Translate this page into:
Chondroitin sulfate produces antinociception and neuroprotection in chronic constriction injury-induced neuropathic pain in rats by increasing anti-inflammatory molecules and reducing oxidative stress
Address for correspondence: Bamidele Victor Owoyele, Department of Physiology, Neuroscience and Inflammation Unit, College of Health Sciences, University of Ilorin, Ilorin, Kwara State, Nigeria. Tel: +2348035065190. E-mail: owoyele@unilorin.edu.ng
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Qassim Uninversity and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objectives:
Damage to the peripheral and central nervous system lead to Neuropathic pain (NP) which is a widespread and devitalizing condition. chondroitin sulfate (CS), has been used in managing joint pain and osteoarthritis. In this study, the effectiveness of CS on NP induced by chronic constriction injury (CCI) is examined.
Methods:
Thirty Wistar rats were distributed at random into six groups (n = 5). Sciatic nerve ligation was carried out by encircling the nerve with four loose ligatures to induce NP. Allodynia (cold and mechanical) and heat hyperalgesia were assessed using Acetone, von Frey filament and Hot plate tests. CCI induction resulted to NP, prominent from the 3rd day after surgery. Structural architecture of sciatic nerves was evaluated via histological examination of the transverse section of the nerves.
Results:
Oral administration of CS (600 mg/kg and 900 mg/kg for 21 days) resulted in significant (P < 0.05) inhibition of allodynia (cold and mechanical) and thermal hyperalgesia. Lipid peroxidation, tumor necrosis factor-α (TNF-α), calcitonin gene related peptide (CGRP), C reactive protein (CRP), and oxidative stress were attenuated by CS. CS also improved interleukin (IL)-6, nitric oxide (NO), total antioxidant capacity (TAC).
Conclusion:
These findings suggest that CS attenuates allodynia, and thermal hyperalgesia induced by CCI by downregulating TNF-α, CRP, CGRP, oxidative enzymes, and upregulating IL-6, NO, and TAC. Nociceptive behavioral studies and histological findings showed significant improvement in the CS treated groups compared to CCI rats. These findings are responsible for the beneficial effect of CS in NP.
Keywords
Chondroitin sulfate
chronic constriction injury
imipramine
neuropathic pain
sciatic nerve
Introduction
Neuropathic pain (NP) is a chronic condition emanating from injury to the somatosensory system.[1] The symptoms associated with it include hyperalgesia and allodynia. Despite immense advancement in NP treatment approaches, effective treatment of patients in this debilitating condition remains a challenge.[2] Hence, it is imperative to explore efficacious and safe options in managing NP.
Accrued evidence have shown the role of reactive oxygen species (ROS) and inflammatory cytokines in the progression of NP.[3] Damage to the nerves stimulates the inflammatory mediators release, such as tumor necrosis factor (TNF)-α and interleukin (IL)-1β.[4] Likewise, nuclear factor (NF)-κB, which plays a key role in the regulation of the inflammatory process, has been reported to be activated in NP.[5] Blockade of the inflammatory cytokines mitigates hyperalgesia and allodynia induced by nerve damage.
Conventional treatments used for NP have limited benefits with several side effects which include ataxia, loss of weight, mood disorders, and slurred speech which all result in poor compliance with treatment that makes the drugs less effective in attenuating pain.[6] Therefore, researchers are in search of alternative remedies that could produce fewer side effects. chronic constriction injury (CCI) model of NP is widely used for the determination of the efficacy of potential drugs against NP. CCI is relatively simple to carry out and gives a resultant hypersensitive pain that is robust, stable and continue for a minimum of 1 month after its initiation.[7] A report have shown that CCI triggers immune cells activation and as well as the production of some mediators of inflammation.[8] These inflammatory mediators play key roles in the progress of neuropathic.[9] Studies have reported a pragmatic link between serum levels of TNF-α and C-reactive protein (CRP) in patients with neuropathy.[10] Thus, a study on the effects of pro-inflammatory and anti-inflammatory mediators as well as those of antioxidants with treatment interventions in a CCI model could play key roles in the elucidation of the pathophysiology of NP. Such studies may provide information in the discovery of new targets for analgesic agents.[11]
Chondroitin sulfate (CS), a member of the family of galactosaminoglycans (GalAGs), are compounds designated as symptomatic slow-acting drugs for the treatment of osteoarthritis due to its chondroprotective and anti-inflammatory properties.[12] GalAGs have a broad spectrum of biological and medical importance. including cell growth and differentiation.[12-15] Due to the many biological and medical importance of GalAGs, many researchers are now conducting research on GalAGs. The mechanism by which CS relieves pain is still unclear. Although, Nemoto et al.[16] while using the partial sciatic nerve ligation (PSNL) model in mice reported that antinociceptive effects of CS is due to inhibition of the phosphorylation of spinal p38 MAPK and activation of Aβ-fibre. The present study investigated whether CS is effective against NP induced by CCI by assessing pain behavioral, biochemical, and histological parameters. The findings from this study could be useful in the development of effective CS based therapies for the treatment of NP.
Methods
Animals
Experimental procedures were approved with the approval number University of Ilorin Ethical Review Committee (UERC/ASN/2019/1949) and carried out according to the guiding principles of the UERC. Wistar (male) rats with a weight range 200–250 g, were selected for this study and were sheltered in the Animal House of the Faculty of Basic Medical Sciences, College of Health Sciences, University of Ilorin. All animals were accustomed to their environment for 2 weeks ahead of the onset of experiments. Experiments were performed between the hours of 9 AM–5 PM. Animals were fed ad libitum and housed (five per cage) in wooden cages with 12 h light/dark cycle. The rats were kept in conducive housing. They were also handled according to the guidelines set by the National Institute of Health in their Guide for the Care and Use of Laboratory Animals.[17]
Chemicals and drugs
Analytical grade CS (Hefe-Joyce, China) was used for oral treatment, sodium pentobarbital (Sigma-Aldrich, USA) was used as anesthesia, Ca2+, LDH assay kit, K+ assay kit (Fortress diagnostics, United Kingdom), TGF and TNF-α (Elabscience, USA) were used for biomarker assays. Other chemicals and materials were of analytical grades and obtained from Bridge Biotech Ltd, Nigeria. 4.0 silk suture was used for CCI and 4.0 chromic catgut suture was used to suture the skin.
Design of the experiment
Animals were allotted into 6 groups (where n = 5 rats per group): (1) Non-ligated Control group: The rats in this group received normal saline (12 ml/kg oral) as treatment without ligation. (2) Sham group: the skin of the rats in this group and the underlining tissues were opened and sutured back without ligating the nerve. The animals were administered normal saline (12 ml/kg oral) as treatment. (3) CCI group: The rats were induced with chronic constriction of the sciatic nerve and treated with normal saline. (4) CS1 group: animals were ligated and treated with low dose of CS (600 mg/kg oral). (5) CS2 group: animals were ligated and treated with high dose CS (900 mg/kg oral). (6) IMI group: animals were ligated and treated with imipramine (10 mg/kg oral) a reference standard.
The duration of treatments was 21 days after the basal assessment tests.
NP induction
The well-documented CCI model of neuropathy was adopted to bring about chronic pain in rats.[18] The rats were benumbed with sodium pentobarbital (90 mg/kg i.p.) and the hair on the skin of the lower back of the rats were barbed. Thereafter, 70% isopropyl alcohol and iodine solution were applied to disinfect barbed area. The incision was made on the right hind limb skin, the sciatic nerves below the femoris muscle were exposed [Figure 1a]. The sciatic nerves were then ligated and the gut ligatures were used to constrict the nerves at four sites with 1-mm gap [Figure 1b]. The skin of the right hind limb of the sham control (SC) group and the femoris muscle was incised to expose rats’ sciatic nerves without ligating the sciatic nerve. The skin was sutured with 4.0 chromic catgut suture [Figure 1c]. The non-ligated control rats had no incision.[19] Pain hypersensitivity examinations were carried out on the 2nd-day pre-CCI and 3rd, 7th, 14th, 21st-day post-CCI across all the groups.
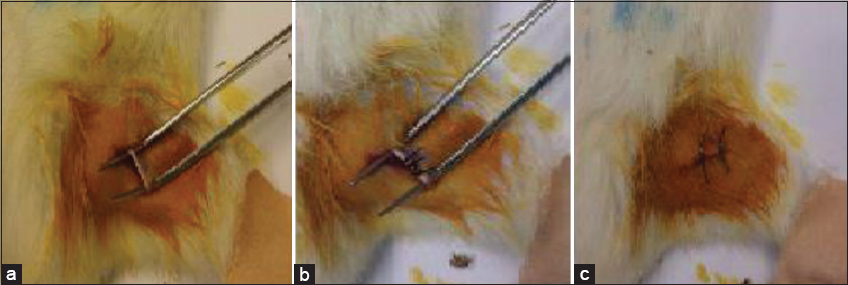
- Chronic constriction injury Induction (a) exposed right sciatic nerve (b) ligated right sciatic nerve (c) sutured skin of the right hind limb
Pain behavioral examinations
NP behavioral examinations composed of three tests: mechanical allodynia, thermal hyperalgesia, and cold allodynia.
Mechanical allodynia (von frey test)
In examining sciatic nerve sensory functionality, the threshold of paw withdrawal was measured in response to mechanical stimulation with the aid of von Frey filament (Ugo Basile, Italy); 10 filaments were selected.[20] The rats were kept in plastic cages with a metal mesh basement and were allowed to be accustomed to the environment for 10 min before testing. von Frey filaments with the order of forces, 4.17 g, 4.31 g, 4.56 g, 4.74 g, 4.93 g, 5.07 g, 5.18 g, 5.46 g, 5.88 g, and 6.1 g, were applied to the plantar surface of the hind paw. Each paw was tested 5 times, mechanical pain threshold was estimated according to the up and down method used by Bonin et al.[21]
Pre-induction (Pre-I or BL) nociceptive threshold were assessed (2 days before CCI) with von Frey filament to determine the basal threshold, post-induction (Post-I) nociceptive threshold were assessed 3 days after CCI intervention and then on the 10th, 17th, and 24th day of the commencement of drug treatments.
Thermal hyperalgesia (hot-plate test)
Hot plate was used to measure the thermal sensitivity of the hind paw (Hefe-joyce, China). The rats were gently placed the surface of the hot plate (55.5°C ± 0.5°C) plate, and paw withdrawal latency in seconds was recorded (a cut-off time was put at 20s).[22]
Cold allodynia
Acetone spray test was used to examine paw withdrawal latency to cold stimulus.[23,24]
Histological analysis
At the end of the of treatment, 5–10 h after the last dose of drug treatment, the rats were anaesthetized and the sciatic nerve was dissected proximal to bifurcation point. All animals were anaesthetized by sodium pentobarbital. Formalin (10%) was used to fix the sciatic nerve, the nerves were included in paraffin. Transverse sections of 7 μm thick were obtained and the sections were subsequently stained with hematoxylin and eosin.[25] The histology slides were analyzed by a blind reading person, based on the following parameters: analysis, nerve constituents, such as epineurium, perineurium and endoneurium, as well as the nerve fiber, presence of inflammatory infiltrates, Schwann cells, fibroblasts, and blood vessels were analyzed based on the images taken from the hind limbs.
Biochemical analysis (sciatic nerve)
At the end of the of treatment, 5–10 h after the last dose of drug treatment, half of the sciatic nerve from the spine to the bifurcation point was dissected, and the proximal part to the spine make up the half that was rinsed in ice-cold saline solution and frozen for biochemical analysis. The sciatic nerve segment was weighed and rinsed in ice-cold saline solution on the day of homogenate preparation. The sciatic nerve was homogenized with a glass homogenizer at 4°C in 2 ml of ice-cold saline (11 mmol/L Tris buffer, pH 7.4).[26] To remove impurities, the homogenate was filtered using a cellulose filter and was divided into fractions for biochemical analysis.[25] Analysis for superoxide dismutase, catalase (CAT), and malondialdehyde (MDA) activities was carried out in the sciatic nerve.
Biochemical analysis (serum and brain)
At the end of treatment, 5–10 h after the last dose of drug treatment, tubes without anticoagulant were used to collect blood serum specimens for biochemical analysis. Serum samples were obtained from collected blood samples after centrifugation at 3000 × g for 10 min at room temperature and then stored at -80°C for subsequent analysis of IL-6, NF-κB-p65, TNF-α, K+, Ca2+. Brains were excised and immediately rinsed with 0.32 M of cold sucrose and immediately put in (1.35 ml × brain weight sample) 0.32 M of cold sucrose solution. The brains were homogenized in cold sucrose solution. The homogenized brain samples were centrifuged at 1500 g × 15 min, after which supernatants were micro-pipetted into plain bottles and immediately stored at a temperature of < −4°C for further analysis of NO and LDH.
Determination of Sciatic nerve MDA
The assay of MDA was by a non-enzymatic colorimetric assay kit obtained from Oxford Biochemical Research Inc., Oxford, USA. MDA assay in the homogenized tissue sample was based on the reaction of a chromogenic reagent, 2-thiobarbituric acid, with MDA at 25°C. One molecule of MDA reacted with 2 molecules of 2-thiobarbituric acid via a Knoevenagel-type condensation to yield a chromophore with an absorbance maximum at 532 nm.[27]
Determination of sciatic nerve CAT, superoxide dismutase, and serum total antioxidant capacity
The assay method of CAT was based on the measurement of the hydrogen peroxide substrate remaining after the action of CAT. First, CAT converted hydrogen peroxide to water and oxygen (catalytic pathway), and then this enzymatic reaction was stopped with sodium azide. An aliquot of the reaction mix was assayed for the amount of hydrogen peroxide remaining by the colorimetric method.[28]
The colorimetric method uses substituted phenol (3,5-dichloro-2-hydroxybenzenesulfonic acid), which couples oxidatively to 4-aminoantipyrine in the presence of hydrogen peroxide and horseradish peroxidase (HRP) to give a red quinoneimine dye (N-(4-antipyryl)-3-chloro-5-sulfonatep-benzoquinone-monoimine) that absorbs at 520 nm.
The principle of the method of assay used for SOD is based on the competition between the pyrogallol autoxidation by O2¯ and the dismutation of this radical by SOD. The enzyme Superoxide dismutase can inhibit the autoxidation of pyrogallol. The autoxidation of pyrogallol in the presence of EDTA in pH 8.2 is 50%.[29]
The principle of the method of assay used for total antioxidant capacity (TAC) is based on the combined action of the antioxidants provided by the sample or standard which acted to reduce Cu2+ to Cu+. This reduced form of copper selectively formed a 2:1 complex with the chromogenic reagent. This complex was stable and had an absorption maximum at ~490 mn. A known concentration of uric acid was used to create a reference curve to compare those readings obtained by the samples.[30]
Determination of brain nitric oxide
Brain nitric oxide (NO) was measured via spectrophotometric quantitation of nitrite using Griess reagent using the kit from Oxford Biomedical Research Inc. (Oxford, USA). The nitric oxide kit employed metallic cadmium for quantitative conversion of nitrate to nitrite before the quantitation of nitrate using Griess reagent — thus providing for accurate determination of total NO production.
Determination of brain lactate dehydrogenase (LDH)
LDH activity were determined in the brain tissue homogenates using an assay kit obtained from Oxford Biomedical Research Inc. (Oxford, USA). LDH catalysis the conversion of pyruvate to lactate; NADH is oxidized to NAD in the process. The rate of decrease in NADH is directly proportional to the LDH activity and is determined by the measurement of the rate of absorbance change at 340 nm due to the reduction.
Determination of serum calcitonin gene related peptide (CGRP) and CRP
Serum CGRP and CRP activity were determined in the brain tissue homogenates using assay kit obtained from Bioassay Technology Laboratory (Shanghai, China). The kit uses the Sandwich- Enzyme-Linked Immunosorbent Assay (ELISA) principle. The micro ELISA plate accompanying the kit was pre-coated with an antibody specific to Rat CGRP and CRP. Standards or samples were added to the micro ELISA plate wells and thereafter combined with the specific antibodies. Then a biotinylated detection antibody specific for Rat CGRP and CRP, and Avidin-HRP conjugate were added consecutively to each micro plate well and incubated. Free components were washed away. The substrate solution was added to each well. Only the wells that contain Rat CGRP and CRP, biotinylated detection antibody, and Avidin-HRP conjugate turned blue. The enzyme-substrate reaction was ended by the addition of stop solution and the color became yellow. The optical density was spectrophotometrically measured at a wavelength of 450 nm ± 2 nm. The optical density value was proportional to the concentration of Rat CGRP and CRP. The concentration of Rat CGRP and CRP in the samples was calculated by comparing the optical density of the samples to the standard curve.
Determination of serum IL-6, TNF-alpha, and NF-κB-p65
Serum IL-6, TNF-alpha, and NF-κB-p65 activity were determined in the serum using an ELISA kit obtained from Elabscience (USA). The plate was pre-coated with Rat IL-6, NF-κB-p65 and TNF-alpha antibodies. IL-6, NF-κB-p65, and TNF-alpha in the sample were added and bonded to antibodies coated on the wells. Thereafter, biotinylated Rat IL-6, NF-κB-p65, and TNF-alpha antibodies were added and bind to IL-6, NF-κB-p65, and TNF-alpha in the sample. Then, Streptavidin-HRP was added and bonded to the Biotinylated IL-6, NF-κB-p65, and TNF-alpha antibodies. Following incubation, the unbound Streptavidin-HRP was washed away. Substrate solution was then added and the color was developed in proportion to the amount of Rat IL-6, NF-κB-p65, and TNF-alpha. The reaction was terminated by the addition of acidic stop solution and absorbance was measured at 450 nm.
Statistical Analysis
Data were reported as mean ± standard errors of the mean. The data obtained from behavioral tests were analyzed with two-way ANOVA followed by Tukey’s post hoc multiple comparison tests. However, the comparison between different groups for biomarkers was analyzed by a one-way ANOVA followed by Tukey’s post hoc test, using Graph Pad Prism 8. P < 0.05 were considered to be statistically significant.
Results
Mechanical allodynia and thermal hyperalgesia test
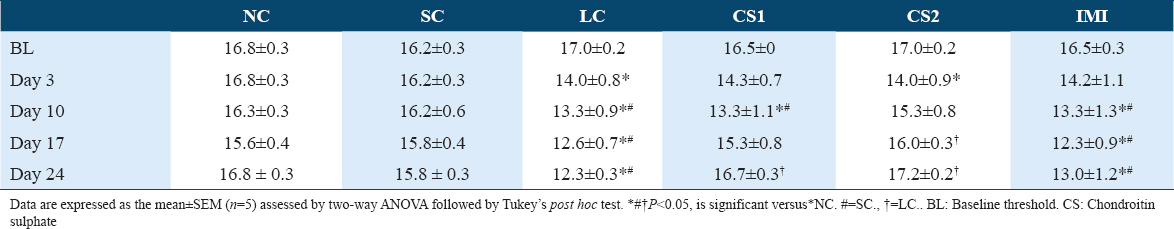
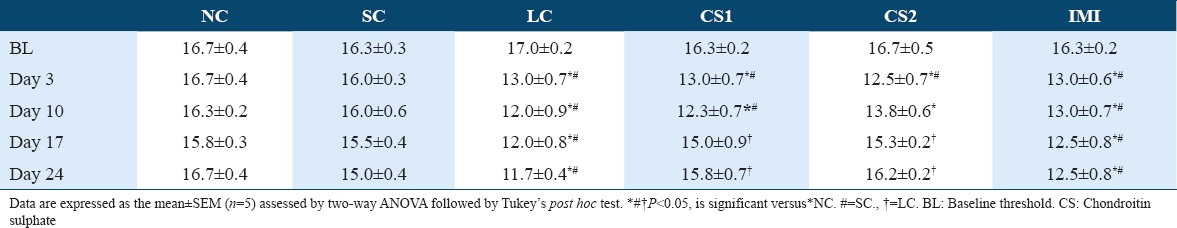
Table 1 shows a significant (P < 0.0004) difference in mechanical pain thresholds of the contralateral paw of non-ligated control (NC) (16.83 ± 0.33 vs. 14.00 ± 0.76) and SC (16.17 ± 0.33 vs. 14.00 ± 0.76) compared to CCI rats. Table 2 shows a significant (P < 0.0001) difference in mechanical pain thresholds of the ipsilateral paw of non-ligated control (NC) (16.67 ± 0.40 vs. 13.00 ± 0.72) and SC (16.02 ± 0.35 vs. 13.00 ± 0.72) compared to CCI rats following CCI intervention. CS at low dose (CS1) significantly reversed contralateral mechanical allodynia on the 17th-day post-CCI (15.33 ± 0.83 vs. 12.67 ± 0.70) and 24th-day post-CCI (16.67 ± 0.31 vs. 12.33 ± 0.31) as shown in Table 1A. Likewise, CS at high dose (CS2) significantly reversed contralateral mechanical allodynia on the 17th-day post-CCI (16.00 ± 0.34 vs. 12.67 ± 0.70) and 24th-day post-CCI (17.17± 0.21 vs. 12.33 ± 0.31) as shown in Table 1A. Also, CS at low dose (CS1) significantly reversed ipsilateral mechanical allodynia on the 17th-day post-CCI (15.00 ± 0.99 vs. 11.67 ± 0.83) and 24th-day post-CCI (15.83 ± 0.67 vs. 11.33 ± 0.40) as shown in Figure 2b. Likewise, CS at high dose (CS2) significantly reversed ipsilateral mechanical allodynia on the 17th-day post-CCI (15.33 ± 0.17 vs. 11.67 ± 0.83) and 24th day post-CCI (16.17± 0.21 vs. 11.33 ± 0.40) [Table 1B]. Imipramine (10 mg/kg) administration did not produce significant difference in paw withdrawal threshold compared to CCI group.
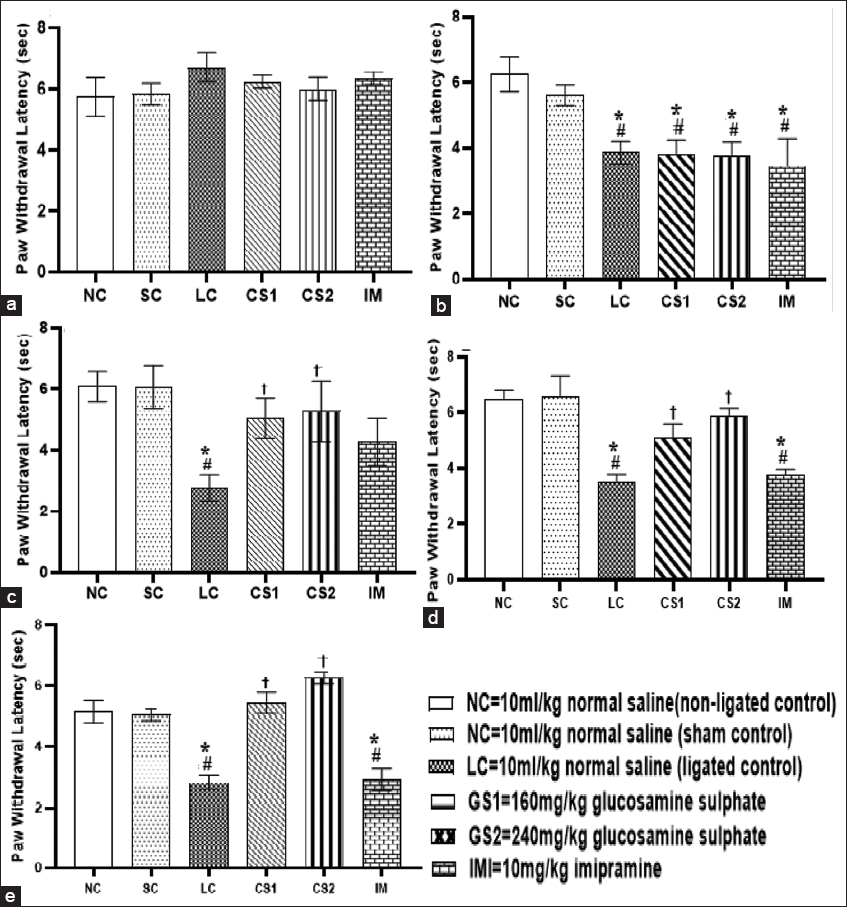
- Chondroitin sulphate reversed thermal hyperalgesia in sciatic nerve-induced neuropathic pain. (a) Pre-surgery thermal test (b) 3rd day thermal test (c) 10th day thermal test (d) 17th day thermal test (e) 24th day thermal test. Data are expressed as the mean ± SEM (n = 5) assessed by two-way ANOVA followed by Tukey’s post hoc test. *#†P < 0.05, is significant versus. *=NC., #=SC., †=LC
Figure 3 shows that CS treatment significantly (P < 0.0001) reduced thermal hyperalgesia which was evidenced by the increase in thermal paw withdrawal threshold of rats following CCI intervention. CCI intervention significantly increased thermal hyperalgesia which was evidenced via significant (P < 0.0001) decrease in paw withdrawal threshold of CCI rats (3.85 ± 0.34 s) compared to the non-ligated control (NC) (6.26 ± 0.52 s) and the SC (5.61 ± 0.30 s) groups 3 days after CCI intervention [Figure 2]. A significant (P < 0.0001) decrease in thermal paw withdrawal threshold of the CCI group was evidenced till day 21 compared to the non-ligated control and SC groups [Figure 2]. A significant increase was observed in the thermal paw withdrawal latency of CS1 rats on the 10th day (5.03 ± 0.67 vs. 2.76 ± 0.43 s), 17th day (5.10 ± 0.49 vs. 3.51 ± 0.26 s), and 24th day (5.43 ± 0.34 vs. 2.80 ± 0.23 s) post-CCI compared to the ligated control. Also, a significant increase in thermal paw withdrawal latency of CS2 rats on the 10th day (5.26 ± 0.41 vs. 2.76 ± 0.43 s), 17th day (5.90 ± 0.25 vs. 3.51 ± 0.26 s), and 24th day (6.26 ± 0.20 vs. 2.80 ± 0.23 s) post-CCI compared to the ligated control [Figure 2a-c respectively].
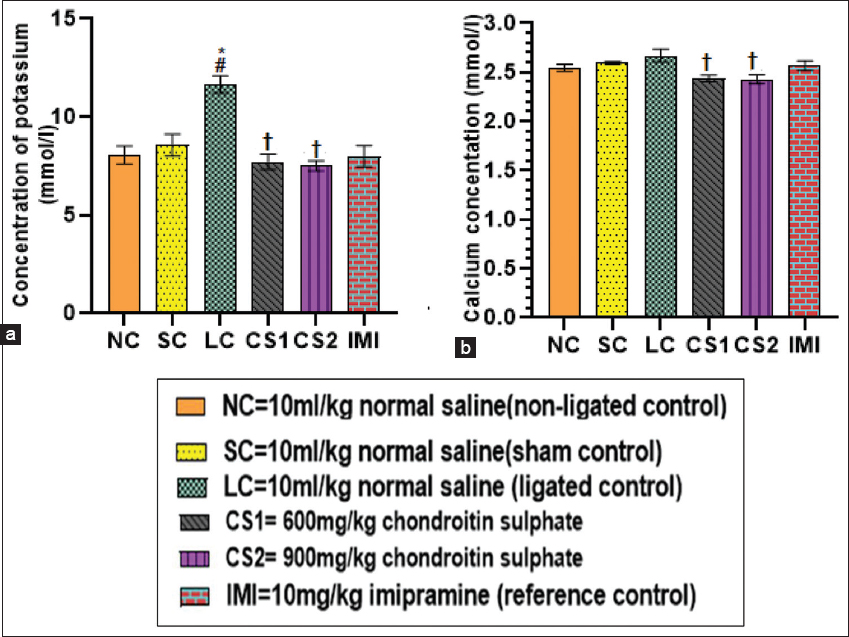
- Chondroitin sulphate reversed serum potassium and calcium (a) potassium concentration and (b) calcium concentration in rats. Data are expressed as the mean ± SEM (n = 5) subjected to one-way ANOVA followed by Tukey’s post hoc test. *#†P < 0.05 is significant versus *NC., #=SC., †=LC
Cold allodynia test
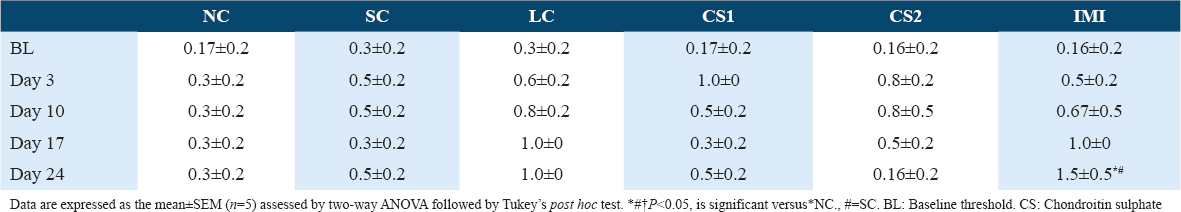
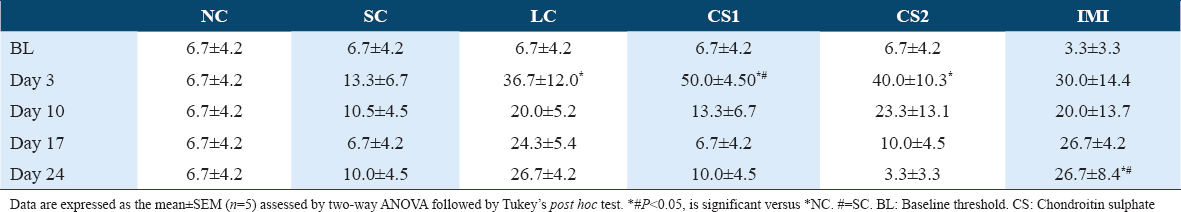
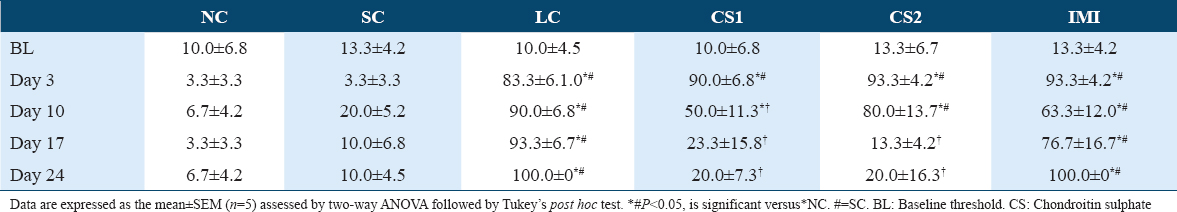
CS treatment significantly (P < 0.0001) attenuated ipsilateral cold allodynia in the ipsilateral paw of rats using acetone drop test [Tables 4 and 6]. This was evidenced by the reduced ipsilateral response score of CS1 groups on day 17 post-CCI (0.83 ± 0.48 vs. 2.67 ± 0.330) and day 24 post-CCI (1.00 ± 0.37 vs. 3.00 ± 0.000) [Table 4] and the ipsilateral response frequencies on day 17 post-CCI (23.33 ± 15.85 vs. 93.33 ± 6.67%) and day 24 post-CCI (20.00 ± 7.30 and vs. 100.00 ± 0.00%) [Table 6]. Also, high dose CS treatment (CS2) reduced ipsilateral response score of CS1 treated groups on day 17 post-CCI (0.67 ± 0.21 vs. 2.67 ± 0.330) and day 24 post-CCI (0.67 ± 0.49 vs. 3.00 ± 0.000) [Table 4] and the ipsilateral response frequencies on day 17 post-CCI (13.33 ± 4.22 vs. 93.33 ± 6.67%) and day 24 post-CCI (20.00 ± 16.33 vs. 100.00 ± 0.00%) [Table 4]. CCI intervention resulted in cold allodynia in the ipsilateral paw of rats on the 3rd day following CCI intervention compared to the non-ligated (NC) control and sham (SC) control groups. This was evidenced by the reduced response score (2.17 ± 0.480) and response frequency (83.33 ± 6.15%) observed in the ipsilateral paws of ligated control (LC) group on the 3rd day following CCI compared to the response scores of NC (0.17 ± 0.170) and SC (0.17 ± 0.170) and the response frequencies of NC (3.33 ± 3.33%) and SC (3.33 ± 3.33%) [Tables 4 and 6]. However, in the contralateral paw of CCI rats, there was no indication of cold allodynia compared to the non-ligated control and SC groups [Tables 3 and 5].

Effect of CS on potassium and calcium ions
Ligated rats showed significant (P < 0.0001) (11.65 ± 0.44 mmol/l) increase in serum concentration of potassium ion compared to non-ligated (8.06 ± 0.46 mmol/l) and SC groups (8.57 ± 0.55 mmol/l) [Figure 3a]. CS1 and CS2 treatment significantly decreased potassium ion concentration (7.71 ± 0.40 mmol/l and 7.51 ± 0.26 mmol/l, respectively) in serum of rats compared to the ligated control group (11.65 ± 0.44 mmol/l) [Figure 3a]. Furthermore, imipramine (10 mg/kg) treated rats also significantly (7.99 ± 0.55 mmol/l) decreased serum potassium ion concentration compared to ligated control rats. Furthermore, the serum concentration of calcium ion of the ligated control rats (2.67 ± 0.07 mmol/l) showed a significant increase compared to CS1 group (2.44 ± 0.03 mmol/l) and CS2 group (2.43 ± 0.05 mmol/l) (P < 0.009) [Figure 3b].
Effect of CS on lipid peroxidation
MDA concentration in the homogenate of the ligated sciatic nerve increased (11.81 ± 1.36 μM) significantly (P < 0.0001) compared to non-ligated (5.13 ± 0.62 μM) and SC (5.44 ± 0.38 μM) rats. CS1 and CS2 rats showed a significant decrease (7.85 ± 0.62 and 7.03 ± 0.25 μM, respectively) in the concentration of MDA compared to the ligated control (11.81 ± 1.36 μM) in the sciatic nerve homogenate [Figure 4a].
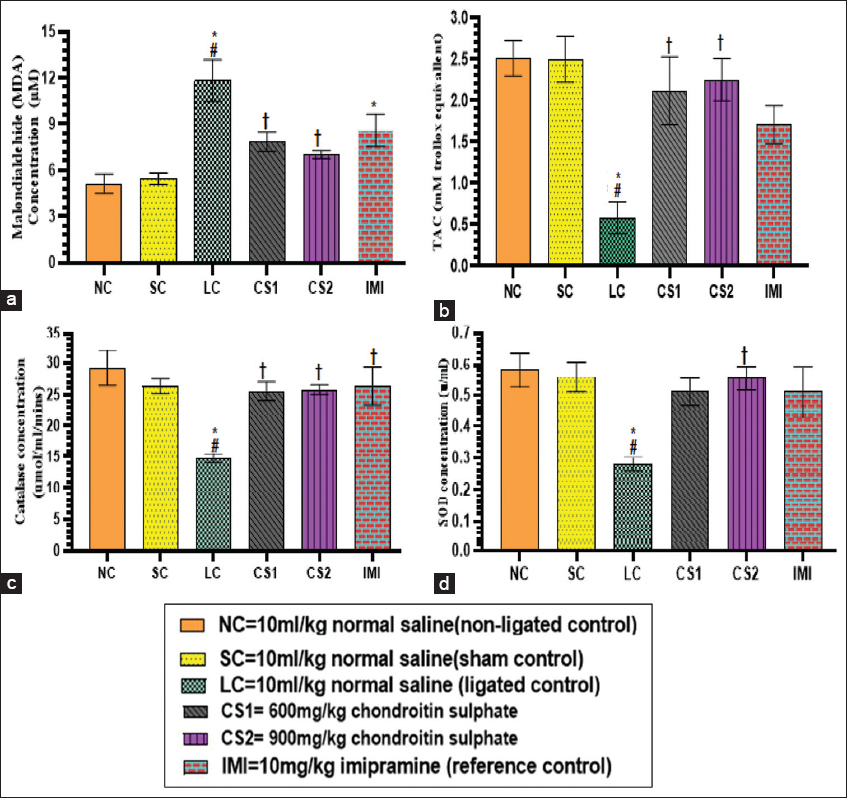
- Chondroitin sulphate reversed lipid peroxidation and antioxidant enzymes in rats. (a) sciatic nerve MDA concentraton (b) serum TAC concentration (c) sciatic nerve CAT concentration (d) sciatic nerve SOD concentration. Data are expressed as the mean ± SEM (n = 5) subjected to one-way ANOVA fllowed by Tukey’s post hoc test. *#†P < 0.05 is significant versus *NC., #=SC., †=LC. CAT: Catalase; SOD: Superoxide dismutase; TAC: Total antioxidant capacity
Effect of CS on antioxidant enzymes
The concentrations of serum TAC and the activities of sciatic nerve CAT and superoxide dismutase (SOD) significantly (P < 0.05) increased in rats administered with CS (600 mg/kg and 900 mg/kg) compared to ligated control rats [Figure 4b-d]. The concentration of TAC in serum of ligated control rats was significantly (P < 0.0004) reduced (0.59 ± 0.19 mM) compared to non-ligated control (2.51 ± 0.21 mM), SC (2.50 ± 0.28 mM) groups as well as CS treated rats. Low dose CS (CS1) and high dose CS (CS2) treated rats showed a significant increase (P < 0.0004) in TAC (2.12 ± 0.41 and 2.25 ± 0.26 mM) compared to ligated control rats (0.59 ± 0.19 mM) [Figure 4b]. Activities of CAT in the homogenate of ligated sciatic nerve of CS1 and CS2 rats (25.58 ± 1.47 and 25.79 ± 0.76 umol/ml/mins, respectively) increased significantly (P < 0.005) compared to ligated control rats (14.74 ± 0.67 umol/ml/mins) [Figure 4c]. Activities of SOD in the homogenate of ligated sciatic nerve of rats treated with CS 900 mg/kg (0.56 ± 0.04 u/ml) increased significantly (P < 0.003) compared to ligated control rats (0.28 ± 0.02 u/ml) [Figure 4d].
Effect of CS on serum c-reactive protein and calcitonin gene-related peptide
The concentration of CRP and CGRP in serum of ligated control rats were increased significantly compared to non-ligated and SC rats. The concentration of CRP in the serum CS1 and CS2 rats (0.43 ± 0.03 ng/ml and 0.39 ± 0.03 ng/ml respectively) decreased significantly (P < 0.0001) compared to the ligated control rats (0.61 ± 0.03 ng/ml) [Figure 5b]. Imipramine 10 mg/kg treated rats showed a significant decrease in CRP concentration (0.47 ± 0.02 ng/ml) compared to ligated control rats (0.61 ± 0.03 ng/ml). The concentration of CGRP in the serum of the CS2 rats decreased significantly (P < 0.0006) (178.17 ± 30.62 pg/ml) compared to ligated control rats (310.01 ± 16.28 pg/ml) [Figure 5c].
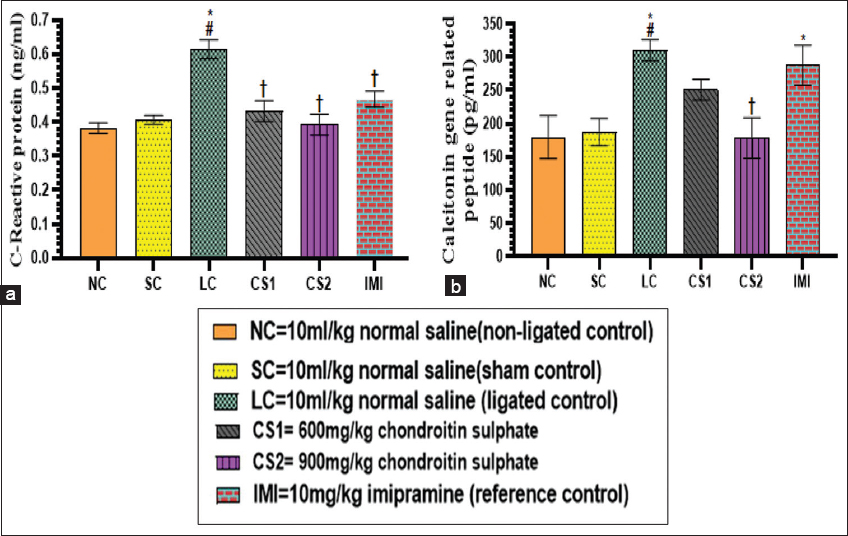
- Chondroitin sulphate reversed serum C-reactive protein and calcitonin gene realated peptide (a) C reactive protein (b) calcitonin gene related peptide concentration in rats. Data are expressed as the mean ± SEM (n = 5) subjected to one-way ANOVA followed by Tukey’s post hoc analysis. *#†P < 0.05 is significant versus *NC., #=SC., †=LC
Effect of CS on brain nitric oxide and LDH
The concentration of nitric oxide (NO) in brain homogenates of ligated control rats was significantly (P < 0.0004) reduced (5.39 ± 0.32 μM) compared to non-ligated control (9.60 ± 0.61 μM) and SC rats (9.25 ± 0.78 μM). The concentrations of NO in the brain of CS1 rats (8.70 ± 0.96 vs. 5.39 ± 0.32 μM) and CS2 rats (9.44 ± 0.56 vs. 5.39 ± 0.32 μM) were more significantly (P < 0.0004) increased compared to ligated control rats [Figure 6a]. The concentrations of LDH in the brain homogenates of CS1 (376.23 ± 51.27 U/L vs. 631.03 ± 14.48 U/L) rats and CS2 rats (245.85 ± 28.70 U/L vs. 631.03 ± 14.48 U/L) were more significantly (P < 0.0001) increased compared to ligated control rats [Figure 6b].

- Chondroitin sulphate reversed brain nitric oxide and lactate dehydrogenase (LDH) (a) nitric oxide concentration and (b) LDH concentration in rats. Data are expressed as the mean ± SEM (n = 5) subjected to one-way ANOVA followed by Tukey’s post hoc test. *#†P < 0.05 is significant versus *NC., #=SC., †=LC
Effect of CS on serum interleukin-6, tumor necrotic factor-α, and nuclear factor kappa B-p65 (NF-κB-p65)
The concentration of interleukin-6 (IL-6) in the serum of ligated control rats (1.73 ± 0.14 ng/L) and imipramine 10mg/kg treated rats (2.08 ± 0.13 ng/L) were significantly (P < 0.0001) reduced compared to the non-ligated (2.84 ± 0.09 ng/L) and SC (2.69 ± 0.15 ng/L) rats. The concentration of IL-6 in the CS1 and CS2 rats were significantly increased (2.45 ± 0.13 and 2.62 ± 0.02 ng/L, respectively) compared to the ligated control rats (1.73 ± 0.14 ng/L) [Figure 7a]. The concentrations of serum TNF-α in the ligated control rats (60.27 ± 3.94) was significantly increased compared to non-ligated (43.91 ± 1.44) and SC rats (44.43 ± 1.78 ng/L). The serum concentration of TNF-α in CS1 and CS2 rats were significantly (P < 0.0001) decreased (50.36 ± 0.71 ng/L and 47.85 ± 1.84 ng/L vs. 60.27 ± 3.94 ng/L, respectively) compared to ligated control rats [Figure 7b]. The concentration of serum NF-κB-p65 in the ligated control rats (0.96 ± 0.02 ng/ml) was significantly (P < 0.005) increased compared to non-ligated (0.79 ± 0.01 ng/ml) and SC (0.80 ± 0.05 ng/ml) rats. The serum concentration of NF-κB-p65 in CS1 and CS2 rats were significantly (P < 0.005) decreased (0.82 ± 0.02 ng/ml and 0.82 ± 0.03 ng/ml vs. 0.96 ± 0.02 ng/ml, respectively) compared to ligated control rats [Figure 7c].
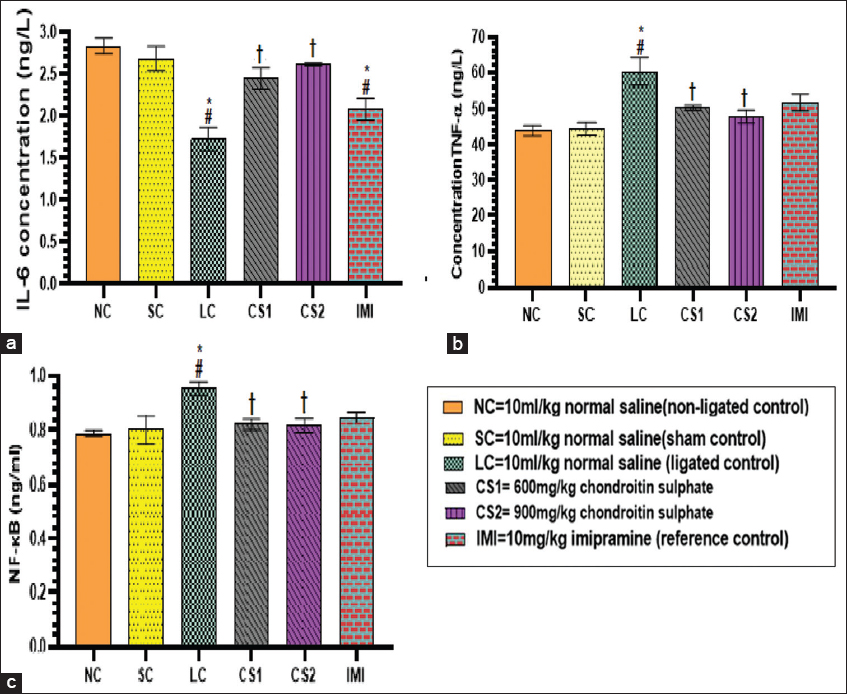
- Chondroitin sulphate reversed serum inflammatory cytokines (a) interleukin -6 (b) Tumor necrosis factor-α (c) NF-κB concentration in rats. Data are expressed as the mean ± SEM (n = 5) subjected to one-way ANOVA followed by Tukey’s post hoc test. *#†P < 0.05 is significant versus *NC., #=CS., †=LC. NC: Non-ligated control: SC: Sham control; LC: Ligated control; CS1: Chondroitin sulphate 600 mg/kg; CS2: Chondroitin sulphate 900 mg/kg; IMI: imipramine 10 mg/kg
Effects of CS on the histology of sciatic nerve
Histological findings from this study showed that the sciatic nerve of non-ligated and sham rats showed properly organized myelin sheets, the presence of round axons and absence of infiltrating cells [Figure 8a and b], sciatic nerve of ligated untreated rat showed several areas of edema, presence of infiltrating cells (polymorphs and lymphocytic) significant inflammation, vacuolization, degraded myelin sheets (myelin ellipsoids) [Figure 8c], ligated sciatic nerve treated with CS 600 mg/kg and ligated sciatic nerve treated with CS 900 mg/kg showed improved edema, vacuolization, infiltrating cells and improved myelin sheets organization compared to the ligated untreated [Figure 8d and e], ligated sciatic nerve treated with Imipramine 10 mg/kg. [Figure 8f] showed few edema, vacuolization, infiltrating cells, and improved myelin sheets organization compared to the ligated untreated.

- Histological analysis of rat sciatic nerves on day 22 after surgery. (a and b) sciatic nerve of non-ligated rat showed that the myelin sheets were well organized round axons and there were absence of infiltrating cells; (c) Sciatic nerve of ligated untreated rat shows several areas of edema (black sphere), presence of infiltrating cells (polymorphs and lymphocytic) (+) (significant inflammation), vacuolization (*), degraded myelin sheets (myelin ellipsoids) (i); (d) ligated sciatic nerve treated with C.S. 600 mg/kg (e) ligated sciatic nerve trated with C.S. 900 mg/kg (h) ligated sciatic nerve treated with imipramine 10 mg/kg. d-f show properly alleviated edema, improved myelin sheets organization, well reduced vacuolization, fewer infiltrating cells compared to the ligated untreated. H and E staining was used
Discussion
The CCI model in rodents is a widely used model of nerve damage that presents with allodynia and hyperalgesia.[18,31] The resulting NP is brought about by constricting the sciatic nerve with four loose ligatures.[18]
CS is the most common components of circulating GalAGs, are most abounding GalAGs in human. They are found in the connective tissues.[31] Previous reports have shown that CS is beneficial in conditions of obesity-induced atherosclerotic plaque inflammation[32] and inflammatory bowel disease.[33] It has been shown that CS, either singly[28] or when combined with glucosamine,[34] relieves pain in osteoarthritis patients. Furthermore, it has been reported that CS mitigates formalin-induced persistent tactile allodynia.[35] It was also reported that administration of CS results in the inhibition of PSNL-induced tactile allodynia.[16] Antinociceptive effect of CS and the possible mechanisms with emphasis on its antioxidant properties in Wistar rats was investigated. This study also examined the involvement of cytokines and other pain mediators in the antinociceptive effects of CS.
Mechanical hypersensitivity[21,26,36] and thermal hyperalgesia are observable facts in NP states that can be modeled in animal studies.[37,38] Acetone spray test can be used as a multimodal stimulus to successfully proof altered nociceptive processing with CCI.[39,40]
This study used the CCI model of NP to probe the effects of CS on allodynia and hyperalgesia and the extent of mediators of inflammation in rats NP induction. It was noticed that CCI induction resulted in significant development of mechanical allodynia, heat hyperalgesia and cold allodynia after surgery. But, CS attenuated CCI-induced behavioural alterations including mechanical allodynia, thermal hyperalgesia and cold allodynia in a dose-dependent manner. These data suggested that CS is effective in attenuating NP symptoms of mechanical allodynia, thermal hyperalgesia and cold allodynia in a CCI model. The observed effect of CS in attenuating CCI-induced mechanical allodynia might be due to the inhibition of spinal p38 MAPK phosphorylation and Ab-fiber activation as reported by Nemoto et al.[16] This also showed that CS is effective in attenuating symptoms of NP of mechanical allodynia, thermal analgesia and cold allodynia in a CCI model. Findings from this study also suggest that CS showed more analgesic effect compared to 10 mg/kg of imipramine.
Prior to the surgical procedure of CCI induction, none of the animals showed any symptoms of cold allodynia, thermal hyperalgesia or mechanical allodynia in both contralateral and ipsilateral paws.
The presence of mechanical allodynia using von Frey filament following CCI as observed in this study have been variously reported.[41,42] Thermal stimulation has often been used to examine pain associated behavior in animals.[43] The benefits of thermal stimulation include the relative constant threshold across body sites, various psychophysical and physiological studies that has clearly established temperature range that result to heat nociception and its underlying mechanisms. Thus, responses to painful thermal stimuli remain one of the valid and best behavioral tools for studying pain in animals.
It has been reported that the pathophysiology of the peripheral nervous system disorder, NP and diabetic neuropathy is associated with anomalous (Ca2+) channel expression and function.[44] In this study, hypercalcemia a condition of increased calcium ion (Ca2+) above normal range in the blood, was observed in the ligated control (untreated) rats. CS however reversed the elevated Ca2+ observed in the serum of CCI-induced rats. It has been reported that elevation of extracellular free Ca2+ concentration or facilitation of its transmembrane flux reduces the opioid antinociception. On the other hand, reduction of extracellular free Ca2+ concentration or of its transmembrane flux increases opioid antinociception or promotes antinociception by itself.[45,46]
Hyperkalemia, a condition of increased extracellular potassium ion (K+) above normal level occurs when renal potassium excretion is restricted due to reductions in glomerular filtrate rate, tubular flow, distal sodium delivery or the expression of aldosterone-sensitive ion transporters in the distal nephron.[47] Evidence emerging have shown that hyperkalemia could affect neuronal excitability and therefore contribute to peripheral neuropathy.[38,47,48] A normal electrophysiological account could be restored by serum K+ lowering.[38] Findings from this study showed that serum K+ level was significantly elevated in the ligated untreated rats and that CS treatment lowered the serum level of K+. It has been documented that hyperkalemia could be a risk factor for peripheral neuropathy.[38,47]
The involvement of free radicals in human disease conditions has been documented by many studies.[49,50] Cellular and tissue oxidative stress are believed to be directly linked to elevated levels of superoxide radicals. The reaction of excess superoxide with SOD result in generation of numerous intracellular hydrogen peroxide. Peroxidation of the cell membrane in the body results to MDA production which can further exasperate damaged cell membrane.[51] MDA levels are a valid marker of lipid peroxidation and can indirectly show the extent of cellular damage due to free radicals.[52,53]
In this study, CS treatment reversed the sciatic nerve MDA level and improved CAT, superoxide dismutase and total antioxidant activities. A significant elevation in LPOs level has been observed following nerve damage.[54,55] The observations shows that CS has antioxidant capacity which is evident by the reductions observed in lipid peroxidation and improving antioxidant enzymes. Previous findings by other authors[56,57] showed that CS has antioxidant capacity. It has been reported that CS inhibit ROS production.[58] CAT and SOD has been implicated in the breakdown of superoxide anion radicals which are potent oxidative stress markers, playing scavenging roles to the superoxide anion radicals.[59] This explains the significant increase in CAT, SOD and TAC in the sciatic nerve following treatment with CS.
The reduced CRP levels following CS treatment could be attributed to its ability to inhibit the activation of NF-κB which might be one of the pathways for the inhibition of inflammation by CS.[60,61] NF-κB a transcription factor alongside CRP play significant roles in many inflammatory processes.[62,63]
Previous studies have reported excessive amount of CGRP may be an indication of sensory afferent activation.[64] This study showed that the level of CGRP was elevated in CCI-induced rats that received no CS treatment. Thus, estimation of CGRP can be regarded as an important marker of sensory afferent activation in tissue during pain.[65] This shows that CGRP could cause the proliferation of CGRP-containing nociceptors and it could sensitize those nociceptors. Further findings from this study showed that 900 mg/kg CS treated rats showed low level of CGRP compared to the ligated control rats. This further confirmed the analgesic property of CS.
Nitric oxide (NO) plays important roles in numerous neurobiological processes. Many physiologic importance of NO have been identified in neurotransmission,[66,67] and in host-defense mechanisms.[68,69] NO has been shown over times to possess both pronociceptive and antinociceptive properties.[70] NO has been reported to elicit neuroprotective effects via kinase Akt and the transcription factor CREB in the survival pathway that is evoked by NO in cerebellar granule cells.[71,72] Various reports have shown that NO expresses not only cytotoxic but also cytoprotective effects in the CNS.[68,69] It was observed that CS treatment led to an increase in the level of brain nitric oxide. This outcome clearly showed that nitric oxide exhibited a neuroprotective effect because the increase in NO level was associated with other beneficial effects of CS in the rats such as antinociception and improved biochemical indices. This is similar to the observation of Džoljić et al.[73] which demonstrated the neuroprotective effects of nitric oxide.[73]
It has been shown that neuronal discharge at high frequencies are triggered by nerve damage during which lactate becomes a preferred substrate.[74] Lactate is transported by monocarboxylate transporters in the brain.[75] These transporters allow neurons to ferry lactate as an efficient fuel even in substrate-poor conditions due to their great affinity for lactate.[74] The brain level of LDH was increased in the ligated but untreated rats but CS administration reduced the level of LDH. Other studies have shown that an elevated level of LDH in the brain or systemic circulation can be a viable marker of neurodegeneration.[76] Therefore, the reductions in the level of brain LDH observed following the administration of CS further demonstrate its neuroprotective potential.
CCI-induced NP produced an increase in TNF-α level in serum in this study. TNF-α appears early in the cytokine cascade, therefore, it is considered to be a prototype proinflammatory mediator.[77,78] The role of TNF-α has been well documented in peripheral as well as central sensitization in NP.[77,78] Results from this study showed elevated levels of TNF-α in serum of CCI-subjected rats. These findings are consistent with previous studies,[79] and those from other laboratories.[80] Oral administration of CS reduced serum level of NF-κB-p65. Studies have implicated NF-κB-p65 as an important transcriptional factor with important roles with direct association with several disease pathogenesis[81] especially in the progression of chronic pain.[82,83] Research findings has shown IL-6 as a multifaceted cytokine that can bring about both pro- and anti-inflammatory effects in a context-dependent fashion.[84] Various studies reported that IL-6 increases circulating levels of the anti-inflammatory cytokines.[85,86] Findings from this study showed that IL-6 played a neuroprotective role following treatment with CS. CCI induction lowered serum level of IL-6. Oral administration of CS elevated serum level of IL-6. Previous studies have shown that IL-6 administration attenuates endotoxin-induced TNF-alpha production, further supporting an anti-inflammatory role of IL-6.[87,88] Furthermore, studies have reported that IL-6 improved nerve dysfunction including sensory, motor nerve conduction velocity, thermal hyperalgesia, tactile allodynia measures, and sciatic nerve endoneurial blood perfusion.[87,89] A lot of studies support the role of IL-6 in initiating and triggering neuroreparative responses.[90] Histological findings with H and E staining of the sciatic nerves of rats subjected to injury and treatment by CS clearly showed the beneficial effect of CS in axonal regeneration, reduced edema area, reduced presence of infiltrating cells, reduced inflammation, reduced vacuolization as well as fewer degraded myelin sheets in CS-administered rats compared to the ligated untreated rats. These results suggest that treatment with CS promotes functional recovery by increasing axonal regeneration after sciatic nerve injury in the rat. The observed effects in this study may be due to the involvement of CS in several physiological processes within the rats[91] which include, the modulation of the differentiation and the proliferation of neurons.[90] As also reported by Sugahara.[92] CCI rats treated with imipramine 10 mg/kg showed few edema, vacuolization, infiltrating cells, and improved myelin sheets organization compared to the ligated untreated rats.
Conclusion
CS mitigates NP in a CCI model, which may perhaps be as a result of its ability to lower the release of proinflammatory mediators, increase antiinflammatory mediators in the course of sciatic nerve damage. It also demonstrated an ability to decrease oxidative stress and increase antioxidants which are important for neuroregeneration.
Authors’ Declaration Statements
Compliance with ethical standards
The study was conducted in accordance with the ARRIVE guidelines. The protocol was also assessed and approved by the (UERC/ASN/2019/1744) before the commencement of the research.
Authors’ Contributions
OFO and BVO conceived and designed research. OFO conducted experiments. BVO contributed new reagents. OFO and BVO analyzed data. OFO and BVO drafted the manuscript. All authors read and approved the manuscript.
Acknowledgment
The authors would like to appreciate the members of Bridge Biotech Ltd, Mr. Bakare OA and members of the Neuroscience Unit who assisted with part of the laboratory work.
ORCID ID: https://orcid.org/0000-0003-3503-9338
Competing Interests
The authors declare that there are no conflicts of interest.
Funding Statement
This study was self-funded.
References
- Mechanisms of neuropathic pain and their implications for the design of clinical trials. Neurology. 2005;65:S66-73.
- [Google Scholar]
- Pharmacological management of neuropathic pain following spinal cord injury. CNS Drugs. 2008;22:455-75.
- [Google Scholar]
- Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain. 2004;111:116-24.
- [Google Scholar]
- Microglia and spinal cord synaptic plasticity in persistent pain. Neural Plast. 2013;2013:753656.
- [Google Scholar]
- Increased activation of nuclear factor kappa B in rat lumbar dorsal root ganglion neurons following partial sciatic nerve injuries. Brain Res. 1998;797:243-54.
- [Google Scholar]
- EFNS guidelines on the pharmacological treatment of neuropathic pain:2010 revison:Treatment of neuropathic pain. Eur J Neurol. 2010;17:113-88.
- [Google Scholar]
- Chronic constriction of the sciatic nerve and pain hypersensitivity testing in rats. J Vis Exp. 2012;61:3393.
- [Google Scholar]
- CCL-1 in the spinal cord contributes to neuropathic pain induced by nerve injury. Cell Death Dis. 2013;4:679-9.
- [Google Scholar]
- Rosmarinic acid attenuates development and existing pain in a rat model of neuropathic pain:An evidence of anti-oxidative and anti-inflammatory effects. Phytomedicine. 2018;40:59-67.
- [Google Scholar]
- Neuropathic pain, depressive symptoms, and c-reactive protein in sciatica patients. Int J Neurosci. 2012;123:204-8.
- [Google Scholar]
- Sphingosine-1-phosphate receptor 1 activation in astrocytes contributes to neuropathic pain. Proc Natl Acad Sci U S A. 2019;116:10557-62.
- [Google Scholar]
- Immunomodulatory and anti-inflammatory effects of chondroitin sulphate. J Cell Mol Med. 2009;13:1451-63.
- [Google Scholar]
- Selectin blocking activity of fucosylated chondroitin sulfate glycosaminoglycan from sea cucumber. Effect on tumor metastasis and neutrophil recruitment. J Biol Chem. 2007;282:14984-91.
- [Google Scholar]
- Cell supports of chitosan/hyaluronic acid and chondroitin sulphate systems. Morphology and biological behaviour. J Mater Sci Mater Med. 2007;18:1719-26.
- [Google Scholar]
- Structural insights into biological roles of protein-glycosaminoglycan interactions. Chem Biol. 2005;12:2003-22.
- [Google Scholar]
- Effect of repeated oral administration of chondroitin sulfate on neuropathic pain induced by partial sciatic nerve ligation in mice. J Pharmacol Sci. 2018;137:403-6.
- [Google Scholar]
- Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109-10.
- [Google Scholar]
- A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87-107.
- [Google Scholar]
- Differential effect of spironolactone in chronic constriction injury and vincristine-induced neuropathic pain in rats. Eur J Pharmacol. 2010;648:102-9.
- [Google Scholar]
- Effect of alcoholic extract of aerial parts of Rosmarinus officinalis L. On pain, inflammation and apoptosis induced by chronic constriction injury (CCI) model of neuropathic pain in rats. J Ethnopharmacol. 2016;194:117-30.
- [Google Scholar]
- Simplified up-down method (SUDO) for measuring mechanical nociception in rodents using von Frey filaments. Mol Pain. 2014;10:26.
- [Google Scholar]
- Method used to evaluate pain behaviours in rodents. Front Mol Neurosci. 2017;10:284.
- [Google Scholar]
- Ethosuximide reverses paclitaxel-and vincristine-induced painful peripheral neuropathy. Pain. 2004;109:150-61.
- [Google Scholar]
- Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain. 1994;59:369-76.
- [Google Scholar]
- Comparison between in situ sciatic nerve grafts and vein grafts with platelet-rich plasma. Experimental study in rabbits. Cir Plast. 2013;23:86-90.
- [Google Scholar]
- Role of calorie restriction in alleviation of age-related morphological and biochemical changes in sciatic nerve. Tissue Cell. 2014;46:497-504.
- [Google Scholar]
- Estimation of lipid peroxidation induced by hydrogen peroxide in cultured human lymphocytes. Dose Response. 2012;10:1-10.
- [Google Scholar]
- Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat Protoc. 2010;5:51-66.
- [Google Scholar]
- Assay of superoxide dismutase activity in animal tissues. J Biosci. 1988;13:305-15.
- [Google Scholar]
- Validation of an automated assay for the measurement of cupric reducing antioxidant capacity in serum of dogs. BMC Vet Res. 2016;12:137.
- [Google Scholar]
- Glycosaminoglycans reduce oxidative damage induced by copper (Cu+2), iron (Fe+2) and hydrogen peroxide (H2O2) in human fibroblast cultures. Glycoconj J. 2004;20:133-41.
- [Google Scholar]
- Treatment with chondroitin sulphate to modulate inflammation and atherogenesis in obesity. Atherosclerosis. 2016;245:82-7.
- [Google Scholar]
- Oral chondroitin sulphate and prebioticsn for the treatment of canine inflammatory bowel disease:A randomised, controlled clinical trial. BMC Vet Res. 2016;10:49.
- [Google Scholar]
- Effect of chondroitin sulphate in symptomatic knee osteoarthritis:A multicentre, randomised, double-blind, placebo-controlled study. Ann Rheum Dis. 2007;66:639-45.
- [Google Scholar]
- Chondroitin sulphate attenuates formalin-induced persistent tactile allodynia. J Pharmacol Sci. 2016;131:275-8.
- [Google Scholar]
- Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55-63.
- [Google Scholar]
- Puerarin ameliorates allodynia and hyperalgesia in rats with peripheral nerve injury. Neural Regen Res. 2018;13:1263-8.
- [Google Scholar]
- Randomized, controlled trial of the effect of dietary potassium restriction on nerve function in CKD. Clin J Am Soc Nephrol. 2017;12:1569-77.
- [Google Scholar]
- An effective and concise device for detecting cold allodynia in mice. Sci Rep. 2018;8:14002.
- [Google Scholar]
- Behavioral and pharmacological validation of the acetone spray test in Gerbils with a chronic constriction injury. Anesth Analg. 2005;101:457-64.
- [Google Scholar]
- Analgesic properties of aqueous bark extract of Adansonia digitata in Wistar rats. Biomed Pharmacother. 2018;97:209-12.
- [Google Scholar]
- Nociceptive neuropeptide increases and periorbital allodynia in a model of traumatic brain injury. Headache. 2012;52:966-84.
- [Google Scholar]
- Objective pain assessment:A key for the management of chronic pain. F1000Res. 2020;9:35.
- [Google Scholar]
- Abnormal calcium homeostasis in peripheral neuropathies. Cell Calcium. 2010;47:130-9.
- [Google Scholar]
- Invovelment of calcium in pain and antinociception. Braz J Med Biol Res. 2001;34:449-61.
- [Google Scholar]
- Hyperkalemia:Pathophysiology, risk factors and consequences. Nephrol Dial Transplant. 2019;34:iii2-11.
- [Google Scholar]
- Has potassium been prematurely discarded as a contributing factor to the development of uremic neuropathy? Nephl dial Transplant. 2004;19:1054-7.
- [Google Scholar]
- Free Radicals in Biology and Medicine (3rd ed). Oxford, United Kingdom: Clarendon Press Oxford; 1999.
- Chemistry and pathophysiology of oxidation of LDL. Rev Physiol Biochem Pharmacol. 1996;127:31-64.
- [Google Scholar]
- Malondialdehyde (MDA) as a lipid peroxidation marker. Wiadomosci Lekarskie. 2004;57:453-5.
- [Google Scholar]
- The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology. 2011;36:2159-68.
- [Google Scholar]
- A morphological and biochemical evaluation of the effects of quercetin on experimental sciatic nerve damage in rats. Exp Ther Med. 2018;15:3215-24.
- [Google Scholar]
- Antinociceptive and neuroprotective effects of bromelain in chronic chronic constriction injury-induced neuropathic pain in Wistar rats. Korean J Pain. 2020;33:13-22.
- [Google Scholar]
- Chondroitin sulfate effect on induced arthritis in rats. Osteoarthritis Cartilage. 2011;19:1373-9.
- [Google Scholar]
- The antioxidant activity of chondroitin-4-sulphate, in carbon tetrachloride-induced acute hepatitis in mice, involves NF-kappaB and caspase activation. Br J Pharmacol. 2008;155:945-56.
- [Google Scholar]
- Chondroitin sulphate:Antioxidant properties and beneficial effects. Mini Rev Med Chem. 2006;6:1311-20.
- [Google Scholar]
- Tolerance of spermatogonia to oxidative stress is due to high level of Zn and Cu/Zn superoxide dismutase. PLoS One. 2011;6:e16938.
- [Google Scholar]
- Effect of a high dose of glucosamine on systemic and tissue inflammation in an experimental model of atherosclerosis aggravated by chronic arthritis. Am J Physiol Heart Circ Physiol. 2009;297:H268-76.
- [Google Scholar]
- Association between use of specialty dietary supplements and C-reactive protein concentrations. Am J Epidemiol. 2012;176:1002-13.
- [Google Scholar]
- Evaluation of the suppressive actions of glucosamine on the interleukin-1β-mediated activation of synoviocytes. Inflamm Res. 2011;56:432-8.
- [Google Scholar]
- Calcitonin gene-related peptide receptor antagonists:Beyond migraine pain-a possible analgesic strategy for osteoarthritis? Curr Pain Headache Rep. 2013;17:375.
- [Google Scholar]
- Presence and distribution of sensory nerve fibers in human peritoneal adhesions. Ann Surg. 2001;234:256-61.
- [Google Scholar]
- Presynaptic cell type-dependent regulation of GABAergic synaptic transmission by nitric oxide in rat insular cortex. Neuroscience. 2015;284:65-77.
- [Google Scholar]
- Nitric oxide signaling modulates synaptic inhibition in the superior paraolivary nucleus (SPN) via cGMP-dependent suppression of KCC2. Front Neural Circuits. 2014;8:65.
- [Google Scholar]
- Nitric oxide as a physiopathological factor in neuropsychiatric disorders. In Vivo. 2004;18:377-90.
- [Google Scholar]
- Nitric oxide neurotoxicity in neurodegenerative diseases. Front Biosci. 2004;9:763-76.
- [Google Scholar]
- Redox regulation of mitochondrial fission, protein misfolding, synaptic damage, and neuronal cell death:Potential implications for Alzheimer's and Parkinson's diseases. Apoptosis. 2010;15:1354-63.
- [Google Scholar]
- Nitric oxide in cell survival:A Janus molecule. Antioxid Redox Signal. 2009;11:2717-39.
- [Google Scholar]
- Role of nitric oxide in the regulation of neuronal proliferation, survival and differentiation. Neurochem Int. 2004;45:903-14.
- [Google Scholar]
- Can lactate serve as an energy substrate for axons in good times and in bad, in sickness and in health? Metab Brain Dis. 2015;30:25-30.
- [Google Scholar]
- Role of monocarboxylate transporters in drug delivery to the brain. Curr Pharm Design. 2014;20:1487-98.
- [Google Scholar]
- Lactate in the brain:An update on its relevance to brain energy, neurons, glia and panic disorder. Ther Adv Psychopharmacol. 2017;7:85-9.
- [Google Scholar]
- Attenuation of neuropathic pain by sodium butyrate in an experimental model of chronic constriction injury in rats. J Form Med Assoc. 2014;113:921-8.
- [Google Scholar]
- Neuropathic pain-attenuating potential of aliskiren in chronic constriction injury model in rats. J Renin Angiotensin Aldosterone Syst. 2013;14:116-23.
- [Google Scholar]
- Effect of pyrroloquinoline quinone on neuropathic pain following chronic constriction injury of the sciatic nerve in rats. Eur J Pharmacol. 2012;697:53.e8.
- [Google Scholar]
- Functional role of human interleukin-32 and nuclear transcription factor-kB in patients with psoriasis and psoriatic arthritis. Int J Health Sci (Qassim). 2018;12:29-34.
- [Google Scholar]
- The IKK-NF-kappaB pathway;a source for novel molecular drug targets in pain therapy? FASEB J. 2008;22:3432-42.
- [Google Scholar]
- In vivo luminescence imaging of NF-kappa B activity and serum cytokine levels predict pain sensitivities in a rodent model of osteoarthritis. Arthritis Rheumatol. 2014;66:637-46.
- [Google Scholar]
- The role of interleukin-6 signaling in nervous tissue. Biochim Biophys Acta. 2016;1863:1218-27.
- [Google Scholar]
- IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am J Physiol Endocrinol Metab. 2003;285:E433-7.
- [Google Scholar]
- Possible inflammatory responses in hypercholesterolemia patients receiving treatment using raw liquid extract of young cashew leaves in herbal homes in Nigeria. Int J Health Sci (Qassim). 2019;13:14-8.
- [Google Scholar]
- Low-dose pulsatile interleukin-6 as a treatment option for diabetic peripheral neuropathy. Front Endocrinol. 2017;8:89.
- [Google Scholar]
- Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humans. FASEB J. 2003;17:884-6.
- [Google Scholar]
- The neurocytokine, interleukin-6, corrects nerve dysfunction in experimental diabetes. Exp Neurol. 2007;207:23-9.
- [Google Scholar]
- gp130 cytokines are positive signals triggering changes in gene expression and axon outgrowth in peripheral neurons following injury. Front Mol Neurosci. 2011;4:62.
- [Google Scholar]
- Purification, characterization and antioxidant activities of chondroitin sulphate extracted from Raja porosa cartilage. Carbohydr Polym. 2020;241:116306.
- [Google Scholar]
- Recent advances in the structural biology of chondroitin sulfate and dermatan sulfate. Curr Opin Struct Biol. 2003;13:612-20.
- [Google Scholar]







