Translate this page into:
Assessing the impact of intermittent fasting and a low-carbohydrate-high-protein diet on metabolic health and pancreatic histopathology in type 2 diabetic rat model
Address for correspondence: Dr. Essam M. Hamad, Department of Food Science and Human Nutrition, College of Agriculture and Food, Qassim University, 51452 Buraidah, Saudi Arabia. Phone: +966 55 311 5819. E-mail: e.hamad@qu.edu.sa
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Qassim Uninversity and was migrated to Scientific Scholar after the change of Publisher.
Abstract
ABSTRACT
Objective:
The current study was conducted to investigate the effect of intermittent fasting (IF) with a low-carbohydrate-high-protein (LCHP) diet on blood glucose control in streptozotocin (STZ)-nicotinamide-induced type 2 diabetic rats (DR).
Methods:
Thirty male Wistar rats were divided into six groups (n = 5) including a group of normal rats (NR) that received a control diet (CD) (50% carbohydrates, 17% protein, and 33% fat) with ad libitum (AL) feeding. The remaining 5 groups were DR injected with STZ and fed on CD or LCHP diet (40% carbohydrates, 30% protein, and 30% fat) for 6 weeks, either AL or IF (with a time-restricted feeding of 16 h followed by 8 h feeding period). There was a standard control group treated with metformin and fed on CD with AL feeding. A random blood glucose was measured. Changes in body weight and feed intake (FI) were monitored weekly.
Results:
Feeding rats on LCHP and IF and their combination significantly reduced FI, body weight gain, blood glucose (P < 0.001), and improved insulin resistance (P < 0.05) with no effect on the insulin levels (P > 0.05). LCHP and IF decreased the levels of triglycerides and very-low-density lipoprotein and showed a possible protection against atherosclerosis by reducing the atherogenic index (P < 0.01). Furthermore, LCHP+IF greatly alleviates the pancreatic histopathological changes induced by STZ and showed the normal histological structure of the Langerhans islets.
Conclusion:
IF with a LCHP diet could be effectively used in improving the indicators of glucose control, and reversing pancreatic histopathological alterations in type 2 diabetes.
Keywords
Low-carbohydrate-high-protein diet
intermittent fasting
streptozotocin
insulin resistance
pancreatic histopathology
Introduction
Diabetes mellitus describes a group of metabolic disorders characterized by hyperglycemia due to insufficient production of insulin secretion, insulin action, or both. With a lack of insulin, the glucose remains in the blood rather than transferred into the cells to provide energy for cellular functions, which leads to high levels of blood glucose above normal (International Diabetes Federation [IDF], 2021). Prevalence estimates of diabetes mellitus reported by the IDF in 2021 showed that 537 million people have diabetes, and this number is projected to reach 783 million by 2045 (IDF, 2021). Furthermore, diabetes mellitus not only has an impact on the individual but also in terms of the economy. Globally, the annual economic burden of diabetes was estimated at 966 billion dollars;[1] In Saudi Arabia, the annual cost of diabetes has been estimated at 17 billion Riyals.[2]
For diet composition, the most recent nutritional guideline from the American Diabetes Association emphasizes that there is not one ideal percentage of calories from macronutrients for all people with diabetes and that macronutrient recommendations should be individualized.[3] The critical review by Feinman et al.[4] suggests that a low carbohydrate diet with a carbohydrate content of <45% of energy plays a role in glycemic control, insulin sensitivity, body weight control, and a positive effect on lipid levels in T2DM study conducted by Skytte et al.[5] investigated the effect of a similar carbohydrate-reduced and high-protein diet. The authors concluded that after 6 weeks reduced fasting glucose concentrations, HbA1c levels, and fat content of the liver and pancreas in weight-stable patients with type 2 diabetes. These results were confirmed by.[6]
On the other hand, intermittent fasting (IF) refers to alternating fasting time with ad libitum (AL) calorie intake time. The most commonly used forms of IF are: Alternate-day fasting (ADF): 24 h of fasting followed by 24 h of AL feeding time; modified alternate-day fasting: calorie intake is restricted to 25% on 1 or 2 days each week alternating with AL feeding time; time-restricted feeding (TRF); and Feeding occurs during specified hours in the day. Typically, eat for 8 h, followed by fasting for the next 16 h. In all instances, non-caloric fluid intake is allowed to reduce the risk of hypotension and dehydration.[7,8] Mechanisms through which fasting affects the body. One such mechanism is the fasting-induced stimulation of NEUROG3 cells that stimulates the regeneration of beta cells and therefore increase insulin secretion.[9,10] In animal studies, it was found that fasting for 16 h protected against hyperinsulinemia and reduced plasma glucose compared with groups with AL feeding to a high-fat diet despite comparable energy intake.[11] A similar study in rodents with streptozotocin (STZ)-induced diabetes[12] reported that TRF for 30 days from (5 PM to 8 AM) increased glucose tolerance, plasma insulin, and an increase in the number of pancreatic β-cells relative to controls.
Therefore, the aim of the current study was to investigate the effects of low-carbohydrate-high-protein (LCHP) versus normal diets, and AL feeding versus IF in type 2 diabetic rats (DR) induced by STZ.
Materials and Methods
All chemicals were acquired from Sigma Chemicals (St. Louis, MO). All chemicals used in the study were analytical grade. Commercial diagnostic kits for estimating serum glucose, lipid profile, and kidney function enzymes were purchased from (Human Diagnostic Worldwide, Germany). Enzyme-linked immunosorbent assay (ELISA) kit for estimating serum insulin was purchased from the Bioassay Technology Laboratories (Shanghai, China). Metformin was purchased from (Merck Pharma GmbH, Darmstadt, Germany). The ingredients of the experimental diets were purchased from MP Biomedicals, Inc (Irvine, CA, USA). Except for cornstarch, sucrose, and sunflower oil, which were locally purchased.
Experimental animals and diets
Thirty male Wistar albino rats weighing between (200 and 250g, 7 weeks old) were obtained from the Animal House, College of Pharmacy, King Saud University, Saudi Arabia. Rats were housed in polyacrylate plastic wire mesh cages having sawdust as bedding (5 rats/cage) at 23 ± 2°C, and under a 12-h light/dark cycle, under a relative humidity of 40–45% in an experimental animal house at the Department of Food Science and Human Nutrition, College of Agriculture and Food, Qassim University, Saudi Arabia. Rats were provided AL water and standard basal diet according to AIN-93 guidelines (Reeves et al., 1993), and allowed to acclimatize to the laboratory environment for 1 week before starting the experiment. The diabetic control diet (CD) was prepared in accordance with the diabetes dietary guidelines by the Diabetes and Nutrition Study Group (DNSG) of the European Association for the Study of Diabetes,[13] and a low-carb-high-protein diet was formulated among acceptable macronutrient distribution range.[14,15] All diets had similar energy densities, CD (4.37 kcal/g) and LCHP (4.29 kcal/g). This approach has been used to provide equivalent energy intakes in previous feeding studies.[16] Table 1 shows the composition of experimental diets.
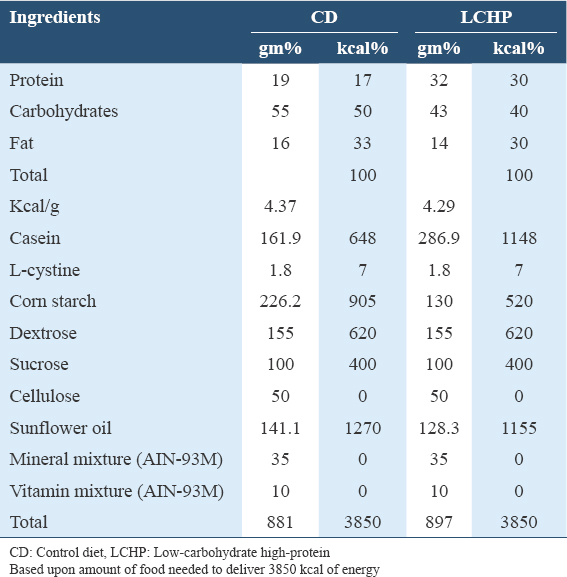
Induction of type 2 diabetes
After the acclimatization period, three rats were used for a pilot study to ascertain that the rats could be made diabetic at the dose level. After that, the rats fasted overnight, and diabetes was induced by intraperitoneal injection with freshly dissolved STZ in citrate buffer (0.1 M, pH = 4.5) at a dose of (45 mg/kg-BW) after 15 min administration of nicotinamide (NA) intraperitoneal (120 mg/kg-BW) according to the method of.[17] In the first 24 h after STZ induction, rats were provided with a 5% glucose solution as drinking water to prevent hypoglycemia. After 72 h, glucose was measured using a glucometer (Contour next one Glucometer, USA) by small incision from the tail vein and loading a drop of blood on glucometer strips. Rats that had reached a considered 200 mg/ dL or above were considered diabetic and were included in the study.[18]
Preparation of metformin
Metformin powder was dissolved in 1 mL of 0.9 % normal saline and orally administered at a dose of (500 mg/kg- BW). The animal dosage (mg/kg) was calculated from the multiplication of the human dosage (mg/kg for a normal 60 kg adult).[19] Metformin was chosen as the reference drug in this study according to a previous study.[20]
IF protocol
The IF groups involve restriction for a specific time during the day without calorie restrictions, 16-h fasting periods with periods of 8-h of AL access to the chow during the active phase (dark period) which is similar to the morning for humans. The time for food access was controlled by removing food daily from (16:00 to 08:00) to implement alternate periods of fasting and feeding. All groups were free to access water during the experimental period. The fasting hours were based on previous studies.[21]
Experimental design
The experimental protocol of this study was approved by the Qassim University Committee for Scientific Research Ethics (Approval #April 21, 2013). The rats were randomly divided into six groups for 6 weeks (n = 5, for each group) as follows: G1: normal rats (NR) as a negative control + CD + AL feeding (NR+CD+AL). While the remaining groups were injected with STZ and NA to induce type 2 diabetes.
After that, diabetic rats (DR) were randomly distributed based on body weight and blood glucose level into five groups: G2: DR as a positive control + CD + AL feeding (DR+CD+AL). G3: DR + CD + IF (DR+CD+IF). G4: DR+ LCHP + AL feeding (DR+LCHP+AL). G5: DR + LCHP diet + IF (DR+LCHP+IF). G6: DR + CD + AL feeding + treated with metformin (DR+CD+AL+MET).
The fasting groups were allowed access to the diet for 8 h per day. After 6 weeks, animals were sacrificed. Figure 1 shows the experimental design of the present study.
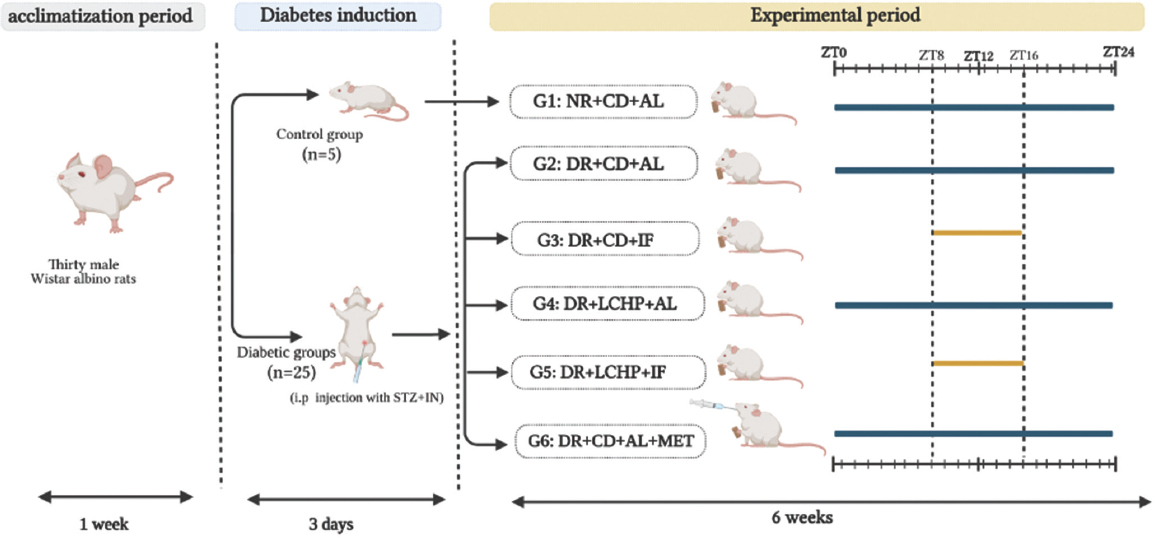
- Experimental design of the present study. NR + CD + AL: normal rats as a negative control + control diet + ad libitum feeding, DR + CD + AL: diabetic rats as a positive control + control diet + ad libitum feeding, DR + CD + IF: diabetic rats + control diet + intermittent fasting, DR + LCHP + AL: diabetic rats+ low-carbohydrate-high-protein diet + ad libitum feeding, DR + LCHP + IF: diabetic rats + low-carbohydrate-high-protein diet + intermittent fasting, DR + CD + AL + MET: diabetic rats + control diet+ ad libitum feeding + administered metformin
Thirty grams per rat of the CD or low-carb-high-protein diet were weighed and presented at ZT 8 for all groups. Subsequently, feed intake (FI) was measured weekly at 16:00 for IF groups and after 24 h for AL groups by the difference between food provided and food remaining in the cage. Residual food was discarded and replaced with a fresh diet. Water and bedding materials were changed daily. Furthermore, random blood glucose (RBG) 2 h after the reintroduction of food to search for possible hypoglycemia and body weight were measured weekly.
After the experimental period, the rats were fasted overnight and sacrificed under anesthetized with a mixture of alcohol, chloroform, and ether (1:2:3) according to.[22] Blood samples were collected in plain tubes and centrifuged at 4000×g for 30 min under cooling to separate the serum, which was stored at −20°C until biochemical analysis.
The liver and kidney were collected and blotted of adhering blood and washed with cold saline solution and dried between two filter papers. After that, the organs’ relative weight was calculated by the body weight of the animals as previously described.[23]
Biochemical measurements
Glucose, insulin and insulin resistance
Serum fasting glucose level was measured by the method of glucose oxidase.[24] Serum insulin level was measured by the ELISA technique according to the method of.[25] The fasting insulin resistance index was determined according to the method of,[26] using the Homeostatic Model Assessment for Insulin Resistance (HOMA-IR).
Lipid profile
Total cholesterol (TC) level
Serum TC level was determined according to the method described by.[27] Serum triglyceride level was determined according to the method described by.[28] Serum high-density lipoprotein cholesterol level was determined according to the method described by.[29] Serum low-density lipoprotein cholesterol level was determined according to the method described by.[30]
Serum very-low-density lipoprotein cholesterol level was determined according to the method described by Friedewald (1972).[30]
Atherogenic index (AI) and percent of protection against atherogenicity
To predict atherosclerosis, AI and % protection were calculated according to the method described by Niroumand et al.[31] using the following formula:
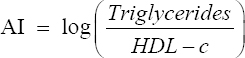
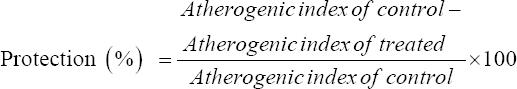
Histological examination
Pancreas histopathological examination was done according to the methods described b[32] The pancreas specimens were fixed in 10% formalin immediately, sections were dehydrated using increasing concentrations of ethanol, xylene and embedded with paraffin wax, then these paraffin blocks were sectioned at 5μm thickness by rotatory microtome (Leica RM 2125RT) and stained with Mayer’s hematoxylin and eosin (H&E). Finally, mounted with diphenylxylene and examined under the microscope for histopathological changes by an observer and micrographs were captured using (Leica DMD108) Standard software.
Statistical analysis
The study results were analyzed statistically by (SPSS, V. 21) Data were expressed as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was used, followed by Tukey’s post hoc test. Results were considered significant if (P < 0.05) according to.[33]
Results
Effect of LCHP diet and IF on body weights and relative organ weights in type 2 DR
The data in Table 2 and Figure 2 demonstrate the effect of different treatment groups on body and organ weight change in DR. At the beginning of the experiment, all groups had similar body weights (P > 0.05). After the 1st week, DR had a lesser weight gain compared to the normal control. Weight loss significantly continued in DR+CD+AL compared to NR+CD+AL for up to 6 weeks (P < 0.001).
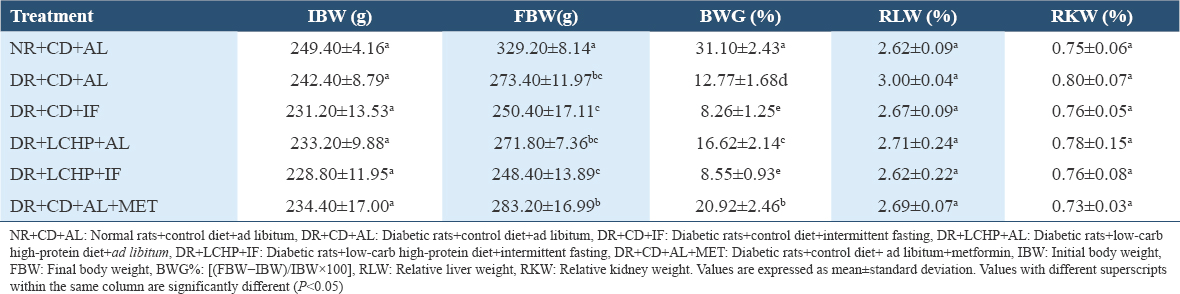
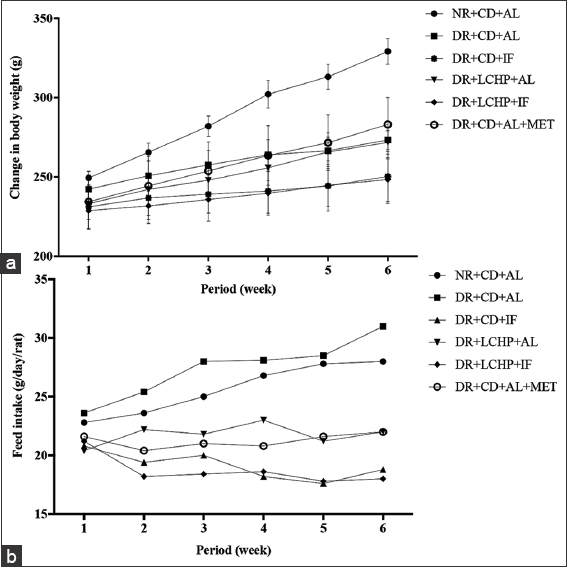
- Effect of a low-carbohydrate, high-protein diet and intermittent fasting for 6 weeks on body weight (a), feed intake (b) changes in type 2 diabetic rats
There was a significant difference in the final body weight (FBW) (P < 0.001). The highest FBW took place in the NR+CD+AL group, whereas the lowest FBW was recorded for the DR+LCHP+IF group. Concerning body weight gain percent (BWG%), the results showed that there was a significant decrease (P < 0.001) in the DR+CD+AL group when compared to NR+CD+AL by 18.33%. Moreover, the DR+CD+AL+MET and DR+LCHP+AL treatments significantly increased BWG by 8.15% and 3.85%, respectively, compared DR+CD+AL group. While the fasting (regardless of the type of diet) in the DR+CD+IF and DR+LCHP+IF groups significantly reduced BWG% by 4.51% and 4.22%, respectively, compared DR+CD+AL group (P < 0.001). In addition, the liver and kidney-to-body weight ratios were not significantly different between all the groups (P > 0.05). On the other hand, there was no significant difference in average FI between the NR+CD+AL and the DC+CD+AL group. In contrast, decreases were observed in FI by fasting rat groups compared to non-fasting groups and it was lower in the LCHP diet group than CD diet group up to 6 weeks. After treatment with metformin, the DR decreased the amount of FI decreased compared with the DR+CD+AL group, but still more than the fasting groups (P < 0.001).
Effect of a LCHP diet and IF on glucose, insulin, and insulin resistance in type 2 DR
RBG, fasting blood glucose, insulin, and insulin resistance are presented in Table 3 and Figure 3. After 1 week, there appeared to be a significant rise in the random glucose level after the induction of diabetes in all groups compared to the NR+CD+AL group (P < 0.001). However, over the 6 weeks, there was a significant decrease in the blood glucose level near to normal in all the treated groups compared to the DR+CD+AL group.
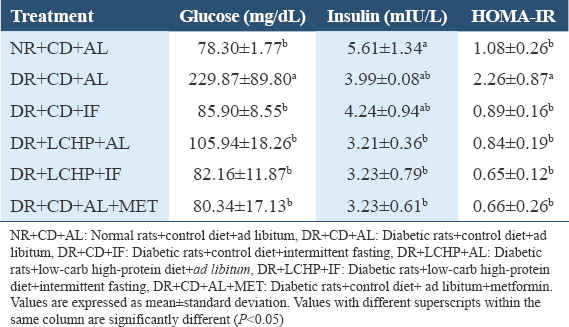
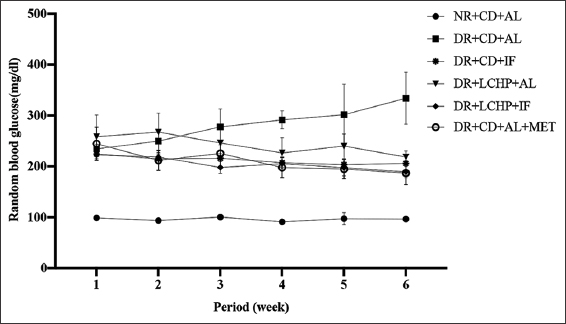
- Effect of a low-carbohydrate, high-protein diet and intermittent fasting for 6 weeks on random blood glucose change in type 2 diabetic rats
Notably, the DR+CD+MET and DR+LCHP-IF groups showed the lowest glucose level, respectively (P < 0.001). Serum fasting glucose level was also considered significantly raised (P < 0.01) in DR+CD+AL compared to the NR+CD+AL group. However, DR+CD+MET, DR+LCHP+IF, DR+CD+IF, and DR+LCHP+AL, respectively, significantly reversed the upraised levels of serum glucose toward normal compared to DR+CD+AL. There was a reduction in serum insulin level in the DR+CD+AL group when compared with the NR+CD+AL group, but this observation was not statistically significant (P > 0.05). Conversely, there was a significant decrease (P < 0.05) in the level of serum insulin in DR+LCHP+AL, DR+LCHP+IF, and DR+CD+AL+ MET compared with NR+CD+AL. Consistent with increased serum glucose and decreased serum insulin levels lead to the insulin resistance index was significantly increased in the DR+CD+AL group (P < 0.05) compared with the NR+CD+AL group. The efficacy of IF (regardless of the type of diet) was similar to the metformin group in the improvement of the HOMA-IR index (P < 0.05).
Effect of a LCHP diet and IF on the lipid profile and AI in type 2 DR
The effect of IF, LCHP, LCHP+IF, and metformin on the lipid profiles including TG, TC, HDL-c, LDL-c, and VLDL-c in type 2 DR was determined; results are illustrated in Table 4. A significant increase in the TG and VLDL-c was noted at 52.28% and 52.35%, respectively, in DR+CD+AL when compared to NR+CD+AL. Furthermore, TC and LDL-c have recorded an insignificant increase of 30.92% and 54.62%, respectively, whereas a non-significant decrease in HDL-c concentration was observed in DR+CD+AL compared to NR+CD+AL (P > 0.05). All experimental treatments showed an improvement in the TG, TC, LDL-c, and VLDL-c levels; this effect was strongest in the LCHP+IF group which was similar to those in the normal control group (P < 0.001). Although none of the experimental groups had any effect on HDL-c.
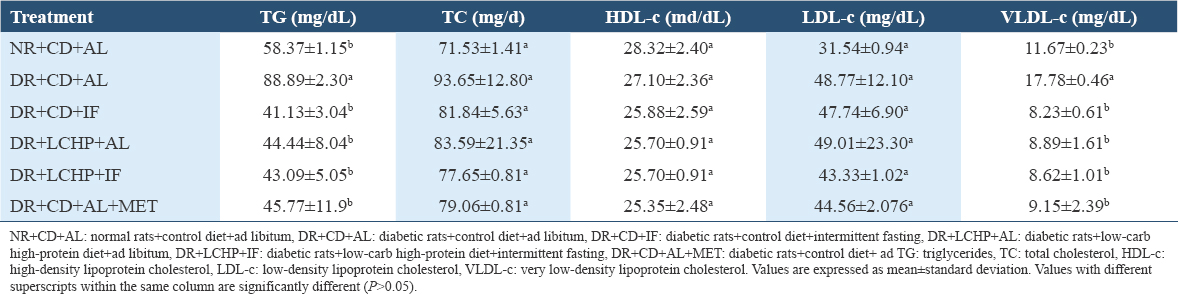
Interestingly, the highest protection against atherogenicity was found by decreasing the AI significantly in DR+CD+IF, DR+LCHP+IF, DR+LCHP+AL, and DR+CD+AL+MET groups, respectively, compared to the positive control group (P < 0.01). However, no significant differences were found between the normal control group and all treated rats (P > 0.05) [Table 5].
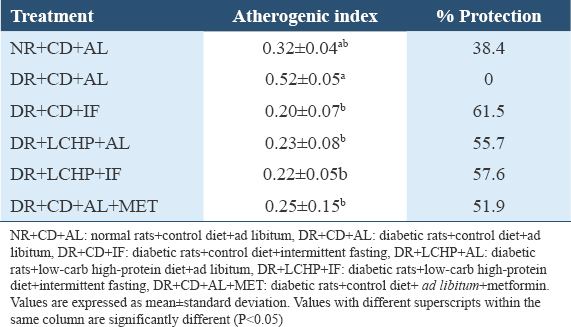
Histopathological examination
The results of histological changes in the structure of the pancreas of the different groups are shown in Figure 4. The histological examination of pancreatic sections from the normal control group (NR+CD+AL) showed a normal histological structure of the endocrine islets of Langerhans consisting of beta and non-beta cells as well as the exocrine acini. (DR+CD+AL): The pancreas of the diabetic control group showed distortion of the general architecture of islet cells with severe degenerative changes in islets of Langerhans and the exocrine part.
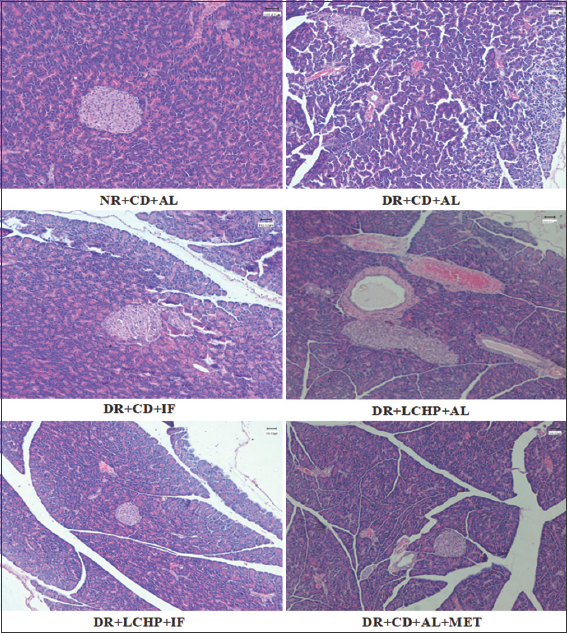
- The histopathological examination of the pancreases from experimental groups. (H&E- Hematoxylin and eosin, ×100). (NR + CD + AL): normal rats + control diet + ad libitum, (DR + CD + AL): diabetic rats + control diet + ad libitum, (DR+CD+IF): diabetic rats + control diet + intermittent fasting, (DR + LCHP + AL): diabetic rats + low-carb high-protein diet + ad libitum, (DR + LCHP + IF): diabetic rats + low-carb high-protein diet + intermittent fasting, (DR + CD+ AL + MET): diabetic rats + control diet+ ad libitum + metformin
The pancreas of the CD + IF group showed a large irregularly shaped islet of Langerhans; it looks newly formed islet of Langerhans with few mononuclear leukocytic infiltrations. (DR + LCHP + AL): The pancreas of LCHP + AL showed a large irregularly shaped islet of Langerhans and dilated irregularly rounded interlobular duct with a congestion area. (DR + LCHP + IF): The pancreas of LCHP+IF showed a nearly normal structure of islets Langerhans which is represented by shrinkage in size and irregular shape with few congestions. (DR+CD+AL+MET): The pancreas of the metformin group showed mild changes in islets Langerhans were relatively small in size and irregular in shape with moderate congestion.
Discussion
The present study aimed to evaluate the combined effect of LCHP diet, and IF in type 2 DR. To our knowledge, this is the first study to investigate such a combined effect in type 2 DR. Our data showed a significant increase in blood glucose, insulin resistance, hypertriglyceridemia, FI, and lower body weight and insulin secretion in STZ/NA-induced DR. Thus, these changes resemble many aspects of type 2 diabetic patients,[34] suggesting that it is considered a good model in this study.
Compared with the CD diet in the current study, the LCHP diet affected body weight recovery and decreased food intake. Indeed, weight loss is a common symptom of untreated diabetes.[35] Occurs due to metabolic dysfunction; tissues cannot absorb glucose; the body creates energy pathways by increasing gluconeogenesis, lipolysis, and ketone production, resulting in weight loss.[36] Therefore, the improved body weight may explain in part the improved glucose homeostasis. On the other hand, Increased food intake is the direct result of glucose accumulation in the blood and depends on energy expenditure, catabolic processes, and urinary excretion.[37] The reduction in food intake after the introduction of the LCHP diet was ascribed to the satiating effect of proteins [38,39] and increasing the gastric emptying time.[40]
In agreement with previous findings that used an LCHP diet with a carbohydrate percentage of 40%–30% and protein of 29%–30%,[5,6,40-42] fasting glucose was significantly lower in LCHP diet as compared with a CD diet. This was partly explained by the reduced efficient glucose output of gluconeogenesis and exogenous glucose.[43] In addition, these studies did not find significant changes in insulin levels.[5,42] One possibility for the insulin concentration to be unaffected is that despite the secretion of insulin stimulated by an increase in protein intake,[44] a corresponding reduction in carbohydrates causes an adverse effect on the insulin response.[45] Contrary to our results,[5,42] found that LCHP did not affect insulin resistance suggest that further study is required on the mechanism associated with insulin resistance.
Regarding hyperlipidemia, our results showed a significant decrease in TG and VLDL-c is in line with those reported,[46,47] and in disagreement with another study.[48] Although there was no significant change in TC and LDL-c in our study, a downward trend was demonstrated, which is congruent with a previous study.[49] Once glycogen stores have been drained during the fasting period, the body then enters ketosis.[50] During ketosis, fat is used for energy, and triglycerides are broken down into glycerol and three fatty acids. However, these changes in blood lipids appear to be variable and may depend on the quality and quantity of food consumption and the degree of weight change.[51]
In the present study, the LCHP diets improve triglyceride levels and reduce the risk of atherogenicity, which agrees with the findings.[5,6] On the other hand, the effect of carbohydrate intake restriction on HDL metabolism is not entirely consistent and may depend on concomitant changes in glucose and body weight. As in the present study,[52] reviewed data from 13 studies and indicated that the LCHP diet does not improve HDL. However, another meta-analysis of 23 studies (from 3 months to 2 years) showed a near-significant increase in HDL,[53] probably due to the that longer periods are needed to manifest changes in LCHP diet-induced HDL metabolism, possibly due to the slow turnover rate of HDL particles.[6]
Similar to,[47,48,54-57] the present study showed a decrease in body weight that may be attributed to the decrease in food intake,[12] and might be indicative of a shift to fat rather than carbohydrates as a source of fuel.[58] Regulation of energy metabolism during fasting and refeeding depends greatly on the interaction between hormones and energy substrates.[59] Fasting leads to adaptive hormonal and metabolic responses, including the conversion to dependence on fatty acids and ketones to produce energy, accompanied by lower triglycerides, blood glucose, and insulin.[60] Accordingly, the obtained results showed decreased blood glucose and insulin resistance.[12,47,55,57,61] Furthermore, some studies have not shown an improvement in insulin secretion.[12,54,57]
As appeared in the histopathological investigation of the sections of the pancreas in the current study, the diabetic group of rats showed pathological changes in both exocrine and endocrine components of pancreatic tissue in comparison to the normal structure observed in the normal control group. This finding was parallel to previous studies that showed STZ seemed highly selectively toxic to the pancreas’s β cells and subsequently reduced insulin secretion.[62] The section of the pancreas of the DR+CD+IF group showed newly formed islet of Langerhans with few mononuclear leukocytic infiltrations, the restoring pancreatic beta cell function is considered strategical approach for the treatment of diabetes.[63] The pancreas of the DR+LCHP+AL group demonstrated a large irregularly shaped islet of Langerhans and dilated irregularly rounded interlobular duct with a congestion area. In contrast, it was observed that the DR+LCHP+IF and DR+CD+AL+MET showed recovery in the structural of pancreatic islet β-cells and tissues. Thus, it could repair islet injury and recuperate the structural integrity of pancreatic islet β-cells and tissues against ROS-mediated damage.[64]
To our knowledge, only one case study has investigated the effects of IF in conjunction with a low-carbohydrate diet for a 69-year-old man diagnosed with type 2 diabetes who is treated using insulin.[65] However, the protocol of IF and macronutrient composition differed from what applied in our study, where used ADF and a very- low-carbohydrate-diet of <50 g/d. However, in agreement with our results, it showed a decrease in body weight, glucose, insulin, and insulin resistance.
Conclusion
In summary, IF and LCHP Diet improved glycemic control, insulin resistance, hyperlipidemia, and reduced predisposition to atherosclerosis. Moreover, regardless of the type of diet, IF resulted in lowered body weight and FI. Moreover, regardless of the type of diet, IF resulted in lowered body weight and FI. Furthermore, the CD diet alone significantly decreases weight when compared with the LCHP diet and metformin. Furthermore, no events of hypoglycemia were connected to LCHP+IF. More work is needed to explore the mechanism(s) of these positive effects.
Ethics Approval and Consent to Participate
The experimental protocol of this study was approved by the Qassim University Committee for Scientific Research Ethics (Approval #April 21, 2013).
Availability of Data and Materials
Relevant data will be provided by the corresponding author upon reasonable request.
Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding Statement
Not applicable.
Authors’ Contributions
AMA, WEA, SAA, and EMH contributed to the conception and design of the study. AMA acquired, analyzed, and interpreted the data under the supervision of WEA, SAA, and EMH. AMA drafted the article. WEA, SAA, and EMH revised the article critically for important intellectual content. All authors revised approved the final version of the manuscript.
References
- International Diabetes Federation (10th ed). Belgium: International Diabetes Federation; 2021.
- Cost of diabetes in the Kingdom of Saudi Arabia, 2014. J Diabetes Metab. 2015;6:575.
- [Google Scholar]
- Nutrition therapy for adults with diabetes or prediabetes:A consensus report. Diabetes Care. 2019;42:731-54.
- [Google Scholar]
- Dietary carbohydrate restriction as the first approach in diabetes management:Critical review and evidence base. Nutrition. 2015;31:1-13.
- [Google Scholar]
- Acarbohydrate-reduced high-protein diet improves HbA(1c) and liver fat content in weight stable participants with type 2 diabetes:A randomised controlled trial. Diabetologia. 2019;62:2066-78.
- [Google Scholar]
- Effects of a self-prepared carbohydrate-reduced high-protein diet on cardiovascular disease risk markers in patients with type 2 diabetes. Nutrients. 2021;13:1694.
- [Google Scholar]
- Effects of fasting intervention regulating anthropometric and metabolic parameters in subjects with overweight or obesity:A systematic review and meta-analysis. Food Funct. 2020;11:3781-99.
- [Google Scholar]
- Effect of various types of Intermittent Fasting (IF) on weight loss and improvement of diabetic parameters in human. Curr Nutr Rep. 2021;10:146-54.
- [Google Scholar]
- Intermittent fasting preserves beta-cell mass in obesity-induced diabetes via the autophagy-lysosome pathway. Autophagy. 2017;13:1952-68.
- [Google Scholar]
- Intermittent administration of a fasting-mimicking diet intervenes in diabetes progression, restores b cells and reconstructs gut microbiota in mice. Nutr Metab (Lond). 2018;15:80.
- [Google Scholar]
- Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15:848-60.
- [Google Scholar]
- Intermittent fasting modulation of the diabetic syndrome in streptozotocin-injected rats. Int J Endocrinol. 2012;2012:962012.
- [Google Scholar]
- Evidence-based nutritional approaches to the treatment and prevention of diabetes mellitus. Nutr Metab Cardiovasc Dis. 2004;14:373-94.
- [Google Scholar]
- The effect of high-protein, low-carbohydrate diets in the treatment of type 2 diabetes:A 12 month randomised controlled trial. Diabetologia. 2011;54:731-40.
- [Google Scholar]
- Classification and diagnosis of diabetes:Standards of medical care in diabetes-2021 Diabetes Care. 2021;44:S15-33.
- Carbohydrate restriction, prostate cancer growth, and the insulin-like growth factor axis. Prostate. 2008;68:11-9.
- [Google Scholar]
- Streptozotocin-nicotinamide-induced diabetes in the rat. Characteristics of the experimental model. Exp Biol Med (Maywood). 2012;237:481-90.
- [Google Scholar]
- Embelia ribes extract reduces high fat diet and low dose streptozotocin-induced diabetic nephrotoxicity in rats. EXCLI J. 2013;12:858-71.
- [Google Scholar]
- Guidance for Industry Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. Maryland: Food and Drug Administration; 2005.
- Metformin improves the glucose and lipid metabolism via influencing the level of serum total bile acids in rats with streptozotocin-induced type 2 diabetes mellitus. Eur Rev Med Pharmacol Sci. 2017;21:2232-7.
- [Google Scholar]
- Time-restricted feeding improves insulin resistance and hepatic steatosis in a mouse model of postmenopausal obesity. Metabolism. 2016;65:1743-54.
- [Google Scholar]
- Hypoglycemic effects of three medicinal plants in experimental diabetes:Inhibition of rat intestinal a-glucosidase and enhanced pancreatic Insulin and cardiac Glut-4 mRNAs expression. Iran J Pharm Res. 2013;12:387-97.
- [Google Scholar]
- Effects of STZ-induced diabetes on the relative weights of kidney, liver and pancreas in albino rats:A comparative study. Int J Morphol. 2010;28:135-42.
- [Google Scholar]
- An improved colour reagent for the determination of blood glucose by the oxidase system. Analyst. 1972;97:142-5.
- [Google Scholar]
- Enzyme immunoassay for intact human insulin in serum or plasma. Clin Chem. 1993;39:578-82.
- [Google Scholar]
- Homeostasis model assessment:Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412-9.
- [Google Scholar]
- National cholesterol education program recommendations for triglyceride measurement:Executive summary. The national cholesterol education program working group on lipoprotein measurement. Clin1 Chem. 1995;41:1421-6.
- [Google Scholar]
- Determination of high-density lipoproteins:Screening methods compared. Clin Chem. 1979;25:939-42.
- [Google Scholar]
- Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499-502.
- [Google Scholar]
- Atherogenic Index of Plasma (AIP):A marker of cardiovascular disease. Med J Islam Repub Iran. 2015;29:240.
- [Google Scholar]
- Theory and Practice of Histological Techniques. New York: Elsevier Health Sciences; 2008.
- Pinciples and Procedures of Statistics a Biometrical Approach. United States: McGraw-Hill; 1997.
- Pathogenesis of fasting and postprandial hyperglycemia in type 2 diabetes:Implications for therapy. Diabetes. 2010;59:2697-707.
- [Google Scholar]
- Diabetes mellitus:Hyperglycemia and its chronic complications. ABCS Health Sci. 2011;36:182-8.
- [Google Scholar]
- Protease inhibitors from marine actinobacteria as a potential source for antimalarial compound. PLoS One. 2014;9:e90972.
- [Google Scholar]
- Ahigh-protein diet enhances satiety without conditioned taste aversion in the rat. Physiol Behav. 2003;78:311-20.
- [Google Scholar]
- Metabolic evidence for adaptation to a high protein diet in rats. J Nutr. 2001;131:91-8.
- [Google Scholar]
- Acarbohydrate-reduced high-protein diet acutely decreases postprandial and diurnal glucose excursions in type 2 diabetes patients. Br J Nutr. 2018;119:910-7.
- [Google Scholar]
- The clinical effects of a carbohydrate-reduced high-protein diet on glycaemic variability in metformin-treated patients with type 2 diabetes mellitus:A randomised controlled study. Clin Nutr ESPEN. 2020;39:46-52.
- [Google Scholar]
- Effects of carbohydrate restriction on postprandial glucose metabolism, b-cell function, gut hormone secretion, and satiety in patients with Type 2 diabetes. Am J Physiol Endocrinol Metab. 2021;320:E7-18.
- [Google Scholar]
- Protein:Metabolism and effect on blood glucose levels. Diabetes Educ. 1997;23:643-51.
- [Google Scholar]
- Effect of protein ingestion on the glucose and insulin response to a standardized oral glucose load. Diabetes Care. 1984;7:465-70.
- [Google Scholar]
- An increase in dietary protein improves the blood glucose response in persons with type 2 diabetes. Am J Clin Nutr. 2003;78:734-41.
- [Google Scholar]
- The effect of intermittent fasting on mortality in patients with type 2 diabetes and metabolic disease with high cardiovascular risk:A systematic review. Clin Diabetol. 2021;10:284-9.
- [Google Scholar]
- Time-restricted feeding improves blood glucose and insulin sensitivity in overweight patients with type 2 diabetes:A randomised controlled trial. Nutr Metab (Lond). 2021;18:88.
- [Google Scholar]
- Metabolic impact of intermittent fasting in patients with type 2 diabetes mellitus:A systematic review and meta-analysis of interventional studies. J Clin Endocrinol Metab. 2021;106:902-11.
- [Google Scholar]
- Time-restricted eating as a nutrition strategy for individuals with type 2 diabetes:A feasibility study. Nutrients. 2020;12:E3228.
- [Google Scholar]
- Meal frequency and timing in health and disease. Proc Natl Acad Sci U S A. 2014;111:16647-53.
- [Google Scholar]
- Effects of high-protein diet on glycemic control, insulin resistance and blood pressure in type 2 diabetes:A systematic review and meta-analysis of randomized controlled trials. Clin Nutr. 2020;39:1724-34.
- [Google Scholar]
- Efficacy and safety of low and very low carbohydrate diets for type 2 diabetes remission:Systematic review and meta-analysis of published and unpublished randomized trial data. BMJ. 2021;372:m4743.
- [Google Scholar]
- Intermittent fasting:Is there a role in the treatment of diabetes?A review of the literature and guide for primary care physicians. Clin Diabetes Endocrinol. 2021;7:3.
- [Google Scholar]
- Effects of intermittent fasting on health markers in those with type 2 diabetes:A pilot study. World J Diabetes. 2017;8:154-64.
- [Google Scholar]
- Eating two larger meals a day (breakfast and lunch) is more effective than six smaller meals in a reduced-energy regimen for patients with type 2 diabetes:A randomised crossover study. Diabetologia. 2014;57:1552-60.
- [Google Scholar]
- Three weeks of time-restricted eating improves glucose homeostasis in adults with type 2 diabetes but does not improve insulin sensitivity:A randomised crossover trial. Diabetologia. 2022;65:1710-20.
- [Google Scholar]
- The effects of Ramadan fasting on activity and energy expenditure. Am J Clin Nutr. 2018;107:54-61.
- [Google Scholar]
- Effects of fasting and refeeding on serum leptin, adiponectin and free fatty acid concentrations in young and old male rats. Gerontology. 2005;51:357-62.
- [Google Scholar]
- Leptin mediates a glucose-fatty acid cycle to maintain glucose homeostasis in starvation. Cell. 2018;172:234-48.e17.
- [Google Scholar]
- Time-restricted feeding improves glucose tolerance in men at risk for type 2 diabetes:A randomized crossover trial. Obesity (Silver Spring). 2019;27:724-32.
- [Google Scholar]
- Histopathological alteration in STZ-nicotinamide diabetic rats, a complication of diabetes or a toxicity of STZ? Int J Diabetes Clin Res. 2018;5:91.
- [Google Scholar]
- Metformin prevents glucotoxicity by alleviating oxidative and ER stress-induced CD36 expression in pancreatic beta cells. J Diabetes Complications. 2017;31:21-30.
- [Google Scholar]
- Therapeutic fasting as a potential effective treatment for type 2 diabetes:A 4-month case study. J Insulin Resist. 2017;2:a31.
- [Google Scholar]







