20 mL/kg, respectively.
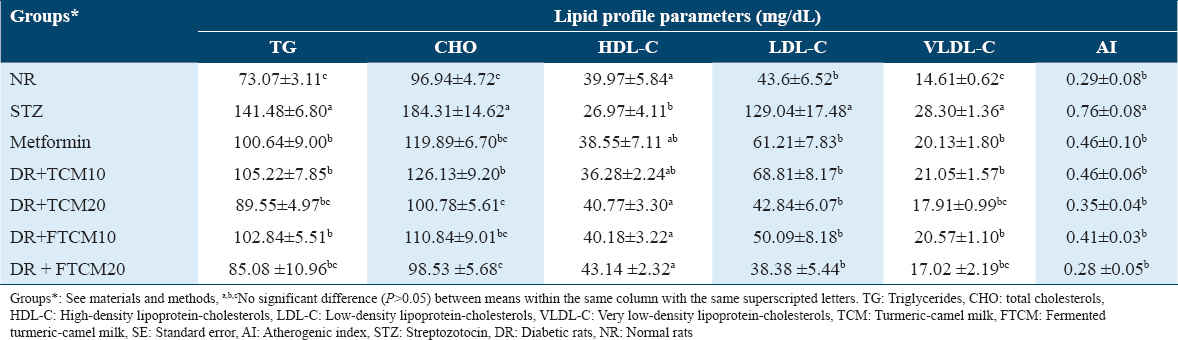
Intriguingly, the HDL-cholesterol (HDL-C) rise rates were 51.16% for TCM and 59.95% for FTCM. The LDL-cholesterol (LDL-C) decrease was 66.80 and 70.25% for TCM and FTCM, respectively. The VLDL-cholesterol (VLDL-C) level was improved associatively with treatments in a type and dose-dependent manner. The effective therapies were TCM and FTCM at 20 mL/kg, lowering VLDL by around 50% compared to the STZ group. Data are shown in Table 4 for the efficacy of TCM and FTCM at 10 and 20 mL/kg and metformin at 50 mg/kg in treating STZ-induced diabetes in rats. Surprisingly, we found a considerable boost compared to NR when comparing AI in STZ-injected rats to normal rats. The most efficient treatments in attenuating the atherogenicity complication were TCM and FTCM at 20 mL/kg (G: TCM20 and G: FTCM20), which present a superior effect than TCM and FTCM at 10 mL/kg (G: TCM10 and G: FTCM10), or even using metformin.
The effect of TCM and FTCM on the liver’s functions
The effect of TCM and FTCM at 10 and 20 mL/kg and metformin at 50 mg/kg on liver functions in the STZ-induced DR were examined; data are illustrated in Table 5. Due to diabetes, STZ injection significantly increased blood ALT, AST, and ALP enzyme levels in STZ rats compared to the NR group. T. bili levels were significantly increased in STZ-treated rats, as shown in Table 5. Administrating TCM and FTCM at 10 and 20 mL/kg improved the liver’s function in a dose-dependent manner. Alterations in liver functions produced by STZ injection were better restored to near-normal levels in NR after treatment with high doses of TCM and FTCM compared to the lower dose [Table 5].
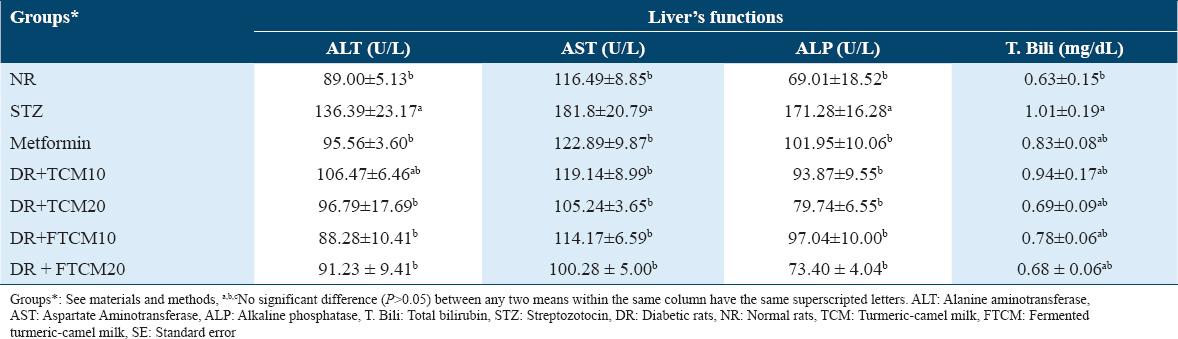
Comparing a lower dose at 10 mL/kg of TCM or FTCM with 20 mL/kg exhibited that administrating 10 mL/kg attenuated ALT by 21.93 and 35.27% against 29.03 and 33.11%, respectively. A similar trend was observed in AST, whereas 34.46 and 42.11 were recorded for TCM at 10 mL/kg and 20 mL/kg. In comparison, 37.2% and 44.84% were recorded for FTCM at 10 mL/kg and 20 mL/kg, respectively. Higher efficiency was noticed for the TCM and FTCM at 10 and 20 mL/kg on ALP. An improvement of 45.19, 53.44, 43.34, and 57.14% for TCM and FTCM at both concentrations was demonstrated, respectively. The best treatment was FTCM at 20 mL/kg since it improved liver enzymes (as shown in ALT, AST, and ALP) and various liver functions (such as T. bili) in a type and dose-dependent manner, outperforming metformin.
In contrast, T. protein, albumin, and globulin levels were drastically reduced in rats given STZ [Table 6]. TCM and FTCM at high doses significantly reduced the changes in T. protein, albumin, and globulin to near or above normal levels in NR [Table 6]. Compared to the NR group, results were significantly improved with 20 mL/kg FTCM, even better than metformin.
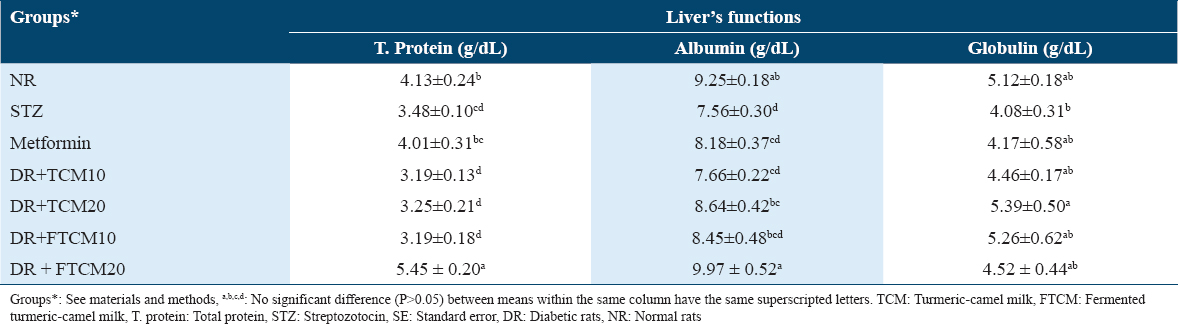
The effect efficiency of TCM and FTCM extracts on the kidneys’ functions
Table 7 shows the findings of an investigation into the nephroprotective effects of TCM and FTCM at 10 and 20 mL/kg and metformin at 50 mg/kg on rats with STZ-induced diabetes. Serum creatinine, urea, and BUN levels in STZ rats were significantly elevated compared to normal rats after STZ injection. Creatinine, urea, and BUN changes brought on by diabetes problems were significantly reduced by high dosages of TCM and FTCM. The most effective improvement was seen with 20 mL/kg FTCM, which was better than utilizing metformin compared to the NR group. The creatinine, urea, and BUN levels were attenuated dose-dependently after administration of TCM and FTCM at 10 and 20 mL/kg. Comparing a lower dose at 10 mL/kg of TCM or FTCM with 20 mL/kg exhibited that administrating 10 mL/kg attenuated creatinine by 25.58 and 27.13% against 37.20 and 34.88%, respectively. A similar trend was observed in the urea level, whereas 47.16 and 38.69 were recorded for TCM at 10 and 20 mL/kg. In comparison, 34.77% and 45.44% were recorded for FTCM at 10 and 20 mL/kg, respectively. Higher efficiency was noticed for the TCM and FTCM at 10 and 20 mL/kg on BUN. An improvement of 37.62, 42.16, 34.79, and 45.43% for TCM and FTCM at both concentrations was remarked.
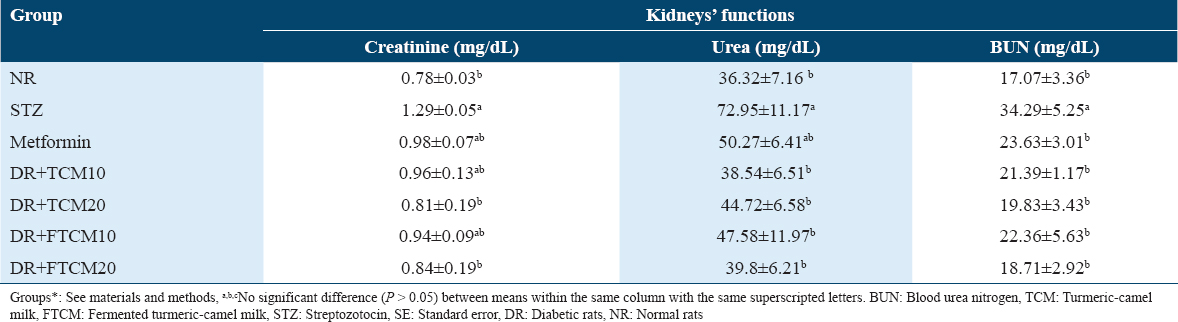
The effect efficiency of TCM and FTCM on the antioxidant biomarkers
Table 8 shows that when compared to NR, STZ-injected rats had significantly lower levels of GSH, CAT, and SOD enzymes and higher amounts of MDA in their blood serum. Antioxidant enzyme activity (GSH, CAT, and SOD) and MDA levels both increased dramatically in TCM and FTCM (10 and 20 mL/kg) and metformin (50 mg/kg) treated rats [Table 8]. TCM treatment at 20 mL/kg increased GSH, MDA, CAT, and SOD by 84.25%, 29.24%, 85.5%, and 57.76%, respectively, compared to the STZ group (STZ). Compared to the STZ-group, GSH, MDA, CAT, and SOD all improved by 102.13, 31.94, 80.76, and 83.91% after treatment with FTCM at 20 mL/kg (STZ). In addition, metformin considerably improved the enzymatic defense system compared to the STZ-rats group.
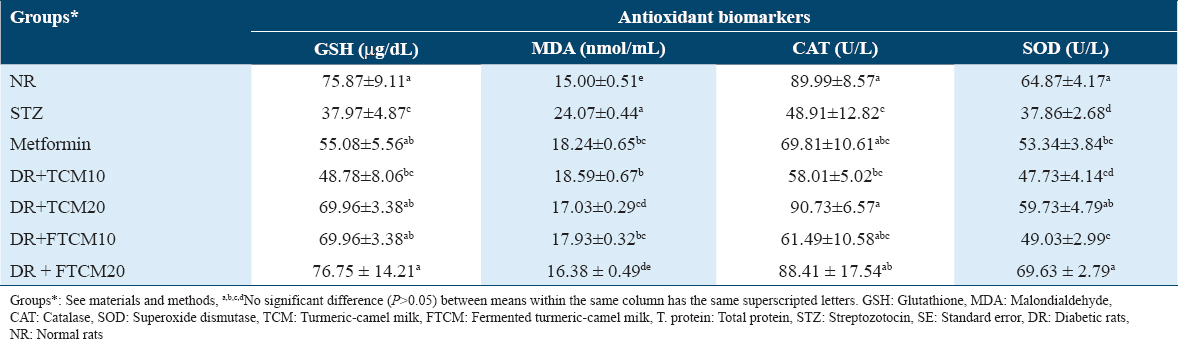
The effects of TCM and FTCM extracts on pancreas histoarchitecture
Table 9 and Figure 1 indicate the extent of histological changes in the rat pancreas after treatment with TCM at 10 and 20 mL/kg and metformin at 50 mg/kg. The control group’s pancreas had normal endocrine (islets of Langerhans cells) and exocrine (acini and ducts) histology [Figure 1, NR]. Histoarchitecture of STZ-treated rats (STZ) showed atrophy, regression in size and shape, or complete absence of the islet of Langerhans cells in most lobules (STZ.a), cystic dilatation of the duct (STZ.b), congestion in the stromal blood vessels (STZ.c), and vascular wall sclerosis (STZ.d). The fatty change was focally observed in the lining epithelium of some acini (STZ.e), and the magnification of this section showed the fatty change in epithelium cells. Only the ducts showed cystic dilatation in (metformin), but the islet of Langerhans cells did not change histopathologically. Histopathological study of islets of Langerhans cells and acini after 10 mL/kg TCM and FTCM showed no alterations [Figure 1, TCM10 and FTCM10]. Histopathological study of islets of Langerhans cells and acini after 20 mL/kg TCM and FTCM showed no alterations [Figure 1, TCM20, and FTCM20].
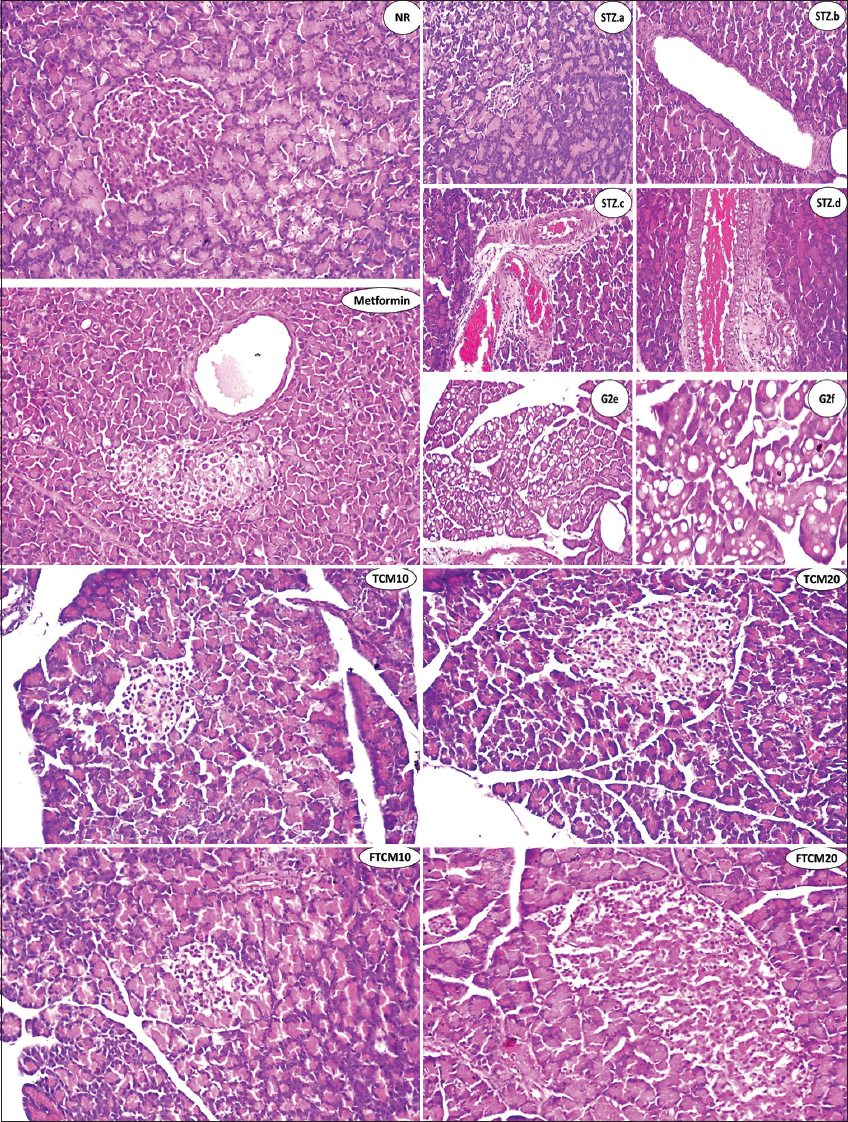
- NR group. There was no histopathological alteration, and the typical histological structure of the islets of Langerhans was recorded as an endocrine portion. The acini and ducts system was recorded as an exocrine one. STZ group, a-f. Showing atrophy and regression in size as well as the shape of the islet of Langerhans cells (STZ.a), showing cystic dilatation of the duct (STZ.b), showing severe congestion in stromal blood vessels between the lobules (STZ.c), showing sclerosis in the wall of congested stromal blood vessels (STZ.d), showing fatty change in the lining epithelium of some acini in the lobules (STZ.e), and magnification of this section to identify the fatty change in epithelium cells was shown (STZ.f). In the metformin group, there was no histopathological alteration in the islet of Langerhans cells, while the ducts showed cystic dilatation. In the TCM10 group, there was moderate size and shape in the islet of Langerhans cells as detected. In the TCM20 group, no histopathological alteration was recorded. In the FTCM10 group, the islet of Langerhans cells showed a small size and shape with regression in the distribution all over the lobules. In the FTCM20 group, there was an increase in the size, shape, and distribution of Langerhans cells in the is let all over the lobules. TCM: Turmeric-camel milk, FTCM: Fermented turmeric-camel milk, TSTZ: Streptozotocin, NR: Normal rats
Discussion
Turmeric milk, known as “golden milk” worldwide, has become popular as people demand natural and healthful meals. Traditional drinks like turmeric milk heal sore throats and fever at home.[50] Duodenal ulcers, asthma, malaria, cough, and cold can be treated with boiled turmeric milk.[51,52] Commercial golden milk is made from cow’s or soybean milk.[52] Golden milk is becoming popular worldwide due to its widespread consumption. Make a CM-based drink with fermented and unfermented turmeric to create a natural product with outstanding preventative and therapeutic properties. This study aimed to combine CM with turmeric and other substances and evaluate its antioxidant and antidiabetic capabilities.[52] The valuable phytochemicals content and antioxidant activities in TCM and FTCM indicated rich polyphenols with high antioxidant activity. Turmeric and additional ingredients added nutritional value and increased biologically active components in TCM. CM is well known to possess antioxidant activity in vitro and in vivo.[53,54] Interestingly, many researchers have confirmed the super antioxidant activity, anti-inflammatory, and immune-boosting efficiency of turmeric (C. longa)[55,56] ginger (Z. officinale L.), cinnamon (C. burmannii L.)[57] clove (S. aromaticum), cardamom (E. cardamomum), and pepper (P. nigrum).[58] Antioxidants like phenolics break down lipid oxidation chain reactions and supply hydrogen to active free radicals. Phenolic hydroxyl groups scavenged and inhibited radicals.[59,60]
Phenolic acids suppress hydrogen peroxide, hydroxyl radicals, and superoxide anion production.[61,62] Idowu-Adebayo et al.[52] indicated that the TPC and AOA ranged from 0.01 to 0.147 GAE g/kg and 7.5 to 17.7 TEAC mmol Trolox/kg samples, respectively. Overall, turmeric added nutritional and chemical value to all the models. However, turmeric-fortified soya milk samples showed the highest protein, iron, zinc, TPC, and antioxidant activity. TCM and FTCM’s metal chelating activity appears to interfere with the Fe2+-ferrozine complex, suggesting they can absorb ferrous ions before ferrozine.
Quantifying phenolic acids and flavonoids in TCM and FTCM showed high levels. The identified phenolics kindes or numbers are a base study as no data about TCM or FTCM were available. A quantitative examination of TCM and FTCM phenolics found eleven acids and four flavonoids in identifiable levels. The most abundant hydroxy-benzoic acid was ellagic acid followed by GA. In contrast, pyrocatechol and chlorogenic acid were observed in a moderate amount. Furthermore, cinnamic acid, vanillic acid, coumaric acid, and caffeic acid were detected. The TCM and FTCM were rich in flavonoid content; catechin and quercetin were detected in higher amounts, followed by daidzein and hesperetin.
Interestingly, the presented data in our study are a pioneering result in this topic and have not been analyzed yet. Thus, there are no findings available to compare with our results. However, Sepahpour et al.[63] studied the effects of various solvents on the extractability and the number of polyphenols in turmeric and some other spices. Curcumin, desmethoxycurcumin, bis-desmethoxycurcumin, rutin, quercetin-3-glycoside, myricetin, quercetin, chlorogenic acid, caffeic acid, p-coumaric acid, and luteolin-7-o-glycoside could be quantifiable compounds. The HPLC profile of turmeric extraction revealed that curcuminoids predominated. Yang et al.[64] indicated that turmeric extracts could be considered a potential source of bioactive compounds for treating cancer-related diseases. During phenolic identification, GA, protocatechuic acid, catechin, chlorogenic acid, epicatechin, ferulic acid, coumarin, rutin, curcumin, myricetin, cinnamic acid, genistein, and quercetin were quantified. Indeed, the differences between the extraction yield of bioactive compounds in our finding and the previous study were likely due to different methods and solvents, extraction, and the turmeric content in TCM.
The results show that STZ, as a diabetes induction agent, exhibits weight loss and decreasing immune organ index in animal models. Due to its cytotoxic nature, it could cause hepatic damage and urotoxicity associated with internal organs.[65] Yu et al.[66] indicated a significant body weight loss in the first 3 days of their experiment. However, oral administration of TCM or FTCM significantly attenuated the body weight, especially in groups administered 20 mL/kg. This finding confirmed that TCM and FTCM helped to improve the bodyweight recovery of STZ-induced rates, as mentioned by Zeng et al.[67] and Hussain et al.[68] Significant improvements were found in kidneys, liver, and spleen relative organ weights after giving TCM and FTCM, especially with 20 mL/kg.[69] The results indicated a significant attenuation in STZ-treated rats after administering FTCM or TCM compared with the STZ group. This may be due to the efficiency of GCM and probiotics as immune-boosting agents.[66,70,71] Previous studies have also reported dose-dependently increased spleen and thymus indexes.[66,67] Based on the above results, TCM and FTCM could significantly increase the immune organ indexes and body weight, restoring the immune function by repairing the STZ-induced damage to immune organs, as previously remarked. Immunomodulatory functions of numerous plant-based derivatives[66,67,72] and CM[54] have been approved.
However, chronic hyperglycemia causes most diabetic complications. Chronic hyperglycemia may cause metabolic abnormalities and oxidative damage in diabetes.[73,74] TCM and FTCM decreased RBG and FBG in experimental rats in a dose- and type-dependent way, as established in our recent in vivo investigation.[75,76] These findings support our study’s results, confirming that CM possesses hypoglycemic effects. TCM and FTCM strongly reduced RBG and FBG because they have polyphenols as effective antioxidants and may have a metformin-like impact, which is helpful in diabetes prevention.[77-79] As a result, TCM and FTCM may have helped diabetic animals restore cognitive function by reducing hyperglycemia.[80]
STZ-DR have elevated blood TGs and cholesterol, indicating decreased fat metabolism due to diabetes;[76] a histopathological examination confirmed this finding. TCM and FTCM at 10 and 20 mL/kg considerably reduced lipid profile alterations.[76,81] TG, CHO, LDL, and VLDL levels improved synergistically with a high dose of TCM and FTCM. Potential causes include phenols, antioxidants, and carotenoids compounds.[82-84] TCM and FTCM may lower cholesterol and enhance lipid profile, which may help manage insulin levels and reduce diabetic complications, according to some clinical trials.[85,86]
Compared to the negative control group, treatment groups had significant liver diagnostic marker alterations (G1). STZ increased ALT, AST, ALP, T. bili, and direct bilirubin in G2 as a normal liver damage deterioration in diabetes mellitus (DM), metformin, TCM, and FTCM at 10 and 20 mL/kg significantly suppressed such increments in groups 3, 4, 5, 6, and 7, while high doses of TCM and FTCM significantly decreased them in G5 and G7. TCM and FTCM include high levels of phenolic acids and flavonoid components, which are liver-friendly and anti-inflammatory and possess attenuation capacity.[83,86,87]
As STZ-injected mice had significantly higher blood glucose levels, their renal function decreased. Higher blood glucose levels harm kidney filtering units, causing kidney failure.[88] Thus, DM became a main cause of diabetic nephropathy, an end-stage kidney disease.[89] Table 7 shows that DR orally treated TCM and FTCM at 10 and 20 mL/kg recovered all liver functions dose-dependently. G7 had significantly higher T. protein, albumin, and globulin and lower creatinine, urea, and BUN than all groups, including the negative control groups (G1) and (G3) with metformin. As previously mentioned, carnosic acid, rosemarinic acid, caffeic acid, and essential oil protect the body from oxidative stress and free radical attack, which helps TCM and FTCM restore kidney function. However, this extract may reduce hepatic glucose generation and increase insulin action in normal and diabetic mice, improving kidney function.[86,88]
Antioxidant enzymes, such as CAT and SOD, and antioxidant peptides, such as GSH, play a critical role in protecting host tissue against oxidative stress initiated by superoxide anions.[90] STZ treatment lowered GSH, SOD, and CAT and increased MDA in DR’ serum compared to normal rats.[73] GSH is a non-enzymatic antioxidant found in all mammals. GPx, GST, and other detoxifying enzymes use GSH’s oxidized form, GSSG, to fight oxidative stress and maintain cellular redox balance.[91] SOD also dismutates two molecules of superoxide anion to hydrogen peroxide and molecular oxygen, making it less toxic.[92] MDA is the initial lipid peroxidation product and a key oxidative stress marker. The catabolite malondialdehyde marker shows ROS damages tissues and peroxidizes lipids.[93] Lipid peroxidation is a well-recognized oxidative stress mechanism in animal tissues and is generated by reactive oxygen species. MDA is a lipid peroxidation end product involved in forming lipid radicals and oxygen uptake and is a marker for endogenous lipid peroxidation.[94] High doses of STZ cause liver injury and hematologic alterations due to an elevation of oxidative stress[95] associated with excessive ROS production.[96] As previously reported, STZ administration lowered GSH, SOD, and CAT and elevated MDA in rats’ serum compared to NR. TCM and FTCM restored SOD, CAT, and GSH activity, alleviating STZ’s varied effects. It may stop MDA production. CM and FCM with turmeric and spices may be effective due to their antioxidant properties. Shakeri and Boskabady[71] proved the preventive effect of curcumin on inflammatory cells, inflammatory mediators, oxidative stress, and immunomodulatory effects in rats. As recently confirmed, its more specific immunomodulatory effect caused increased Th1/Th2 balance, with promising therapeutic potential against asthma disease.[50] The possible mechanisms of GCM in their antioxidant activities contain two hypotheses. First, antioxidants could directly mediate free radical activity by interacting with different membrane receptors and/or modulating various post-receptor intracellular signaling pathways.[50,71] Second, antioxidants may indirectly exert their antioxidant effects, such as prebiotics, by contributing to the synthesis and release of antioxidants by probiotic bacteria and inhibiting inflammation.[70,71]
In our recent study, TCM and FTCM exhibited antioxidant activities in vitro and in vivo. The oral administration of TCM and FTCM showed significant antioxidant activity in vivo, but the detailed mechanism needs further study. Evidence suggests that antioxidants have immunomodulatory activity. Moreover, it has been proved that the ingredients included could act as prebiotics to promote the growth and metabolism of probiotics. Butyrate, one of the microbial metabolites, could activate immune effector molecules to enhance intestinal mucosal immunity.[72]
Meanwhile, intestinal microbiota could modulate oxidative stress by releasing antioxidants.[73] Antioxidants could maintain the membrane fluidity of cells, which is beneficial for exerting the immune response.[74] Interestingly, CM peptides have a strong antioxidant potential that reduces the effects of oxygen free radicals and lipid peroxidation by orchestrating the overall antioxidant system to the optimum in vivo.[53] Moreover, it may aid in the recovery of crucial antioxidant enzymes that play critical roles in innate immune responses, implying an immunopotentiation effect in diseases or conditions linked with leukopenia or drug-induced toxicity.[54]
TCM and FTCM at 10 and 20 mL/kg and metformin at 50 mg/kg restored antioxidant activities such SOD, CAT, and GSH, alleviating STZ effects. It may stop MDA production.[75] As previously approved, TCM and FTCM have a metformin-like action, preventing oxidation better than metformin at 10 and 20 mL/kg.[77] In this work, oral TCM and FTCM reduced lipid peroxidation and raised SOD and CAT levels in STZ-DR;[75] a high dose of TCM markedly increased the efficiency, and FTCM were given, presenting small molecules of “metformin-like” substances in addition to rich phenolic content that capable of modulating diabetes and attenuating its complications.[78,79,81] In STZ-treated rats, TCM and FTCM reduced MDA and restored antioxidant power. TCM and FTCM’s high antioxidative action in the presence of rich polyphenols may successfully reduce oxidative stress consequences.[62,77,84]
As appeared in the pancreas histoarchitectures under the present study, photomicrographs of the G1 section show normal histological structure. The decrease in size and irregular borders of normally developed islets of Langerhans in G2 (a and b) compared to G1 has markedly appeared. As a positive control among study groups, the STZ-treated rats (45 mg/kg, i.p.), histoarchitecture of the STZ-treated rats (STZ) showed atrophy, regression in size as well as shape or complete absence of the islet of Langerhans cells noticed in most of the lobules, cystic dilatation of the duct was shown, associated with congestion in the stromal blood vessels as shown, as well as sclerosis the vascular wall. The fatty change was detected in the lining epithelium of some acini in a focal manner, and fatty change in epithelium cells was shown. Thus, STZ seemed highly toxic to the pancreatic cells, severely damaging the β-cells.[97] STZ has been shown to significantly lower β-cell mass and pancreatic islet volume in experimental animals.[98]
Moreover, El-Sheikh et al.[99] indicated that DR exhibit significant damage and loss of architecture in their pancreatic cells, resulting in atrophy of the islets of Langerhans and a decrease in β-cell number and size. A similar result was explained by Abunasef et al.[100] and Alharbi et al.[101] who mentioned that STZ produced severe degenerative alterations by lowering islet size and quantity, especially in the core. In metformin, only the ducts displayed cystic dilatation, while the islets of Langerhans cells did not change histopathologically. Restoring Langerhans activity is crucial to treating DM,[102] and targeting pancreatic β-cells is a possible intervention for diabetes treatment.[22] TCM and FTCM also helped STZ-DR. The injection of TCM and FTCM restored insulin production in DR, indicating that Langerhans islet’s β-cell function was restored. This may be because TCM and FTCM extract are high in phenolic and flavonoid components.[103,104]
Both islets of Langerhans cells and acini showed no histopathological changes after 10 or 20 mL/kg TCM administration. The islets of Langerhans cells and acini revealed no histological changes after 20 mL/kg FTCM, although the cells increased in size, shape, and distribution across the lobules. Indeed, to accurately define the antidiabetic potential of TCM and FTCM, collected histopathological data and biochemical examination data should be combined and discussed in parallel. As previously remarked, the potential antidiabetic of CM was confirmed[105-107] and agreed with our results. Therefore, consuming or incorporating TCM and FTCM into diet or feed is highly encouraged. Importantly, different bacteria and the metabolites they produce (postbiotics) can play a role in combating metabolic syndrome diseases. For example, patients with type 2 diabetes have reduced levels of SCFA-producing bacteria, and some SCFAs (e.g., butyrate) facilitate improved insulin sensitivity, muscle fatty acid oxidation, and increased satiety.[17] FCM by Lactococcus lactis subsp. cremoris increased SOD, CAT, and GPx activity, oxidative stress, and decreased cardiac damage induced by acute exposure of mouse heart tissues to CCl4.[18] Fermented CM protects heart tissue due to its antioxidant chemical composition. Examples include oligosaccharides, vitamins, bioactive peptides, and conjugated linoleic acid.[19] Fermented CM contains lots of magnesium, which aids GSH biosynthesis.[20] It may be a good explanation of the efficiency of FCM and FTCM as antidiabetes and antioxidative stress agents.
Conclusion
The antioxidant, antidiabetic, and hypolipidemic effects of TCM and FTCM were investigated in rats with STZ-induced type 2 diabetes and oxidative stress. FTCM and TCM have pungent phenolics and super antioxidants. HPLC study revealed ten phenolic acids and four flavonoids, with ferulic acid and resveratrol dominating. A 6-week animal model with seven treatment groups investigated TCM and FTCM at 10 and 20 mL/kg for antidiabetic and hypolipidemic effects. RBG, FBG, liver, kidney, antioxidant biomarkers, and histology were examined. TCM and FTCM significantly decreased RBG, FBG, and weight gain recovery. TCM and FTCM at 20 mL/kg significantly bettered hypolipidemia more than 10 mL/kg, reducing TG, CHO, HDL-C, LDL-C, and VLDL-C. TCM and FTCM at 20 mL/kg enhanced liver and kidney functions more than metformin or 10 mL/kg. Amazingly, TCM and FTCM dose-dependently increased antioxidant enzyme activity, such as GSH, CAT, and SOD, and decreased MDA levels. Histopathologically, TCM and FTCM at 10 and 20 mL/kg showed normal Langerhans cell and acini structure, outperforming metformin. Finally, turmeric-CM TCM and FTCM exhibited therapeutic efficacy and could be profitable for diabetes complications and oxidative stress.
Ethical Approval
The study was approved by the Committee of Research Ethics, Deanship of Scientific Research, Qassim University (Approval No. 22-06-03 on Monday, October 10, 2022), SA.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Authors’ Contributions
Conceptualization, H.B., and Y.M.A.; methodology, H.B., and M.A.A.; investigation, H.B., and M.A.A.; data curation, H.B, and Y.M.A.; writing – original draft preparation, H.B., and M.A.A.; review and editing, H.B., and Y.M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by the Deanship of Scientific Research, Qassim University, Saudi Arabia, under the number (CAVM-2022-1-2-J-29785).
Acknowledgments
The authors gratefully acknowledge Qassim University, represented by the Deanship of Scientific Research, on the financial support for this research under the number (CAVM-2022-1-2-J-29785) during the academic year 1444 AH/2022 AD.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Trends of the prevalence of pre-gestational diabetes in 2030 and 2050 in Belgrade cohort. Int J Environ Res Public Health. 2022;19:6517.
- [Google Scholar]
- Use of insulin degludec/insulin aspart in the management of diabetes mellitus:Expert panel recommendations on appropriate practice patterns. Front Endocrinol (Lausanne). 2021;12:616514.
- [Google Scholar]
- Genetics of diabetes mellitus and diabetes complications. Nat Rev Nephrol. 2020;16:377-90.
- [Google Scholar]
- Comparative effects of microvascular and macrovascular disease on the risk of major outcomes in patients with type 2 diabetes. Cardiovasc Diabetol. 2017;16:95.
- [Google Scholar]
- Processing challenges and opportunities of camel dairy products. Int J Food Sci. 2017;2017:9061757.
- [Google Scholar]
- Effect of heat treatment on camel milk proteins with respect to antimicrobial factors:A comparison with cows'and buffalo milk proteins. Food Chem. 2000;68:227-32.
- [Google Scholar]
- Nutritional and Medicinal Value of Camel (Camelus dromedarius) Milk. England: WIT Press; 2013.
- [Google Scholar]
- Antigenotoxic and anticytotoxic effect of camel milk in mice treated with cisplatin. Saudi J Biol Sci. 2010;17:159-66.
- [Google Scholar]
- Antibacterial and antiviral activity of camel milk protective proteins. J Dairy Res. 1992;59:169-75.
- [Google Scholar]
- Evaluating the nutritional and immune potentiating characteristics of unfermented and fermented turmeric camel milk in cyclophosphamide-induced immunosuppression in rats. Antioxidants (Basel). 2022;11:792.
- [Google Scholar]
- Lactoferrin of camel milk of Kazakhstan. In: Desertification Combat and Food Safety:The Added Value of Camel Producers. Vol Vol. 362. Amsterdam: IOS Press; 2005. p. :158-67.
- [Google Scholar]
- Probiotic-enriched milk and dairy products increase gut microbiota diversity:A comparative study. Nutr Res. 2020;82:25-33.
- [Google Scholar]
- A 100-year review:Yogurt and other cultured dairy products. J Dairy Sci. 2017;100:9987-10013.
- [Google Scholar]
- Beneficial health effects of milk and fermented dairy products--review. Folia Microbiol (Praha). 2008;53:378-94.
- [Google Scholar]
- A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55-60.
- [Google Scholar]
- Intestinal microbiota and faecal transplantation as treatment modality for insulin resistance and type 2 diabetes mellitus. Clin Exp Immunol. 2014;177:24-9.
- [Google Scholar]
- Beneficial effects of fermented camel milk by Lactococcus lactis subsp cremoris on cardiotoxicity induced by carbon tetrachloride in mice. Biomed Pharmacother. 2018;97:107-14.
- [Google Scholar]
- Impact on human health of microorganisms present in fermented dairy products:An overview. Biomed Res Int. 2015;2015:412714.
- [Google Scholar]
- Effect of metformin on glutathione and magnesium in normal and streptozotocin-induced diabetic rats. J Appl Toxicol. 1995;15:387-90.
- [Google Scholar]
- How does ascorbic acid prevent endothelial dysfunction? Free Radic Biol Med. 2000;28:1421-9.
- [Google Scholar]
- Camel milk as a potential therapy for controlling diabetes and its complications:A review of in vivo studies. J Food Drug Anal. 2015;23:609-18.
- [Google Scholar]
- Turmeric (Curcuma longa L.). volatile oil inhibits key enzymes linked to type 2 diabetes. Int J Food Sci Nutr. 2012;63:832-4.
- [Google Scholar]
- Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care. 2003;26:3215-8.
- [Google Scholar]
- Cloves improve glucose, cholesterol and triglycerides of people with type 2 diabetes mellitus. FASEB J. 2006;20:A990.
- [Google Scholar]
- Effect of cinnamon, cardamom, saffron and ginger consumption on blood pressure and a marker of endothelial function in patients with type 2 diabetes mellitus:A randomized controlled clinical trial. Blood Press. 2016;25:133-40.
- [Google Scholar]
- An herbal formulation containing Zingiber officinale rhizomes and Allium sativum cloves can increase oral glucose tolerance in mice. Biomed Mater Eng. 2017;2:1-3.
- [Google Scholar]
- Turmeric and curcumin:Biological actions and medicinal applications. Curr Sci. 2004;87:44-53.
- [Google Scholar]
- Phytotherapeutic potential of bi-herbal extract of cinnamon and turmeric:In vivo antidiabetic studies. Clin Phytosci. 2021;7:38.
- [Google Scholar]
- Antioxidant activity of various extracts and fractions of Chenopodium quinoa and Amaranthus spp. Seeds. Food Chem. 2008;106:760-6.
- [Google Scholar]
- Effects of different cooking methods on health-promoting compounds of broccoli. J Zhejiang Univ Sci B. 2009;10:580-8.
- [Google Scholar]
- Phenolics extracted from potato, sugar beet, and sesame processing by-products. Int J Food Prop. 2013;16:1148-68.
- [Google Scholar]
- In vitro antioxidant activities of methanol extracts of five phyllanthus species from India. LWT Food Sci Technol. 2007;40:344-52.
- [Google Scholar]
- Evolution of phenolic compounds and antioxidant activity during malting. J Agric Food Chem. 2007;55:10994-1001.
- [Google Scholar]
- Screening of plant extracts for antioxidant activity:A comparative study on three testing methods. Phytochem Anal. 2002;13:8-17.
- [Google Scholar]
- Effects of extraction solvent mixtures on antioxidant activity evaluation and their extraction capacity and selectivity for free phenolic compounds in barley (Hordeum vulgare L.) J Agric Food Chem. 2006;54:7277-86.
- [Google Scholar]
- Phenolic acid profiles and antioxidant activities of wheat bran extracts and the effect of hydrolysis conditions. Food Chem. 2006;95:466-73.
- [Google Scholar]
- Renoprotective effect of aged garlic extract in streptozotocin-induced diabetic rats. Indian J Pharmacol. 2013;45:18-23.
- [Google Scholar]
- An improved colour reagent for the determination of blood glucose by the oxidase system. Analyst. 1972;97:142-5.
- [Google Scholar]
- National cholesterol education program recommendations for triglyceride measurement:Executive summary. The national cholesterol education program working group on lipoprotein measurement. Clin Chem. 1995;41:1421-6.
- [Google Scholar]
- Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499-502.
- [Google Scholar]
- Atherogenic index of plasma as useful predictor of cardiovascular risk among postmenopausal women in Enugu, Nigeria. Afr Health Sci. 2010;10:248-52.
- [Google Scholar]
- Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61:882-8.
- [Google Scholar]
- Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351-8.
- [Google Scholar]
- Superoxide dismutases:I Occurrence in higher plants. Plant Physiol. 1977;59:309-14.
- [Google Scholar]
- Principles and Procedures of Statistics:A Biometrical Approach. USA, Boston: McGraw-Hill; 1997.
- [Google Scholar]
- The effect of Curcuma longa on inflammatory mediators and immunological, oxidant, and antioxidant biomarkers in asthmatic rats. Evid Based Complement Alternat Med. 2021;2021:4234326.
- [Google Scholar]
- Effects of bioactive substance from turmeric on growth, skin mucosal immunity and antioxidant factors in common carp, Cyprinus carpio. Fish Shellfish Immunol. 2019;92:612-20.
- [Google Scholar]
- Turmeric-fortified cow and soya milk:Golden milk as a street food to support consumer health. Foods. 2022;11:558.
- [Google Scholar]
- Camel milk peptide improves wound healing in diabetic rats by orchestrating the redox status and immune response. Lipids Health Dis. 2015;14:132.
- [Google Scholar]
- Immune potentiating and antitoxic effects of camel milk against cyclophosphamide-induced toxicity in BALB/C mice. Int J Health Sci (Qassim). 2017;11:18-22.
- [Google Scholar]
- Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa:A review of preclinical and clinical research. Altern Med Rev. 2009;14:141-53.
- [Google Scholar]
- The therapeutic potential of curcumin:A review of clinical trials. Eur J Med Chem. 2019;163:527-45.
- [Google Scholar]
- Study of antioxidant, antibacterial and anti-inflammatory activity of cinnamon (Cinnamomum tamala), ginger (Zingiber officinale) and turmeric (Curcuma longa) Am J Life Sci. 2013;1:273-77.
- [Google Scholar]
- Antioxidant activity of selected Indian spices. Prostaglandins Leukot Essent Fatty Acids. 2000;62:107-10.
- [Google Scholar]
- Extraction of sage (Salvia officinalis L.) by pressurized hot water and conventional methods:Antioxidant activity of the extracts. Eur Food Res Technol. 2002;215:158-63.
- [Google Scholar]
- Antioxidant properties and total phenolic content of eight Salvia species from Turkey. Biol Res. 2009;42:175-81.
- [Google Scholar]
- Antioxidant potential of Salvia officinalis l. Residues as affected by the harvesting time. Ind Crops Prod. 2014;54:78-85.
- [Google Scholar]
- Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts. Ind Crops Prod. 2013a;43:827-31.
- [Google Scholar]
- Comparative analysis of chemical composition, antioxidant activity and quantitative characterization of some phenolic compounds in selected herbs and spices in different solvent extraction systems. Molecules. 2018;23:402.
- [Google Scholar]
- Phenolic profiles, antioxidant, and antiproliferative activities of turmeric (Curcuma longa) Ind Crops Prod. 2020;152:112561.
- [Google Scholar]
- Immunomodulatory effects of phosphorylated radix Cyathulae officinalis polysaccharides in immunosuppressed mice. Molecules. 2019;24:4150.
- [Google Scholar]
- Immunomodulatory activity of low molecular-weight peptides from Nibea japonica skin in cyclophosphamide-induced immunosuppressed mice. J Funct Foods. 2020;68:103888.
- [Google Scholar]
- Immune enhancement and antioxidant effects of low molecular-weight peptides derived from Nibea japonica muscles on immune-deficient mice induced by cyclophosphamide. Process Biochem. 2021;102:42-50.
- [Google Scholar]
- Protective effects of Picrorhiza kurroa on cyclophosphamide-induced immunosuppression in mice. Pharmacognosy Res. 2013;5:30-5.
- [Google Scholar]
- Ovotransferrin enhances intestinal immune response in cyclophosphamide-induced immunosuppressed mice. Int J Biol Macromol. 2018;120:1-9.
- [Google Scholar]
- Dietary litchi pulp polysaccharides could enhance immunomodulatory and antioxidant effects in mice. Int J Biol Macromol. 2016;92:1067-73.
- [Google Scholar]
- Anti-inflammatory, antioxidant, and immunomodulatory effects of curcumin in ovalbumin-sensitized rat. Biofactors. 2017;43:567-76.
- [Google Scholar]
- The role of intestinal microbiota in the development and severity of chemotherapy-induced mucositis. PLoS Pathog. 2010;6:e1000879.
- [Google Scholar]
- Diabetes, oxidative stress, and antioxidants:A review. J Biochem Mol Toxicol. 2003;17:24-38.
- [Google Scholar]
- Dietary antioxidants:Immunity and host defense. Curr Top Med Chem. 2011;11:1752-66.
- [Google Scholar]
- Potential antidiabetic effect of camel milk. In: Handbook of Research on Health and Environmental Benefits of Camel Products. United States: IGI Global; 2020. p. :185-96.
- [Google Scholar]
- Improved glycemic control and lipid profile in hyperlipidemic type 2 diabetic patients consuming Salvia officinalis L leaf extract:A randomized placebo. Controlled clinical trial. Complement Ther Med. 2013;21:441-6.
- [Google Scholar]
- Metformin-like effect of Salvia officinalis (common sage):Is it useful in diabetes prevention? Br J Nutr. 2006;96:326-33.
- [Google Scholar]
- A comparative study on antidiabetic effect of buffalo and camel fermented milk in induced diabetic rats. Adv Food Sci. 2017;39:124-32.
- [Google Scholar]
- Systematic review of clinical trials assessing pharmacological properties of Salvia species on memory, cognitive impairment and Alzheimer's disease. CNS Neurosci Ther. 2014;20:485-95.
- [Google Scholar]
- Antidiabetic effect of milk fermented using intestinal probiotics. Nutr Food Sci. 2019;49:1063-74.
- [Google Scholar]
- Effect of Salvia officinalis L. leaves on serum glucose and insulin in healthy and streptozotocin-induced diabetic rats. J Ethnopharmacol. 2005;100:310-3.
- [Google Scholar]
- Pharmacological properties of Salvia officinalis and its components. J Tradit Complement Med. 2017;7:433-40.
- [Google Scholar]
- Antioxidant capacity and polyphenolic composition as quality indicators for aqueous infusions of Salvia officinalis L. (sage tea). Front Pharmacol. 2011;2:79.
- [Google Scholar]
- Effects of Salvia officinalis L (common sage) leaves tea on insulin resistance, lipid profile, and oxidative stress in rats with polycystic ovary:An experimental study. Avicenna J Phytomed. 2020;10:263-72.
- [Google Scholar]
- Phytochemical and biochemical studies of sage (Salvia officinalis l.) Int J Pharma Bio Sci. 2016;5:56-62.
- [Google Scholar]
- Study the molecular mechanism of salvia species in prevention of diabetes. IJPSR. 2018;9:4512-21.
- [Google Scholar]
- Interactions between kidney disease and diabetes:Dangerous liaisons. Diabetol Metab Syndr. 2016;8:50.
- [Google Scholar]
- Evaluation of serum glucose and kidney disease progression among patients with diabetes. JAMA Netw Open. 2021;4:e2127387.
- [Google Scholar]
- Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44-84.
- [Google Scholar]
- Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1-40.
- [Google Scholar]
- Characterization of lipid oxidation products in quinoa (Chenopodium quinoa) Food Chem. 2007;101:185-92.
- [Google Scholar]
- Study on Dendrobium officinale o-acetyl-glucomannan (dendronan®):Part vi. Protective effects against oxidative stress in immunosuppressed mice. Food Res Int. 2015;72:168-73.
- [Google Scholar]
- Gamma-glutamylcysteine ethyl ester protects against cyclophosphamide-induced liver injury and hematologic alterations via upregulation of pparg and attenuation of oxidative stress, inflammation, and apoptosis. Oxid Med Cell Longev. 2016;2016:4016209.
- [Google Scholar]
- Casein glycomacropeptide hydrolysate exerts cytoprotection against H2O2-induced oxidative stress in raw 264.7 macrophages via ros-dependent heme oxygenase-1 expression. RSC Adv. 2015;5:4511-23.
- [Google Scholar]
- Comparative evaluation of pancreatic histopathology of rats treated with olanzapine, risperidone and streptozocin. Braz J Pharm Sci. 2018;54:1-5.
- [Google Scholar]
- Quantitative assessment of proliferative effects of oral vanadium on pancreatic islet volumes and beta cell numbers of diabetic rats. Iran Biomed J. 2016;20:18-25.
- [Google Scholar]
- Cooperation of Nicotinamide with mesenchymal stem cells to control diabetes mellitus-induced by streptozotocin in rats. J Sci Res Sci. 2015;32:250-68.
- [Google Scholar]
- A histological and immunohistochemical study of beta cells in streptozotocin diabetic rats treated with caffeine. Folia Histochem Cytobiol. 2014;52:42-50.
- [Google Scholar]
- Antioxidative, antidiabetic, and hypolipidemic properties of probiotic-enriched fermented camel milk combined with Salvia officinalis leaves hydroalcoholic extract in streptozotocin-induced diabetes in rats. Antioxidants (Basel). 2022;11:668.
- [Google Scholar]
- Protective effect of camel milk as anti-diabetic supplement:Biochemical, molecular and immunohistochemical study. Afr J Tradit Complement Altern Med. 2017;14:108-19.
- [Google Scholar]
- Evaluation of in vitro a-amylase and a-glucosidase inhibitory potential and hemolytic effect of phenolic enriched fractions of the aerial part of Salvia officinalis L. Diabetes Metab Syndr. 2020;14:689-94.
- [Google Scholar]
- Evaluation of bioactive properties and phenolic compounds in different extracts prepared from Salvia officinalis L. Food Chem. 2015;170:378-85.
- [Google Scholar]
- Inhibitory effect of effective fraction of Salvia officinalis on aldose reductase activity:Strategy to reduce complications of type 2 diabetes. Orient Pharm Exp Med. 2019;19:211-6.
- [Google Scholar]
- Preventive effects of Salvia officinalis L against learning and memory deficit induced by diabetes in rats:Possible hypoglycaemic and antioxidant mechanisms. Neurosci Lett. 2016;622:72-7.
- [Google Scholar]
- An investigation of the inhibitory effects of dichloromethane and methanol extracts of Salvia macilenta, Salvia officinalis, Salvia santolinifola and Salvia mirzayanii on diabetes marker enzymes, an approach for the treatment diabetes. Clin Phytosci. 2022;8:7.
- [Google Scholar]







