β-caryophyllene attenuates oxidative stress and hepatocellular mitochondrial dysfunction in type-2 diabetic rats induced with high fat and fructose diets
Address for correspondence: Dr. Vadivel Mani, Department of Biochemistry, Konaseema Institute of Medical Science and Research Foundation, Amalapuram, East Gothwari - 533 201, Andhra Pradesh, India. Phone: +91-9566888598. E-mail: velvdm.vel5@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Qassim Uninversity and was migrated to Scientific Scholar after the change of Publisher.
Abstract
ABSTRACT
Objective:
Hyperglycemia, hyperlipidemia, and systemic resistance to insulin are typical manifestations of type 2 diabetes mellitus. One of the main pathophysiological alterations in insulin-sensitive organs is mitochondrial malfunction associated with oxidative stress and diminished fuel utilization. β-Caryophyllene (BCP) has qualities that are anti-inflammatory, anti-tumor, antioxidant, hypolipidemic, and hypoglycemic. In this work, rats suffering from type 2 diabetes were given a diet high in fat and sugar with the aim of examining the ameliorative effects of BCP on oxidative stress-mediated hepatic mitochondrial dysfunction.
Methods:
The diabetic condition was experimentally induced by feeding rats a high-calorie diet. The rats were then administered the recommended doses of BCP and metformin (MET) once every day for 30 days at 200 mg and 50 mg concentrations per kg of body weight, respectively, to prove the hypothesis of the study that BCP ameliorates mitochondrial dysfunction induced by oxidative stress in diabetic rats. Mitochondrial dysfunction can be identified by indicators such as oxidative stress, cardiolipin dienes, membrane phospholipid concentration, and mitochondrial enzymes.
Results:
The mitochondria in the liver of rats with diabetes exhibit elevated redox imbalance-related parameters and malfunctioning mitochondria with peroxided cardiolipin, while their amounts of glutathione and phospholipids are lowered. Oxidative stress indices, ameliorated mitochondrial activities, and peroxided cardiolipin were drastically decreased in rats with diabetes treated with BCP or MET.
Conclusions:
The present research demonstrated that BCP improved the vital role of mitochondria by reducing free radical dominance in type 2 diabetic experimental rats fed high-fat and high-sugar diets.
Keywords
Cardiolipin
ETC complex
mitochondrial dysfunction
protein carbonyls
TCA cycle enzyme
b-caryophyllene
Introduction
Diabetology research has made considerable advancements in our understanding of the diabetes phenomena over the past 20 years, which offers hope that the disease’s symptoms and functional effects may worsen.[1] Due to a lack of insulin secretion or activity, diabetic-ill patients have no ability to metabolize carbs and other macronutrients.[2] The enzymes that participate in the primary hexose-utilizing pathways, the EMP pathway, the denova-lipogenic pathway, and the phosphogluconate pathway are inhibited, while liver neo-glucogenic, liver-glycogenolytic, and adipo-lipolytic pathway processes are enhanced, altering the process of metabolism in non-diabetics due to the cells’ inability to recognize and absorb glucose in the blood.[3] Hereditary, elevated sugar levels, hypertension, weight gain, bloodstream lipid abnormalities, and oxidative stress are all associated with an elevated likelihood of morbidity as well as mortality from vascular problems in diabetes patients.[4] Abnormal biological changes in the cells, like self-oxidation of glucose, abnormal glycation of enzymes and structural proteins non-enzymatically, and unwanted stimulation of minor polyol pathways with enhanced oxidative stress, are all effects of uncontrolled diabetes.[5] Electron leakage from respiratory complexes I, II, and III of the electrochemical respiratory chain has been demonstrated in vitro to be the primary cause of reactive oxygen species (ROS) production in the mitochondrion.[6] Numerous studies have emphasized their role in the etiology of numerous illnesses, including autoimmune diseases, cancer, cardiovascular diseases, metabolic syndrome, and neurological disorders.[7] Diabetes-related insulin resistance tissues may experience changes in mitochondrial shape and function as a result of oxidative stress.[8] It has been demonstrated, for example, that a high-fat, high-sugar diet, a prevalent lifestyle choice, particularly in Western nations, may change cellular function.[9] Animal models for diet-induced liver function impairment have been the subject of numerous investigations.[10] Although liver disease is more common in the diabetic population (42.6%), there is little information regarding the impact of western diets on physico-chemical manifestations of the emergence of liver disease.[11] This is true even though obesity is a life phase in which animals are more exposed to developing diet-induced insulin resistance and liver dysfunction.[12] The hepatic parenchyma is the main insulin-dependent tissue that is more susceptible to vulnerable substance (ROS) injury, and because of its critical functions like managing circulatory forms of lipids and the glycemic index of blood, it has an important contribution to the growth of metabolic diseases, including insulin resistance.[13] Previous research has demonstrated that the onset of β-cell dysfunction and lipid metabolic impairment is significantly impacted by 60 days of feeding on a high-fat diet and high fructose.[14] Indicating that further research is need to understand the relationship between oxidative stress and changes in metabolism caused by eating obesogenic diet.
β-Caryophyllene (BCP) is a sesquiterpene that is extensively dispersed in the plant kingdom.[15] They are a key ingredient in the essential oils that are derived from plants used to make spices and foods.[16] The bioactivities of these compounds have received a lot of attention over the years, with BCP receiving particular focus because of its analgesic, anti-inflammatory, antioxidant, neuroprotective, antidiabetic, and chemopreventive properties. Its capacity to modulate cannabinoid CB2 receptors has also received attention. The Food and Drug Administration and the European Food Safety Authority have both given their approval for this plant chemical, which is used in cosmetics and as a flavor enhancer.[17]
Therefore, the objective of the current study was to investigate how BCP affected liver metabolic alterations brought on by oxidative stress. To learn more about the ameliorate action of BCP and the significance of oxidative stress in mitochondrial dysfunction in diabetic rats, researchers specifically looked at the livers of experimental rats treated with high fat and fructose levels.
Materials and Methods
Chemicals
The analytical-grade chemicals and reagents were purchased from standard companies. The traditional antidiabetic drug metformin (MET) was purchased from Sigma Chemicals and BCP from the Japanese-based company TCI Chemicals. In addition, ACON Laboratories provided strips for glucose monitoring.
Animals
The research activities were accepted by the Meenakshi University animal ethical board under the regulation of CPCSEA. Approval certificate number 007/2019, dated April 11, 2019. At the Meenakshi University research wing, the Central Facility for Caring Animal Unit collected and cared for healthy adult male Wistar albino rats weighing 180–200 g. They were given regular feed in the form of pellets, and free access to clean drinking water was provided.
Induction of type-2 diabetes
By supplementing rats with high-calorie food, the fed was prepared under hygienic with fortified with 2 g of cholesterol, 1 g of bile salt, cholic acid to increase digestive power, 30 g of coconut oil for the 100 g of fed preparation, and 25% fructose supplemented through drinking water. This procedure was followed under the guidance as described previously.[14] Rats are biologically made to be diabetic at the 9th week of supplementation, and conformed by the study fasting blood sugar level reached more than 120 mg/dL. The study’s conclusion saw a continuation of the high-calorie feeding, and we found that 80% of rats were induction with type-2 diabetes. In the healthy control group, animals were fed regular pelleted rat food and given unlimited access to water.
Experimental design
Healthy rats and HFFD-induced diabetic rats are subjected to an experimental design for 1 month. Healthy adult rats were randomly divided into two groups (control group I and drug control group V). Diabetic rats were randomly divided into three groups: Diabetic (group II), BCP treatment (group III), and MET treatment (group IV). Group-I subjected with normal fed, Group-II animals subjected with HFFD, Group-III subjected with HFFD and BCP 200 mg/kg of b.wt once in day, Group-IV subjected with HFFD with MET 50 mg/kg of b.wt once in day, and Group-V animals subjected with BCP 200 mg/kg of b.wt once in day. Each group had six animals, and based on the results of our earlier research, the ideal dosage of BCP was chosen.[18]
The drugs were administrated orally using an 18-gauge ball-tipped gavage needle for 30 days, and the animals were fasted overnight. Physiological saline was injected into the anesthetized animals after sodium thiopentone 40 mg/kg of body mass was introduced intraperitoneally to anesthetize them, and the liver was cut out to assess various qualities. Blood was withdrawn from the cardiac puncture method, and serum was separated without hemolysis for biochemical analysis.
Tissue homogenate preparation
The liver was removed from the rats as soon as they died, washed in cold isotonic saline, dried, and weighed. A homogenate was created using minced liver tissue and homogenized with physiological buffer (PBS buffer, 0.1 M, pH 7.4). To extract the protein part of the hepatic homogenate, it was subjected to centrifugation at 16,000 rpm for 20 min under ice-cold temperatures. The lipid peroxidation (LPO), protein carbonylation, and antioxidant reactivity of this supernatant were all measured.
Oxidative stress parameters
An assay using spectrophotometry was used to determine the parameters of oxygen-dependent stress.[19] Tissue protein content: Tissue proteins are the source and evidence for metabolic alteration. Such biomolecules were measured by the pioneering method of Lowry et al.; proteins were intensified with blue color development, sensitized at 640 nm, and expressed as mg/g of hepatic tissue. LPO assay: The interaction between the heterocyclic ring of thiobarbituric acid (TBA) and the aldehyde group of malondialdehyde (MDA) to create a pink-to-brown color TBA reactive derivative complex captures the light maximum at 535 nm. This reaction intensified depending on the amount of peroxidation in lipid membranes. It was employed to quantify the degree of lipid rancidity in the liver tissue homogenate of all the experimental animals, and it is expressed in the form of nmol of MDA/mg protein. Assay for protein carbonyls (PCO): Brady’s reagent, a hydrazine derivative, was used in a spectrophotometric manner to measure PCO in accordance with the methodology published previously,[20] two of the field’s pioneering researchers in antioxidant biology. A colored precipitate was produced when PCO compounds and the hydrazine ring in DNPH interacted. Centrifugation was used to extract the precipitate, and a 370 nm color measurement was made. The findings were presented as carbonyl group nanomoles per mg of liver tissue protein.
Tissue antioxidant assays
Antioxidant enzyme activities were determined by a spectrophotometric enzyme assay.[21] By using the Marklund and Marklund method, the superoxide dismutase enzyme (SOD) (EC 1.15.1.1) activity of the enzyme was indirectly measured by the oxidation of pyrogallol. The oxidized dye developed a pink color intensity measured at 420 nm. Catalase (CAT) (EC 1.11.1.6) activity was measured indirectly by reduction of hydrogen peroxide level by the CAT enzyme. By using the Sinha et al. technique, the rate of dichromate oxidation reduction was indicated and measured at 570 nm. Glutathione peroxidase enzyme (GPx) (EC 1.11.1.9) activity was assessed. Glutathione (GSH)-dependent peroxidase neutralizes hydrogen peroxide. The rate of peroxide oxidation was determined by changes in the color of DTNB at 420 nm by using the Rotruck et al. technique. The Staal et al. method was used to measure the glutathione reductase enzyme (GR) (EC 1.6.4.2) activity by the rate of NADH autoxidation, which is calculated by the drop in absorbance at 420 nm/30 s. The Habig et al. method was utilized for testing the glutathione-S-transferase enzyme (GST) (EC 2.5.1.18) activity. The substrate dinitrobenzene (CDNB) is an electrophilic xenobiotic conjugated with the thiol group of GSH, leading to a rise in absorbance at 340 nm. The Moron et al. technique was employed for quantifying reduced GSH by adding DTNB that had been oxidized to yellow nitrobenzoic acid by GSH. The yellow color intensity measured the change in absorbance at 412 nm.
Mitochondrial dysfunction analysis
Mitochondrial dysfunction parameters were accessed by spectrophotometry methods.[20] Isolation of mitochondria: The Johnson and Lardy approach was used to separate the mitochondrion of liver tissue. The sucrose gradient developed at physiological pH 7.4, the initial gradient of elementate cellular debris, and the supernant collected and centrifuged at high speed to pellet the mitochondrion. TCA cycle enzyme assay: The mitochondrial oxidoreductase assay’s kinetic principle was utilized to quantify enzyme activity using a spectrophotometer technique. According to the Bell and Baron Method, mitochondrial oxidoreductase-isocitrate dehydrogenase enzyme (EC 1.1.1.42) was measured for enzyme activity by the production of α-ketoglutarate and expressed as nmoles of α-ketoglutarate liberated per minute/mg protein. Using the Reed and Mukherjee method, the rate of maximum velocity of the enzyme α-ketoglutarate dehydrogenase (EC.1.2.4.2) was measured. The kinetic rate of succinate oxidation from The measurement of α-ketoglutarate was conducted by monitoring the production of ferrocyanide and the color intensity at 540 nm. The Slater and Bonner method was employed for assaying the succinate dehydrogenase enzyme (EC.1.3.5.1). Reducing the succinate amount through measurement of the absorbance reduction at 420 nm at 30-s intervals for five minutes allowed us to calculate the rate of fumarate formation. Malate dehydrogenase enzyme (EC.1.3.5.1) was evaluated using Mehler et al.’s methodology, and the kinetic rate of oxaloacetate formation was determined by UV absorption at 340 nm. Mitochondrial respiratory chain enzyme activities were determined by a spectrophotometric enzyme assay. ETC complex-I (NADH dehydrogenase enzyme, EC 1.6.5.3) was examined using Paradies et al.’s methodology. Activity was measured by the reduction of NADH content by UV absorption in spectrophotometry. The activity of the enzyme was represented by the amount of (nmol) NADH oxidized per min/mg of protein. As per the Heales 1996 and Wharton 1967 methods, the Complex IV (cytochrome c-oxidase enzyme, EC 1.9.3.1) activity was determined by potassium cyanide oxidation and depended on the amount of reduced cytochrome c availability at 550 nm. In accordance with the technique of cytochrome c oxidase enzyme activity performed with potassium cyanide and without potassium cyanide, the difference in enzymatic rate was expressed as specific enzyme activity.
Analysis of cardiolipin and peroxidized cardiolipin in liver
Mitochondrial phospholipids, including peroxidized cardiolipin, were measured based on the procedure of Paradies et al.[22] Lipids from liver tissue were extracted with chloroform and methanol by the Bligh and Dyer method. Mitochondrial membrane lipids were separated and quantified using the adsorption principle of the high-performance liquid chromatography (HPLC) technique. Hexane and propanol were used as the mobile phase and a silicon column as the stationary phase to achieve a gradient elution rate of 2 mL/min. At 206 nm, the eluent was measured. Analysis of peroxidized cardiolipin: Bovine cardiolipin extracted from liver tissue was matured to form oxidized product conjugated dienes when exposed to oxidizing agents overnight at 37°C. Cardiolipin-conjugated dienes’ peroxydized product was recognized and measured for liquid chromatography absorbance, which is a measure of UV absorption at 235 nm.
Statistical analysis
Descriptive statistics were accessed, and results are expressed as an arithmetic mean ± standard error. Statistical significance between the control and experimental groups was assessed using the statistical procedure one-way ANOVA, followed by the Tukey-Kramer multiple comparison tests. P < 0.05 was considered significant.
Results
BCP modulates liver oxidative stress markers in experimental diabetic rats
In experimental diabetic rats, we looked at how BCP affected oxidative stress by measuring the concentrations of MDA and PCO. The expressions of PCO and LPO are higher in experimental diabetic rats compared to non-diabetic rats. However, LPO and PCO in diabetic rats supplemented with 200 mg of BCP for 30 days were considerably reduced [Figure 1].
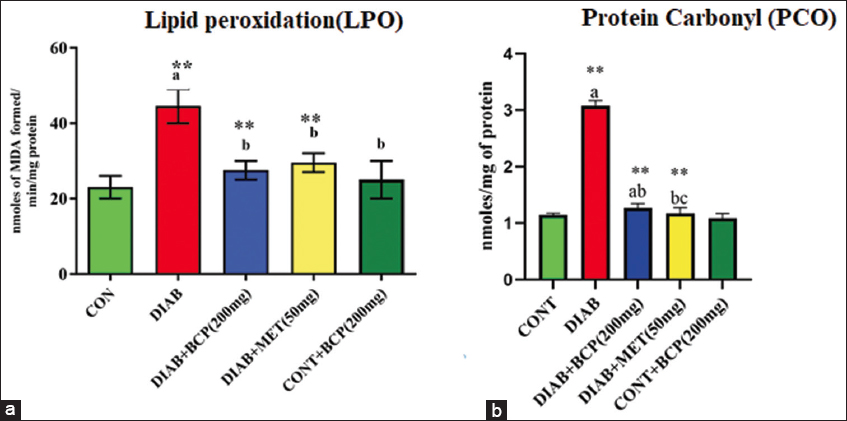
- (a and b) Illustration of oxidative marker: lipid peroxidation and protein carbonyls in experimental diabetic rats
In the bar diagram, the results represent the arithmetic mean with ± predictable error of six experimental animals. The statistical drift of the study was set at P < 0.05* or P < 0.01**. ANOVA between the experimental groups was performed and mentioned in the bar diagram as a symbol of a-compared with the diabetic group mean and c-compared with the mean of 200 mg/b.wt BCP-treated animals.
BCP supplementation improves metabolic stress management capacity in the liver of experimental diabetic rats
The biological activities of antioxidant enzymes in lever were modulated by the treatment with BCP in diabetic rats, as shown in Figures 2 and 3. In our study, the diabetic group’s amount of biologically active GSH was remarkably lower than that of the experimental control rats. In addition, diabetic experimental rats’ biological desire for polar nature-free radical scavenging enzymes with glutathione-dependent peroxidase (GPx), reductase (GR), sulfur transferase (GST), and GSH non- dependent enzymes like superoxide neutralizer (SOD) and CAT were significantly decreased in comparison to non-diabetic healthy control rats. However, BCP-supplemented experimental animals considerably increased their enzymatic antioxidant system with GSH. BCP has been illustrated to show the ability to debate oxidative stress in diabetic experimental rats [Figures 2 and 3].
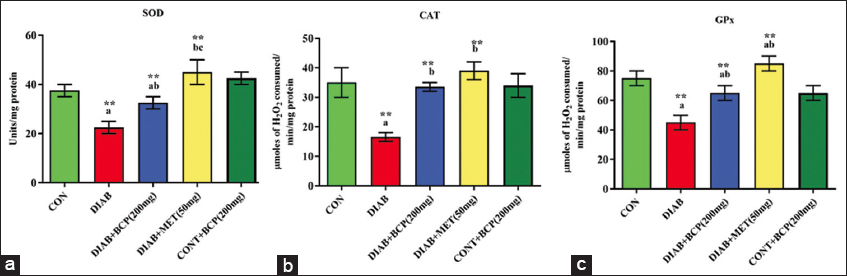
- (a-c) Illustration of non-glutathione-dependent antioxidant enzymes in experimental diabetic rats
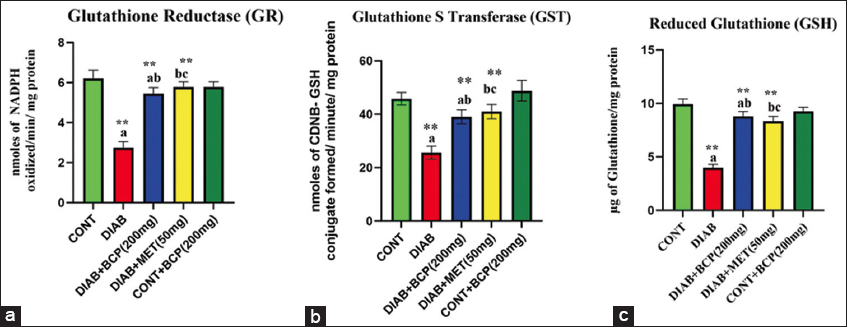
- (a-c) Illustration of glutathione-dependent antioxidant enzymes in experimental diabetic rats
In the bar diagram, the results represent the arithmetic mean with ± predictable error of six experimental animals. The statistical drift of the study was set at P < 0.05* or P < 0.01**. ANOVA between the experimental groups was performed and mentioned in the bar diagram as a symbol of a-compared with the diabetic group mean and c-compared with the mean of 200 mg/b.wt BCP-treated animals.
In the bar diagram, the results represent the arithmetic mean with ± predictable error of six experimental animals. The statistical drift of the study was set at P < 0.05* or P < 0.01**. ANOVA between the experimental groups was performed and mentioned in the bar diagram as a symbol of a-compared with the diabetic group mean and c-compared with the mean of 200 mg/b.wt BCP-treated animals.
BCP enhances liver mitochondrial oxidoreductase enzyme activity in experimental diabetic rats
A bar diagram [Figure 4] shows how BCP stabilizes the mitochondrial oxidoreductase enzymes. The reduced electron carries generating enzymes in the matrix of mitochondria, like NAD and FAD-dependent oxidoreductase enzymes, were markedly decreased in experimental diabetic rats liver extracts. However, the level of hepatic mitochondrial oxidoreductase enzymes in the BCP-treated group was significantly higher than in diabetic experimental rats. It was proven that BCP can improve mitochondrial oxidoreductase enzyme activity in experimental diabetic rats [Figure 4]. The levels of this mitochondrial oxidoreductase activity in experimental diabetic rats supplemented with BCP are statistically considered if a P < 0.05 increased when the level was comparable to that of the common drug MET.
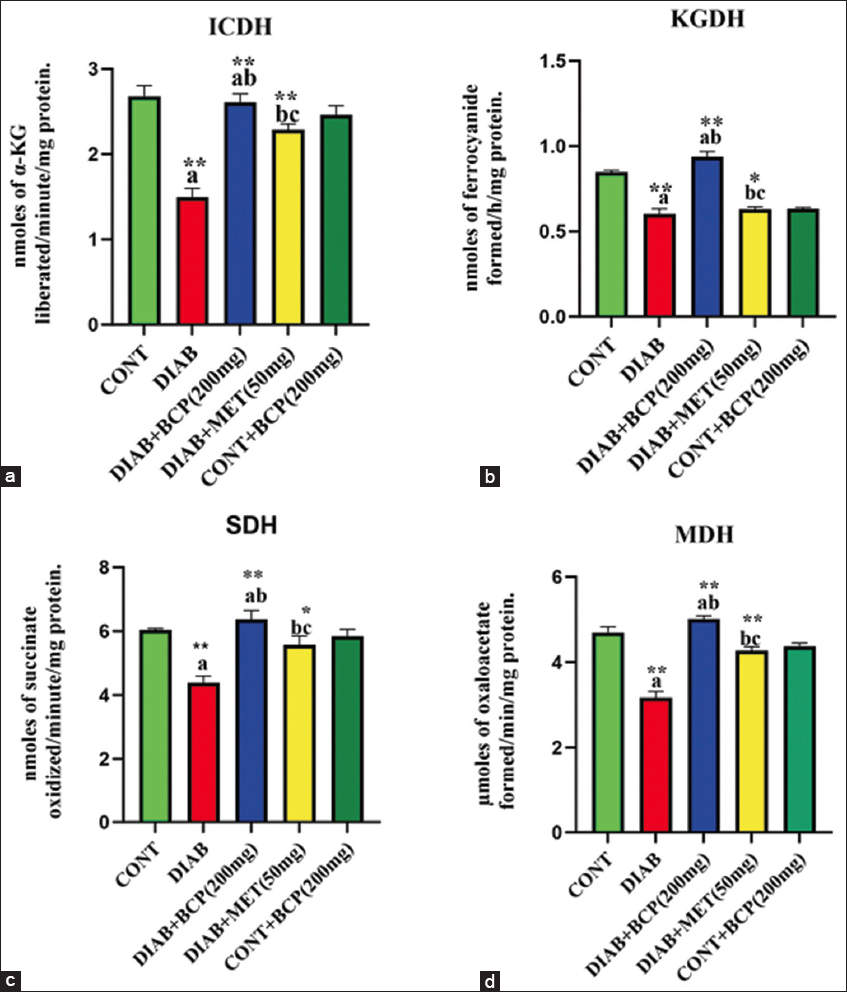
- (a-d) Hepatic mitochondrial oxidoreductase activity in experimental diabetic rats
In the bar diagram, the results represent the arithmetic mean with ± predictable error of six experimental animals. The statistical drift of the study was set at P < 0.05* or P < 0.01**. ANOVA between the experimental groups was performed and mentioned in the bar diagram as a symbol of a-compared with the diabetic group mean and c-compared with the mean of 200 mg/b.wt BCP-treated animals.
BCP improves liver mitochondrial ETC complex activity in experimental diabetic rats
NADH-reductase (Complex-I) and cytochrome-c-oxidase enzymes (Complex-IV), a key component, were involved in the metabolic consequences of mitochondrial respiration and free radical generation, primarily in metabolically active organs like the liver. The upshot of BCP on NADH-reductase and cytochrome-c-oxidase enzymes in the liver was illustrated through the statistical bar diagrams in Figure 5a and b. The high-fat and fructose-treated diabetic animals show a considerable reduction in mitochondrial electron consumers NADH-reductase enzyme (Complex-I) and cytochrome-c-oxidase enzyme (Complex-IV) activity. Whereas BCP-supplemented diabetic animals show increased electron consumption activity in hepatic mitochondria, to a level comparable to that of the conventional medication MET.
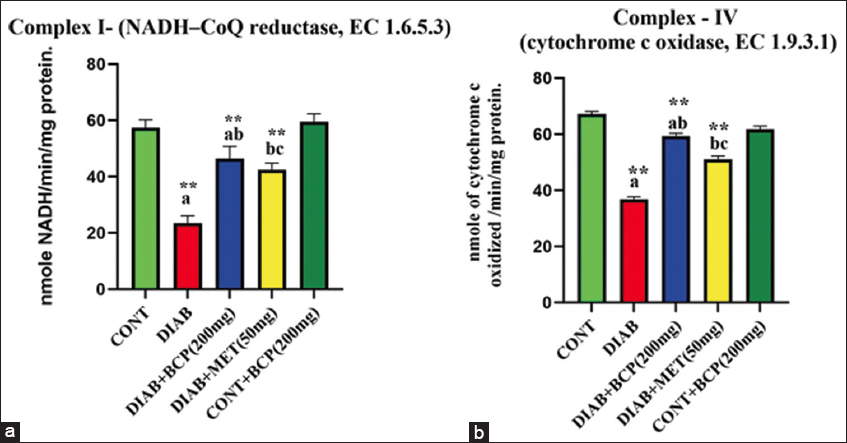
- (a and b) Hepatic tissue ETC complex activity in β-caryophyllene supplemented experimental diabetic rats
In the bar diagram, the results represent the arithmetic mean with ± predictable error of six experimental animals. The statistical drift of the study was set at P < 0.05* or P < 0.01**. ANOVA between the experimental groups was performed and mentioned in the bar diagram as a symbol of a-compared with the diabetic group mean and c-compared with the mean of 200 mg/b.wt BCP-treated animals.
BCP attenuates cardiolipin content and cardiolipin dienes in HFFD-induced experimental diabetic rats
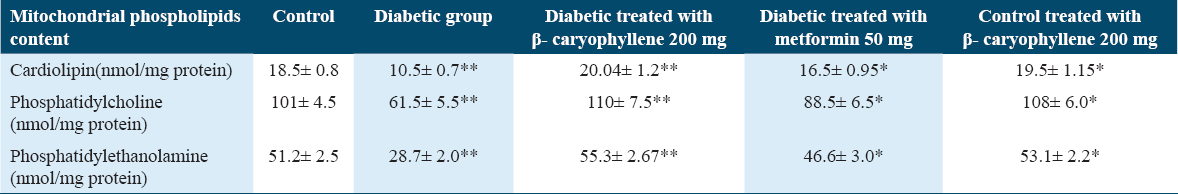
To ascertain if BCP may modify the phospholipid content in diabetic rats, we used a HPLC approach to access the phospholipid content in mitochondrial extraction in rats treated for 30 days. The study reports indicated that the diabetic group holds less phospholipids and a higher amount of peroxided cardiolipin. The quantities of phospholipids were improved, and peroxided cardiolipin dramatically decreased after 30 days of therapy with BCP. Figure 6 and the ability of the supplemented natural phytonutrient BCP against mitochondrial sub-cellular oxidative stress in experimental diabetic animals are illustrated in Figures 6 and Table 1.
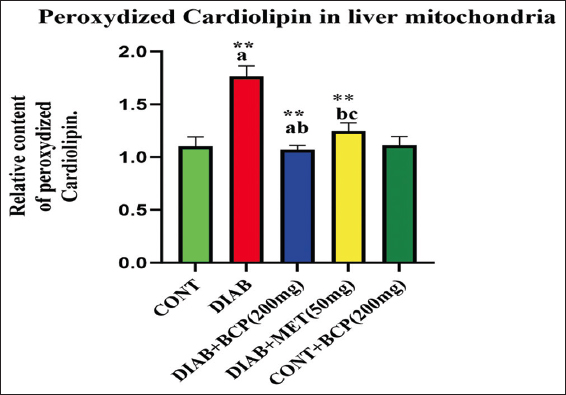
- Impact of β-caryophyllene on mitochondrial cardiolipin diene in experimental rats
In the bar diagram, the results represent the arithmetic mean with ± predictable error of six experimental animals. The statistical drift of the study was set at P < 0.05* or P < 0.01**. ANOVA between the experimental groups was performed and mentioned in the bar diagram as a symbol of a-compared with the diabetic group mean and c-compared with the mean of 200 mg/b.wt BCP-treated animals.
A comparison was made between rats from the control and experimental groups regarding the quantity of peroxidized cardiolipin found in their liver tissue during mitochondrial extraction. The concentration of cardiolipin dienes is expressed as peak area (at 235 nm) per mg of membrane phospholipids in mitochondrial extract, assuming that the peak area of the control serves as the unit. In the bar diagram, the results represent the arithmetic mean with ± predictable error of six experimental animals. The statistical drift of the study was set at P < 0.05* or P < 0.01**. ANOVA between the experimental groups was performed and mentioned in the bar diagram as a symbol of a-compared with the diabetic group mean and c-compared with the mean of 200 mg/b.wt BCP-treated animals.
Discussion
Blood glucose monitoring and control strategies are helpful in reducing clinical complications noticeably, according to the World Health Organization (WHO), the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, and the Diabetes Control and Complications Trial (DCCT). However, issues did not completely resolve even with the best blood glucose control.[23-26] Diabetes mellitus (DM) is a mild inflammatory condition associated with a rise in the production of free radicals and a decrease in antioxidant defenses,[22,27] it indicating the need for other treatment approaches.
The naturally occurring sesquiterpene BCP has biological consequences that are antioxidant, anti-inflammatory, hypolipiedimic, and hypoglycemic.[26] Because of these biological effects, BCP is an excellent choice for treating DM when combined with other oral hypoglycemic medications to manage complications of DM. In our research, the impact of BCP on mitochondrial dysfunction brought on by high fat and high fructose levels in the hepatocytes as a result of free radical generation in type-2 diabetic experimental rats was examined. The antioxidant BCP reduces mitochondrial dysfunction.
Reduced GSH provides a primary defense against polar oxidants, as it can capture water-soluble free radicals and reduce hydrogen peroxide formation in cells.[28] The level of MDA [Figure 1a] along with PCO [Figure 1b] is the first-line indicator of membrane peroxidation, particularly PCO, which begins as a more early and stable marker than other oxidative parameters.[20] GSH [Figure 3c] levels in HFFD-induced type-2 diabetic rat mitochondria treated with BCP were safeguarded significantly compared to diabetic control rats. In the present study, mitochondrial MDA was discovered that PCO formation was decreased when HFFD and BCP were co-incubated with mitochondria in comparison with type-2 diabetics who only received HFFD treatment.
In the current investigation, elevated ROS levels were seen in the mitochondria of diabetic rat livers produced by HFFD. In diabetic rats, the elevated ROS level may reduce the essential activity of the inner biomembrane of mitochondria and result in the leakage of hydrogen ions due to a loss of permeability.[20] Oxidative stress affected the inner mitochondrial membrane’s physiological processes.[29] Elevated ROS during diabetic conditions stimulates the rancidity of the mitochondrial biomembrane and the generation of lipid-peroxy radicals, which, when combined with PUFA, cause swelling and structural changes to the mitochondrial membrane.[30] In addition, a reduction in the antioxidant defense system may result from increased ROS generation [Figures 2 and 3]. The synergistic action of decreased antioxidant and increased ROS targets to damage the unsaturated double bonds in lipid bilayers leads to a loss of the physical and biological role of biomembrane as well as cellular function. The enzymatic antioxidant systems SOD [Figure 2a], CAT [Figure 2b], GPx [Figure 2c], GR [Figure 3a], GST [Figure 3b], and reduced GSH [Figure 3c] make up the cellular defense mechanism against oxidative stress. Oxidative stress-produced peroxides can be removed using an enzyme system.[31] The liver’s enzymatic antioxidant capacity is increased in HFFD-induced diabetic (type-2) experimental rats given 200 mg of BCP, which also reduces protein carbonylation and lipid peroxidation. This might be because BCP has strong antioxidant properties.
In hepatic lipid metabolism, mitochondria contribute to numerous oxygen-dependent metabolic activities, inclusive of the transport and oxidation of free fatty acids, and they are essential for preserving a healthy lipid metabolism.[32] Oxidative stress plays an essential part in the pathophysiology of insulin resistance and diabetes through a variety of molecular pathways, including beta cell failure, inflammatory responses, and mitochondrial malfunction.[33] Additionally, studies have demonstrated that the etiology of chronic inflammatory liver disorders involves oxidative stress.[34] In the diabetic rats in our investigation, we also observed decreased GSH content, increased LPO, increased PCO, and decreased respiratory chain activity that produced ROS from mitochondrial enzyme activity and ROS. Increased mitochondrial oxidation, which takes place in the initial phases of NAFLD due to increased lipid flux, worsens ROS generation and creates hazardous lipid intermediates.[35] Superoxide anions are produced by the mitochondrial respiratory chain’s complexes I, II, and III, which are a key source of pro-oxidants.[36] The potential for enzymatic and non-enzymatic antioxidants can be decreased by electron leakage from the mitochondrial respiratory chain.[37] A number of substrates are oxidized by aerobic metabolic enzymes like oxidoreductase in the mitochondria, which is used in the Krebs cycle to produce reduced electron carriers that donate electrons to ETC for ATP generation by chemiosmotic mechanisms.[37] The activities of mitochondrial oxidoreductase enzymes were significantly reduced in the hepatocytes of HFFD-induced experimental diabetic animals in our study [Figure 4]. Our results are consistent with earlier research (García-Berumen et al., 2019).[38] Elevated levels of ROS may be a causative agent for the decreased activity of these mitochondrial enzymes because they can cause oxidative protein damage that renders the NADH-reductase and cytochrome-c-reductase enzymes of the electron transport chain inactive. The decreased mitochondrial enzyme activity during diabetes may reduce mitochondrial function. When HFFD-induced diabetic experimental animals were supplemented with BCP compared to diabetic control animals, the TCA cycle enzymes’ activity improved. This result implies that by enhancing their antioxidant capacity and reducing the mitochondrial damage linked to diabetic issues, BCP may have restored mitochondrial functionality.[39]
Changes in the ETC complex’s activities are key factors in ROS production under physiologically normal circumstances.[37] The increased production of ROS caused changes in ETC complex activity in a number of clinical diseases.[40] According to many research, oxidative stress, hyperglycemia, and problems linked to diabetes may all be closely related.[41] An essential ETC membrane enzyme complex is called Complex I; it captures electrons from reduced NADH and transfers them to ubiquinone, whereas Complex IV does the same to catalyze the electrons from cytochrome c to oxygen.[42] Since ATP synthesis is known to be a chemiosmotic process, it is imperative that positive charges be separated across the mitochondrial biomembrane and electrons flow over the inner mitochondrial membrane.[43] According to the study’s findings, there was a noteworthy decrease in NADH-reductase (Complex-I) and cytochrome-c-oxidase enzymes (Complex-IV) involvement in the hepatic mitochondrial tissue of diabetic rats [Figure 5]. This result is consistent with recent studies (Zhang et al., 2023).[44] Because TCA cycle oxidoreductase enzyme activity produces less of Complex I’s substrate, NADH, the activity of Complex I may have decreased [Figure 5]. Contrarily, when compared to diabetic rats who were not treated, those who were treated with BCP had higher Complex-I activity. Throughout this investigation, the cytochrome-c-oxidase enzyme activity in the diabetic experimental rats was noticeably reduced [Figure 5]. These findings concur with those of earlier research (Ramesh, 2021).[20] In this work, when diabetic rats were given BCP, the activity of the mitochondrial enzyme cytochrome c oxidase was increased in their livers. A different strategy to lessen excessive ROS formation and prevent the entirety of the ETC activity, Complex IV, is crucial, and it works well with Complex III. Potential degradation of crucial cell components is the activation of ETC complexes by BCP.
Conclusion
In the final analysis, our findings show that oxidative injury impairs mitochondrial function in diabetic rats. The peroxidative attack of oxygen-free radicals on the unsaturated fatty acids in cardiolipin appears to be at least partially to blame for this decline’s molecular cause. Complexes I and IV are thought to be the controlling steps of the cellular respiratory chain and a key location for the production of oxygen-dependent free radicals in the cell. Cardiolipin damage brought on by complex-I and IV dysfunction brought on by ROS in the diabetic liver may worsen electron leakage from the mitochondrial respiratory chain, lower antioxidant potential, increase oxidative stress, and continue a periodic evolution of oxygen species-induced mitochondrial biological membrane injury that eventually results in hepatocyte mitochondrial dysfunction. This demonstrates the antioxidant potential of the naturally occurring sesquiterpene BCP. In contrast, diabetic experimental rats supplemented with the natural phytonutrient BCP had increased biocatalysts-based and GSH-dependent antioxidant potential and preserved mitochondrial functions.
Our findings may be helpful in explaining why metabolic changes like fatty liver changes in diabetes are caused by mitochondrial dysfunction, which was prevented by the natural compound BCP, as well as in informing the development of effective treatment regimens.
Author Declaration Statements
Ethics approval
Ethical approval of this study was taken from the Meenakshi University Animal ethical committee (# CPCSEA-007/2019).
Availability of data and materials
Will be provided by the corresponding upon reasonablee request.
Conflicts of Interest
None.
Financial resources
Not funded.
Author’s Contributions
ID created the experimental study design and carried out the experiment; SC analyzed the data and edited the manuscript. VM composed the scientific draft and performed the hypothesis analysis. MN reviewed and verified the text. Approval of this article was given by all authors.
References
- Diabetes 2030:Insights from yesterday, today, and future trends. Popul Health Manag. 2017;20:6-12.
- [Google Scholar]
- Diabetes mellitus and its metabolic complications:The role of adipose tissues. Int J Mol Sci. 2021;22:7644.
- [Google Scholar]
- Protective role of mitochondrial uncoupling proteins against age-related oxidative stress in type 2 diabetes mellitus. Antioxidants (Basel). 2022;11:1473.
- [Google Scholar]
- Is mitochondrial dysfunction a common root of noncommunicable chronic diseases? Endocr Rev. 2020;41:bnaa005.
- [Google Scholar]
- Mitochondrial dysfunction in the liver of type 2 diabetic Goto-Kakizaki rats:Improvement by a combination of nutrients. Br J Nutr. 2011;106:648-55.
- [Google Scholar]
- Awareness of type 2 diabetic patients about the importance of exercise and diet on diabetes type 2 in the Western Region of Saudi Arabia. Mater Sociomed. 2021;33:276-81.
- [Google Scholar]
- The potential application of Chinese medicine in liver diseases:A new opportunity. Front Pharmacol. 2021;12:771459.
- [Google Scholar]
- Prevalence and markers of advanced liver disease in type 2 diabetes, QJM. 2012;105:425-32.
- Dietary patterns and risk of non-alcoholic fatty liver disease. BMC Gastroenterol. 2021;21:41.
- [Google Scholar]
- Ferulic acid exerts its antidiabetic effect by modulating insulin-signalling molecules in the liver of high-fat diet and fructose-induced type-2 diabetic adult male rat. Appl Physiol Nutr Metab. 2015;40:769-81.
- [Google Scholar]
- A systematic review on the neuroprotective perspectives of beta-caryophyllene. Phytother Res. 2018;32:2376-88.
- [Google Scholar]
- Essential oils chemistry. In: de Sousa DP, ed. Bioactive Essential Oils and Cancer. Switzerland: Springer International Publishing; 2015. p. :19-28.
- [Google Scholar]
- Beta-caryophyllene is a dietary cannabinoid. Proc Natl Acad Sci U S A. 2008;105:9099-104.
- [Google Scholar]
- Dose-dependent effect of β-caryophyllene on Glycemic control of high-fat diet and fructose-induced type-2 diabetic rats. J Kerman Univ Med Sci. 2022;29:341-7.
- [Google Scholar]
- Antioxidant and hepatoprotective effects of Capparis spinosa L. fractions and Quercetin on tert-butyl hydroperoxide-induced acute liver damage in mice. J Tradit Complement Med. 2017;8:120-7.
- [Google Scholar]
- Oxidative stress and hepatocellular mitochondrial dysfunction attenuated by asiatic acid in streptozotocin-induced diabetic rats. J King Saud Univ Sci. 2021;33:101369.
- [Google Scholar]
- Spectrophotometric methods for measurement of antioxidant activity in food and pharmaceuticals. Antioxidants (Basel). 2022;11:2213.
- [Google Scholar]
- The effect of reactive oxygen species generated from the mitochondrial electron transport chain on the cytochrome c oxidase activity and on the cardiolipin content in bovine heart submitochondrial particles. FEBS Lett. 2000;466:323-6.
- [Google Scholar]
- Action to control cardiovascular risk in diabetes (ACCORD) trial:Design and methods. Am J Cardiol. 2007;99:21i-33i.
- [Google Scholar]
- DCCT/EDIC Research Group. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years:Overview. Diabetes Care. 2014;37:9-16.
- [Google Scholar]
- Diabetes. Available from: https://www.who.int/news-room/fact-sheets/detail/diabetes
- Inflammation, oxidative stress and mitochondrial dysfunction in the progression of type II diabetes mellitus with coexisting hypertension. Front Endocrinol (Lausanne). 2023;14:1173402.
- [Google Scholar]
- Beta-caryophyllene protects against diet-induced dyslipidemia and vascular inflammation in rats:Involvement of CB2 and PPAR-g receptors. Chem Biol Interact. 2019;297:16-24.
- [Google Scholar]
- Organic Zn and Cu supplementation imprints on seminal plasma mineral, biochemical/antioxidant activities and its relationship to spermatozoal characteristics in bucks. Reprod Biol. 2020;20:220-8.
- [Google Scholar]
- Role of membrane disturbance and oxidative stress in the mode of action underlying the toxicity of differently charged polystyrene nanoparticles. RSC Adv. 2014;4:19321-30.
- [Google Scholar]
- Mitochondrial electron transport chain, ROS generation and uncoupling (Review) Int J Mol Med. 2019;44:3-15.
- [Google Scholar]
- The pathomechanism, antioxidant biomarkers, and treatment of oxidative stress-related eye diseases. Int J Mol Sci. 2022;23:1255.
- [Google Scholar]
- Role of oxidative stress in the pathogenesis of non-alcoholic fatty liver disease:Implications for prevention and therapy. Antioxidants (Basel). 2021;10:174.
- [Google Scholar]
- Pathophysiology, phenotypes and management of type 2 diabetes mellitus in Indian and Chinese populations. Nat Rev Endocrinol. 2022;18:413-32.
- [Google Scholar]
- Mitochondrial metabolic dysfunction and non-alcoholic fatty liver disease:New insights from pathogenic mechanisms to clinically targeted therapy. J Transl Med. 2023;21:510.
- [Google Scholar]
- The alterations of mitochondrial function during nafld progression-an independent effect of mitochondrial ROS production. Int J Mol Sci. 2021;22:6848.
- [Google Scholar]
- Mitochondrial dysfunction and oxidative stress in metabolic disorders-a step towards mitochondria based therapeutic strategies. Biochim Biophys Acta Mol Basis Dis. 2017;1863:1066-7.
- [Google Scholar]
- Mitochondrial TCA cycle metabolites control physiology and disease. Nat Commun. 2020;11:102.
- [Google Scholar]
- The severity of rat liver injury by fructose and high fat depends on the degree of respiratory dysfunction and oxidative stress induced in mitochondria. Lipids Health Dis. 2019;18:78.
- [Google Scholar]
- Beta-caryophyllene as an antioxidant, anti-inflammatory and re-epithelialization activities in a rat skin wound excision model. Oxid Med Cell Longev. 2022;2022:9004014.
- [Google Scholar]
- Is mitochondrial dysfunction a common root of noncommunicable chronic diseases?Endocr Rev. . 2020;41:bnaa005.
- [Google Scholar]
- A review on oxidative stress, diabetic complications, and the roles of honey polyphenols. Oxid Med Cell Longev. 2020;2020:8878172.
- [Google Scholar]
- Biochemistry, electron transport chain. 2023. StatPearls. Treasure Island, FL: StatPearls Publishing; Available from: https://www.ncbi.nlm.nih.gov/books/NBK526105
- [Google Scholar]
- 2.10-ATP production II:The TCA cycle and oxidative phosphorylation. In: Quantitative Human Physiology (2 nd ed). United States: Academic Press; 2017. p. :227-40.
- [Google Scholar]
- The impact of oxidative stress-induced mitochondrial dysfunction on diabetic microvascular complications. Front Endocrinol (Lausanne). 2023;14:1112363.
- [Google Scholar]







