Translate this page into:
Clinical and epidemiological characteristics and outcomes of Coronavirus disease-19 patients in a large longitudinal study
Address for correspondence: Deldar Morad Abdulah, Lecturer at Community and Maternity Health Unit, College of Nursing, University of Duhok, Iraqi Kurdistan, Iraq. Phone: +9647507443319. E-mail: deldarmorad@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Qassim Uninversity and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective:
This study aimed to determine the clinical and epidemiological characteristics and outcomes of Coronavirus disease (COVID)-19 patients.
Methods:
In this large cohort study, 15,409 confirmed patients with the COVID-19 of different severities were followed-up from three specialized COVID-19 hospitals between March 18 and October 11, 2020 in Iraqi Kurdistan. The predictors of mortality and severity were examined in binary logistic regression analysis.
Results:
The incidence rate of severe/critical status was 12.3% with a median age of 36.0 and case fatality rate (CFR) of 1.98%. The incidence rate of severe/critical conditions and CFR rose with increased age groups; except for 0–14 years (11.9%). The incidence rate of severe/critical patients and CFR was 8.3% and 0.5%, 21.1% and 4.0%, and 23.7% and 8.7% in 15–49 years, 50–64 years, and 65 and older age groups, respectively. The severity of the disease and CFR was associated with coexisting chronic diseases such as cardiovascular diseases (18.2% and 3.1%) and diabetes mellitus (19.8% and 3.4%). The asymptomatic patients (8400 and 54.5%) had statistically higher CFR; 2.3% versus 1.6% (P = 0.006). The most common symptoms on diagnosis were fever (31.9%), cough (23.5%), loss of smell/taste (16.3%), sore throat (15.7%), shortness of breath (9.8%), and headache (9.5%). The results showed that being older was the only predictor of mortality and severity in COVID-19 patients.
Conclusions:
This region has a low incidence of severe-critic status and CFR. The patients with coexisting medical conditions are more likely to have severe conditions and die of COVID-19. The older age predicts severe/critic status and higher CFR.
Keywords
COVID-19
case fatality rate
outcome
severity
Introduction
The novel coronavirus infection outbreak known as Coronavirus disease (COVID)-19 emerged from Wuhan in China in December 2019.[1] The coronavirus has been spread to other countries, including Iraqi Kurdistan.[2,3] Globally by November 11, 2020, there have been 50,810,763 confirmed cases of COVID-19, including 1,263,844 deaths, reported to the World Health Organization (WHO).[4] The WHO has presented deep concern about the spread of this pandemic.[5]
Health-care systems are encountered with rapidly increasing demands due to the COVID-19 pandemic. The health systems are being overwhelmed, both directly from the coronavirus outbreak and indirectly from vaccine-preventable and treatable medical conditions. Maintaining the population’s trust in the capability of the health system is important to provide the safe essential required medical necessities and to control infection risk in medical settings. Infection control is a key step to ensuring appropriate care-seeking behavior and adherence to preventive measures.
The Kurdistan Regional Government (KRG) reported the first confirmed cases of the COVID-19 among the persons who returned from Iran in early February 2020. After that, the KRG released preventive health measures for travelers and community members. In this regard, public and private schools and universities were closed from February 26 to May 2, 2020. The curfew was applied between March 13 and April 23, 2020. The KRG has applied some other preventive measures; including reducing business hours; referring suspected cases of the COVID-19 to special medical settings; quarantining the persons with close contact with the suspected cases; and increasing awareness about preventive measures through mass media.[6]
The current disease has occurred by a different virus than previously known one. The clinical course of the COVID-19 disease caused by this new virus could be different and was not known completely.[7] The common clinical features are fever, dry cough, shortness of breath (SOB), and pneumonia. The less common clinical features are headache, diarrhea, productive cough, runny nose, and hemoptysis.[8] The clinical outcomes of the patients are different from mild to severe illnesses. The persons aged 65 years and older, smokers, and those with comorbid diseases such as diabetes mellitus (DM) and hypertension are more likely to have a severe situation.[9] The median incubation period of the COVID-19 is between 5 and 6 days, but it could take up to 24 days[10] with an uncertain period of infectivity.[11] The primary goal of controlling this virus is preventing transmission. The patients with COVID-19 show various degrees of laboratory abnormalities such as leukopenia, leukocytosis, and lymphopenia.[12]
The studies have shown that preventive measures such as lockdown and social distance are effective strategies to impede the COVID-19 outbreak across the countries.[13-15] In England, many areas with previously high case incidence reduce sharply. However, the lockdown had not the same effect in every region. For instance, in Kent, the cases were continuously increased during the lockdown, despite having the same restrictions as other regions.[16] Viruses constantly change by mutation result in new variants of a virus over time and sometimes the new variants emerge and disappear. Multiple variants of the virus caused by COVID-19 have been documented in the United States and globally during this pandemic. A new variant of Severe acute respiratory syndrome-coronavirus (SARS-CoV-2) emerged has been responsible for up to 70% more transmissible than the previously known virus. In September 2020, the new variant represented one in four new diagnoses, while by mid-December, this rate was increased to almost two-thirds of new cases in London.[17] A similar pattern was observed in South Africa as well.[18]
The demographic and anthropometric characteristics of the diseases are different across geographic locations[19] and these differences could affect clinical outcomes. We need to analyze the data from a large investigation to find out the disease, establish a specific treatment plan, and optimize resource allocation. There are several studies about COVID-19 from countries over all continents characterizing the nature of the disease. However, we have only one small study in Iraqi Kurdistan focused on the demographic and clinical outcomes of the COVID-19 patients published at the early stage of the outbreak.[3] This study aimed to determine the clinical and epidemiological characteristics of COVID-19 patients. Besides, the association of clinical and epidemiological characteristics of COVID-19 patients with mortality rate was examined in this study.
Methods
Study design and patients
In this large follow-up study, the confirmed patients with the COVID-19 of different severities were followed up by the discharge date from three specialized COVID-19 hospitals. The patients with non-severe medical status who were not admitted to the hospitals were followed up by the mobile medical teams. The patients whose medical conditions were escalated were admitted to the hospital as well. The patients were diagnosed by a certified physician in medical settings in the Duhok governorate of Iraqi Kurdistan between March 18 and October 11, 2020. The governorates of Iraqi Kurdistan are presented in a map in Figure 1.[20] A total of 15,409 patients were diagnosed during the mentioned time. The medical doctors diagnosed the COVID-19 cases based on the WHO interim guidance for COVID-19[21] and local guidelines of the Ministry of Health. The patients were included in this study regardless of disease severity, coexisting medical conditions, and socio-demographic aspects.
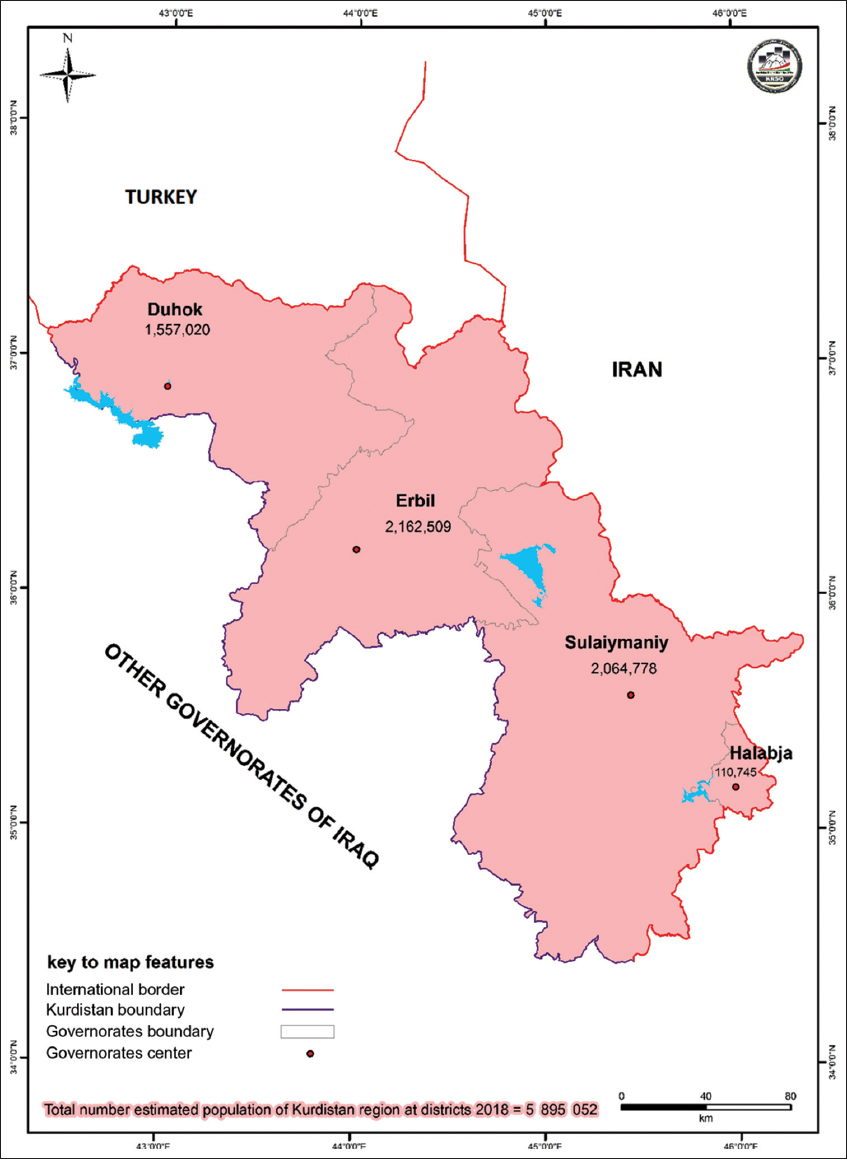
- Map of kurdistan region with its estimated population at governorate level
The individuals were tested for the COVID-19 for the following reasons in this region. The patients crossed the international border, had contact with a confirmed case, or became ill following visiting health facilities (suspected case). They returned from other governorates/places or they need a certificate of health to work in the private sector. Several persons were tested for the COVID-19 as a routine random screening/surveillance for the suspected cases included those persons who were in quarantine settings.
The patients who were included in this study were selected from different medical settings in the Duhok governorate. The patients who were diagnosed with severe disease were admitted to the following health settings. In early March 2020, the Burn and Plastic Surgery hospital was assigned for COVID-19. In addition, a new 100 beds hospital was made called “Lalav Infectious Diseases hospital” for treatment of the COVID-19 patients with severe and critical conditions. Thereafter, Azadi Teaching Hospital a main tertiary hospital in this region was devoted to treating the COVID-19 patients in early June 2020.
Classification of the disease severity
An infected case was defined as a SARS-CoV-2 positive real-time reverse-transcriptase polymerase chain reaction (RT-PCR) taken from a nasal and/or throat swab together. The cases were defined on having signs, symptoms, or radiological findings suggestive of COVID-19 pneumonia.[22]
The criteria for severity of COVID-19 were defined according to the diagnosis and treatment protocol for novel coronavirus pneumonia (Version 7) as mild, moderate, severe, and critical.[23] We classified as non-severe and severe/critic in this investigation.
The non-severe cases
The non-severe cases compromised mild and moderate cases in this study. The patients with no sign of pneumonia on imaging were considered mild and patients with fever and respiratory symptoms and confirmed radiological outcomes on imaging were considered moderate cases. The severe and critical cases of children and adult populations were determined based on the criteria given in the diagnosis and treatment protocol for novel coronavirus pneumonia (Version 7).[23]
Management
The patients who were diagnosed in this region had different severities and were managed as follows. The management of the cases was performed based on the medical regulation issued by the Ministry of Health of KRG numbered 4504 on February 30, 2020, and the Ministry of Health of Iraq numbered 27,429 on June 1, 2020. The patients were managed according to COVID-19 National Clinical guidelines.[3]
Diagnostic criteria
The RT-PCR diagnostic tests were performed according to the mean recovering time of the patients (14 days). The patients were discharged from the hospital according to the clinical improvement and viral clearance over RT-PCR (two negative results at least 24 h apart). The patients were informed of the possible recurrence of the disease. We did not document the recurrence in this study, because the study was so large.
Data collection
The information of the patients was collected in one of the following categories. The general information of the patients included age, gender, hospital stay (day), occupation (healthcare worker, or non-healthcare worker), and residence was recorded in the first category. The epidemiological and coexisting disorders included pregnancy in childbirth, reasons for testing, and medical conditions were recorded in the second category. At the end, the symptoms of the patients and outcomes were recorded in the third category. The outcomes were documented as recovered or death.
Statistical methods
The general and epidemiological information of the patients was presented in the median (interquartile range) or no. (%). The incidence rates of symptoms and mortality were calculated by dividing the infected or dead patients by the total number of included patients and determined in no. (%). The comparison of incidence rates of severity and mortality in patients with different epidemiological characteristics, coexisting disorders, and symptoms was examined in Pearson Chi-squared tests. The predictors of mortality in patients with COVID-19 were examined in binary logistic regression. The significant level of difference was determined in a P < 0.05. The statistical calculations were performed by statistical package for social sciences version 25 (IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp). The box plots of this study were drawn by JMP SAS 14.3.
Ethical considerations
This study was supported and approved by the institutional ethical board of Duhok General Directorate of Health and Ministry of Health of the KRG registered as 13,878 in 2020. We protected the confidentiality of the personal information of patients through de-identification of the patients’ personal information. We did not apply any intervention for patients who were included in this study. The study has no risk to the patents.
Results
The total number of COVID-19 patients was 15,409, of whom 12.3% (n = 1888) were severe/critical. The median age of the patients was 36.0 aged between 0 and 103 years; the majority were 15–49 years. The patients were males (9868, 64.0%) and females (5541, 36.0%). The incidence rate of severe/critical conditions rose with increased age groups; except for 0–14 years (11.9%). The patients with severe/critical disease were older than those with non-severe illness with a median of 9 years. The incidence rate of severe/critical patients was 8.3%, 21.1%, and 23.7% in 15–49 years, 50–64 years, and 65 and older age groups, respectively. Female and non-healthcare worker patients were more likely to have severe/critical condition; 13.0% and 12.4% compared to males and healthcare workers; 11.9% and 9.2%, respectively. Concerning pregnancy, 123 (0.08%) were infected in different trimesters (P = 0.434). The patients were from Duhok (97.8%), other Kurdish governorates (1.0%), Iraqi governorates (1.1%), and other countries (0.2%), as shown in Table 1.
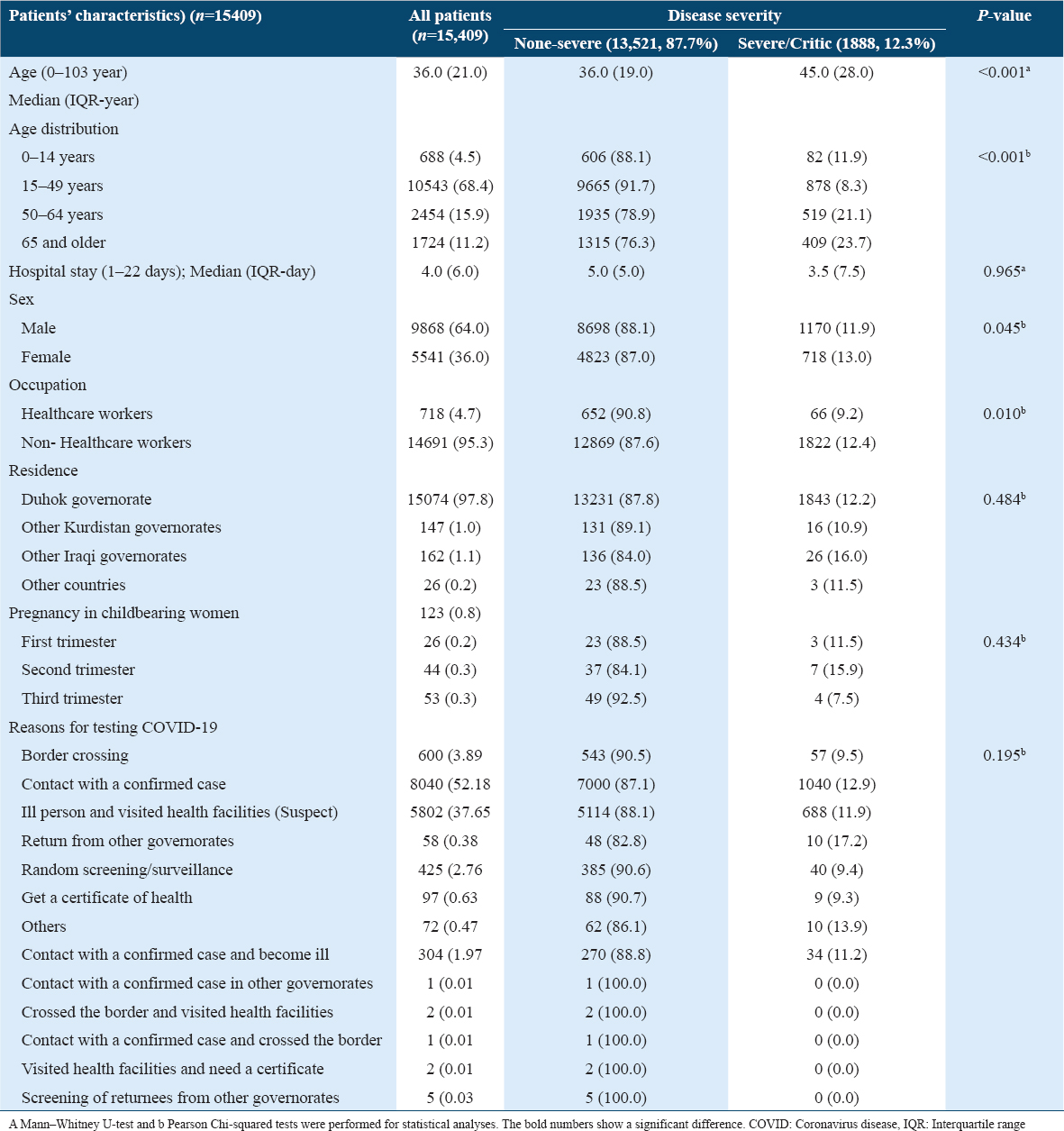
The severity of the disease was more likely to be associated with certain coexisting chronic diseases such as cardiovascular diseases (18.2%) and DM (19.8%); however, chronic lung disease, renal impairment, malignancy, and immunodeficiency did not show this association [Table 2].
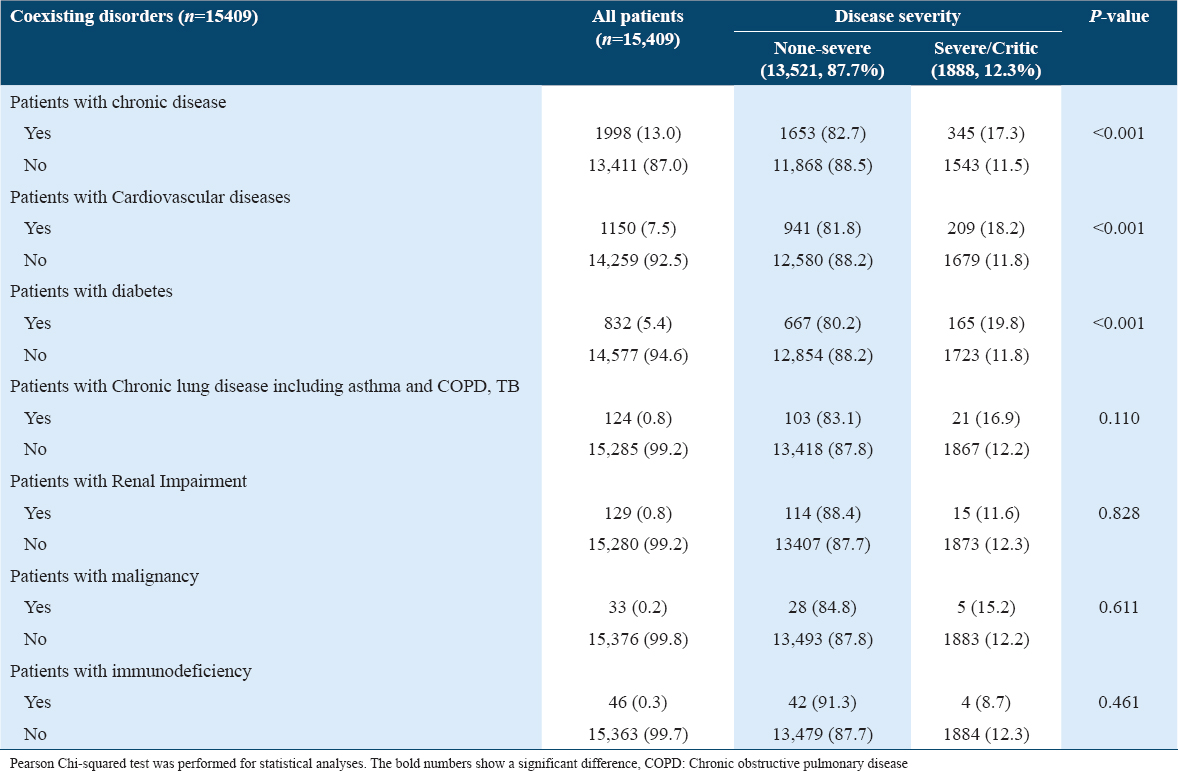
There were 8400 (54.5%) asymptomatic and 7009 (45.5%) symptomatic patients. The most common symptoms on diagnosis were fever (31.9%), cough (23.5%), loss of smell/taste (16.3%), sore throat (15.7%), SOB (9.8%), and headache (9.5%). Severe COVID-19 was more likely found in patients with SOB (11.6%), loss of appetite (2.7%), or headache (8.0%), Table 3 and Figure 2.
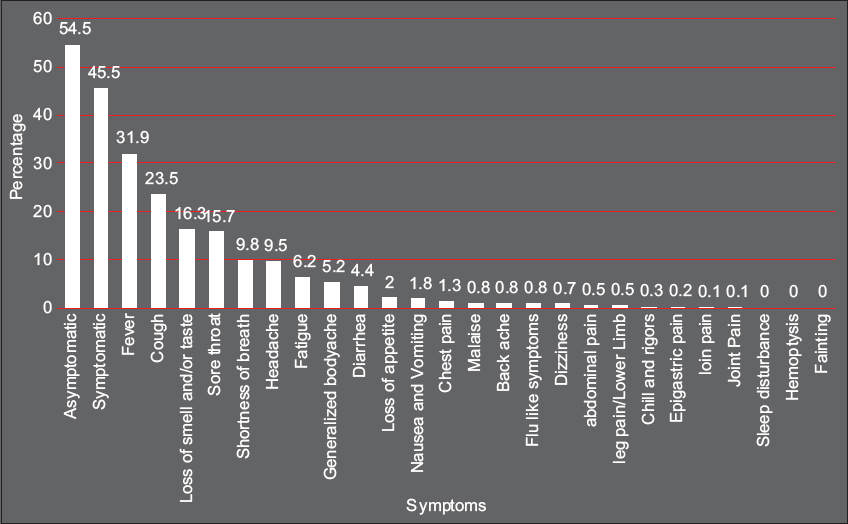
- Prevalence rates of symptoms in patients with COVID-19
The case fatality rate (CFR) was 1.98%, with a higher rate of aged patients. Lethality increased with age, except for the 0–14 age group (0.7%). The CFR was 0.5%, 4.0%, and 8.7% in 15–49 years, 50–64 years, and 65 and older, respectively. The median hospital stay was 4.0 days in dead patients. The mortality rate was significantly higher in non-healthcare workers compared to healthcare workers; 2.0% versus 0.6; P = 0.005. The lethality was higher in pregnant women during the second trimester (2.3%). However, the overall comparison of mortality was not statistically substantial in women with different trimesters. Regarding reasons for COVID-19 testing, there was no significant difference between patients who attended the clinic settings and those in different governorates (P = 0.258), Table 4 and Figure 3.
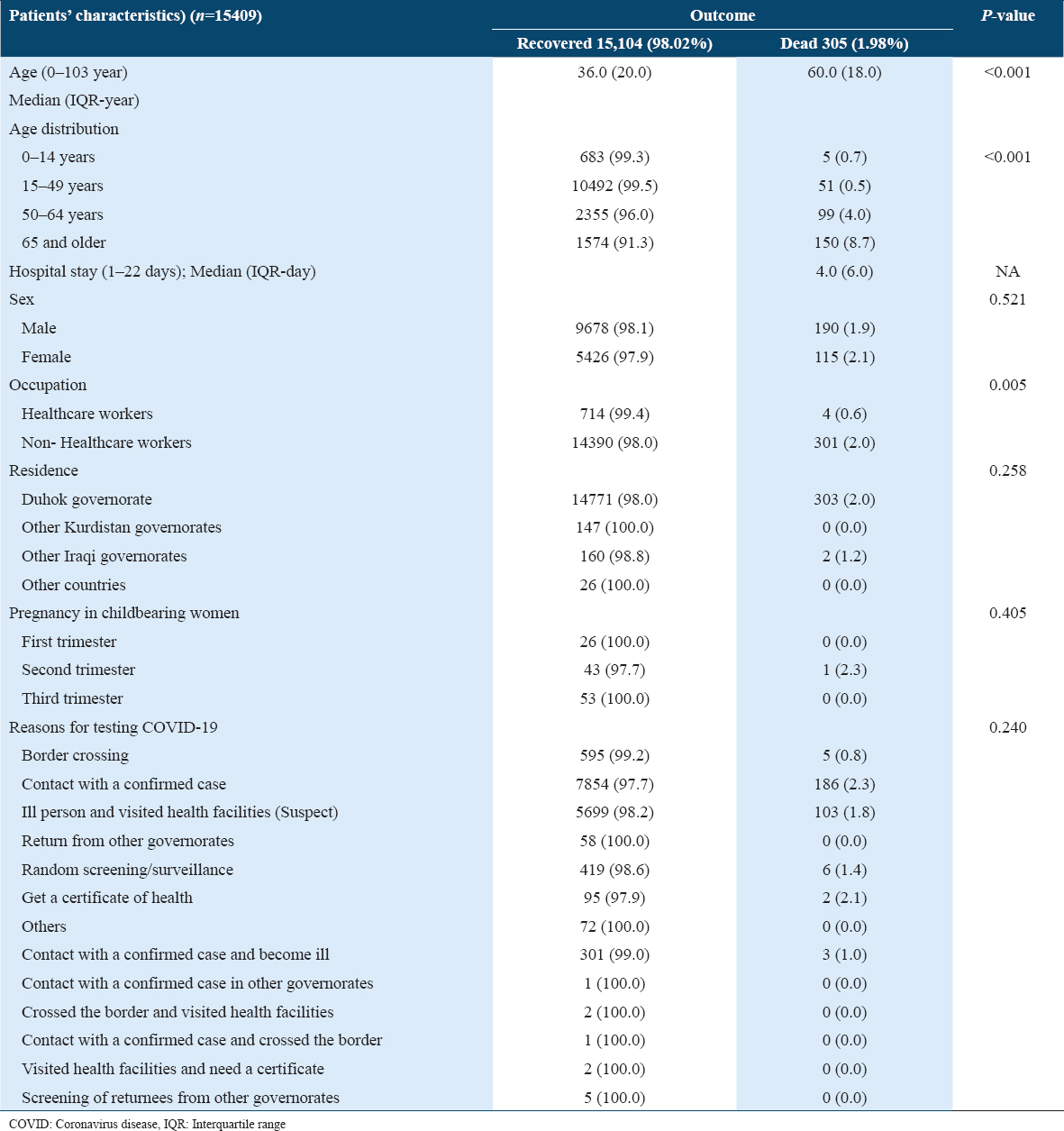
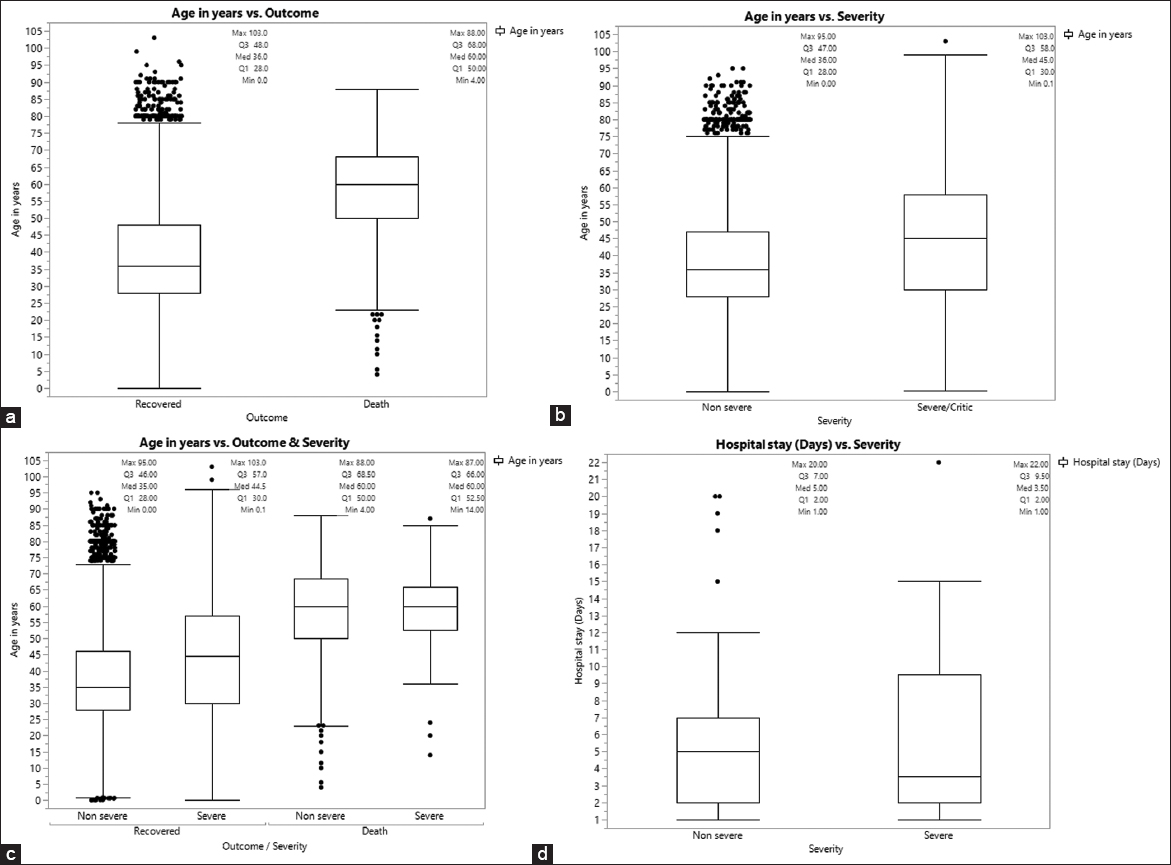
- Comparison of median age between patients with different outcomes, (a) age of recovered and dead patients, (b) age of non-severe and severe/critic patients, (c) age of non-severe and severe/critic patients in recovered and dead patients, (d) hospital day of non-sever and sever/critic patients
The patients with chronic diseases, cardiovascular diseases, or type 2 DM had a significantly higher CFR rate; 3.1%, 4.4%, and 3.4%, respectively. The patients with other coexisting disorders have not had significantly higher CFR. The asymptomatic patients had statistically higher CFR compared to symptomatic patients; 2.3% versus 1.6 (P = 0.006). Furthermore, the patients with SOB had higher CFR compared to patients without SOB; 2.8% versus 1.9%; P = 0.012 [Table 5].
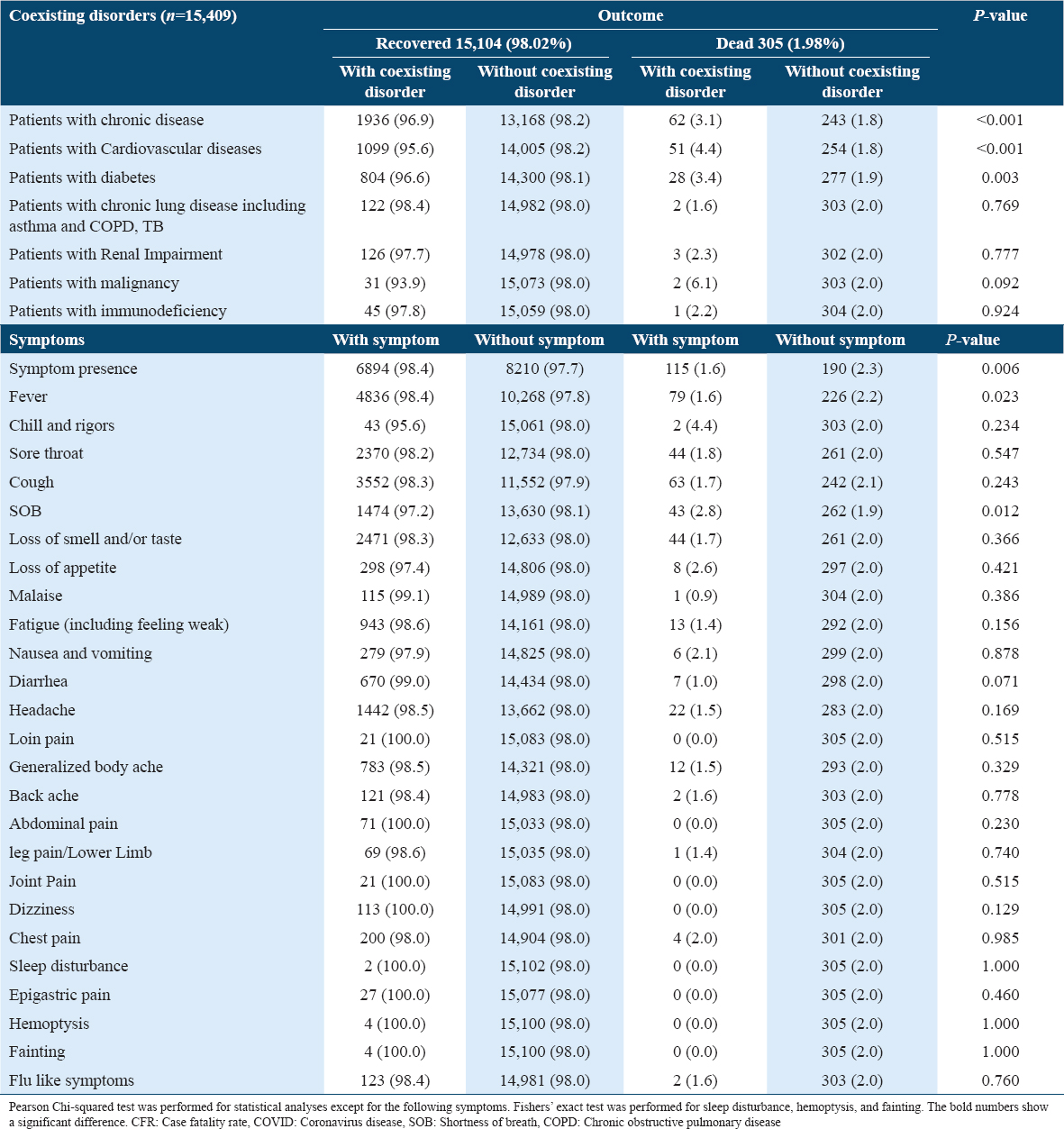
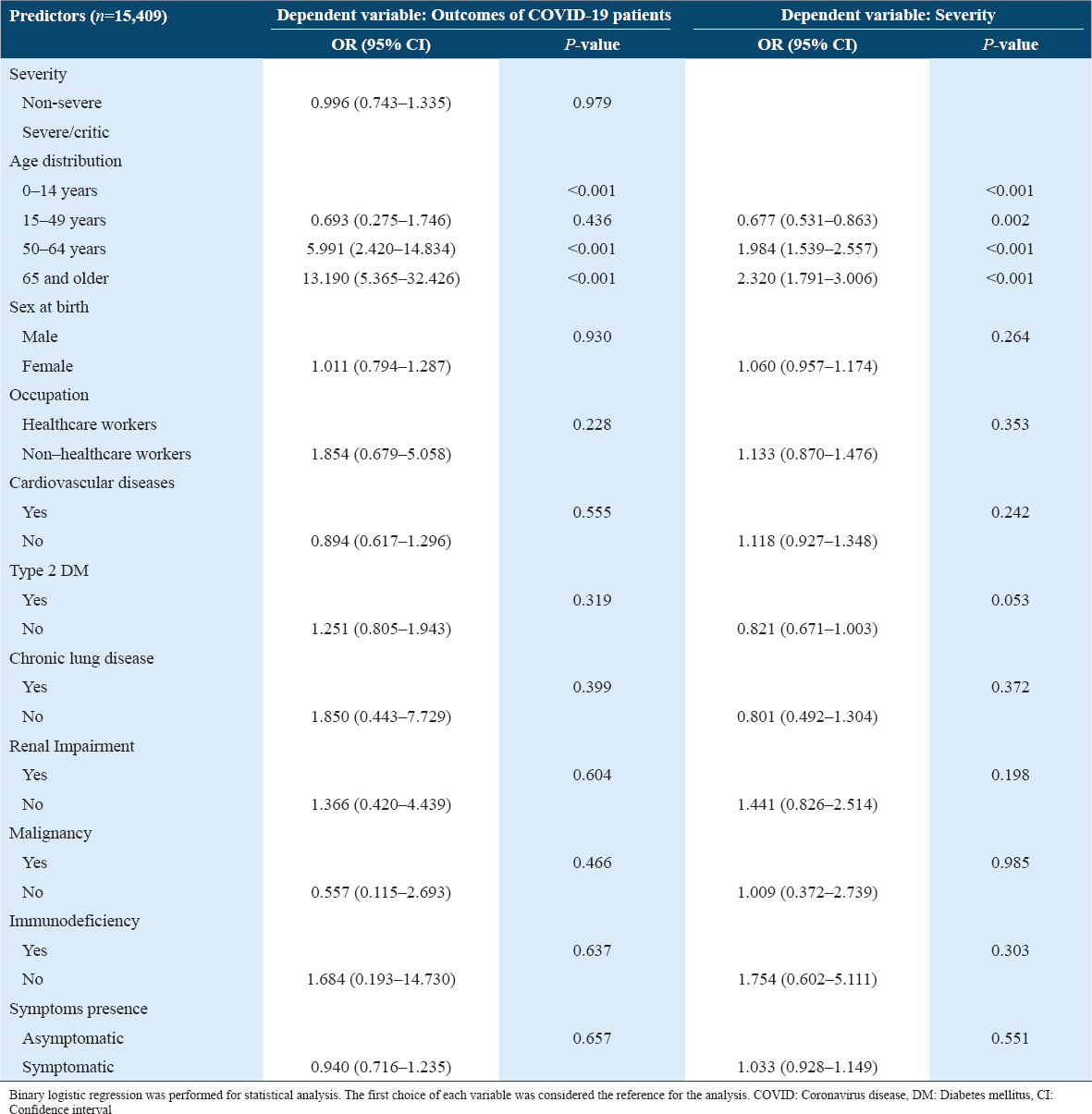
The results showed that being older was the only predictor of mortality and severity in COVID-19 patients (Except for 15–49 years old). Ironically, being severe or non-severe, asymptomatic or symptomatic, or having coexisting chronic diseases were not predictors for mortality and severity in patients with COVID-19.
A similar pattern was found in the analysis of mortality predictors in patients aged ≥ 65 years old. However, older age was shown to associate with severe/critical medical conditions in patients aged ≥ 65 years old [Table 6].
Discussion
To the best of our knowledge, this is the first large observational study of confirmed COVID-19 patients in Iraq. The median age of the patients was 36.0 aged 0-103 years, though it is different across the world. The median age was lower compared to studies from Italy (65.0 between 18 and ≥75 years);[22] China (47.0 between 0 and ≥65 years);[24] and (56.0 between 18 and 87 years);[25] and the United States (63.0 between 0 and 107 years).[26] The lower median age of the affected persons could be due to the median age of 20 in Iraq’s population.[27]
In this study, the incidence rate of severe/critical COVID-19 patients was 12.3% and it was linked with increasing age group except for 0–14 years old. It is well documented in the literature that worse outcomes are associated with increasing age.[24,28] Overall, increasing age is associated with impaired immunity and there is an increased prevalence of comorbid diseases such as DM and hypertension. The different rates of severe/critical conditions were reported in the literature, for instance, 15.74%.[24] The studies have reported that COVID-19 patients with severe/critical conditions are older with different median years, 9 years in this study (Iraqi Kurdistan), 7 years in China.[24]
The study showed that healthcare workers (HCWs) are at risk of getting an infection by the COVID-19. In our study, 4.7% of the affected patients were HCWs. The different infection rates have been reported in the literature, 3.5%.[24] Nguyen et al.[29] followed up the general community in the UK and USA including front-line HCWs, using the self-reported technique. At the end of the follow-up time, they included 2,035,395 community individuals and 99,795 front-line healthcare workers. They recorded 5545 incident reports of a positive COVID-19 test over 34,435,272 person-days. The front-line HCWs had an increased risk for reporting a positive COVID-19 test (adjusted HR 11·61, 95% confidence interval [CI] 10·93–12·33). Therefore, health policymakers must pay special attention to protect the medical staff against the COVID-19 outbreak. The shortage of medical staff leads to a remarkable burden on health-care services in this region. Importantly, the risk of infection is doubled in household members of front-facing workers, in adjustment for age, gender, comorbidity, and other epidemiological factors.[30] In agreement with this study, the literature has reported similar findings.[29-31]
This study reveals that 54.5% of the patients were asymptomatic on visiting a medical doctor. Fever, cough, loss of smell/taste, sore throat, SOB, and headache are prevalent symptoms in COVID-19 patients. Those with SOB, loss of appetite, or headache are more likely to have severe/critical situations. The literature has reported that fever and cough on admission are the most prevalent symptoms in COVID-19 patients. Guan et al.[24] reported that 43.8% and 88.7% had a fever on admission and during hospitalization, respectively, and 67.8% had a cough. In addition, the patients with coexisting illness were more likely to have the severe disease compared to non-severe patients, 38.7% versus 21.0%, respectively.[24] Similar findings were reported in other parts of the words in terms of symptoms.[22,32]
The common symptoms reported by systematic reviews were fever (85.6%), cough (68.7%), and fatigue (39.4%). The frequent comorbidities are hypertension (17.4%), diabetes (3.8%), and coronary heart disease (3.8%). The critical cases with complications are 9%, intensive care unit admission is required in 7.3%, invasive ventilation in 3.4%, and mortality is 2.4%.[33] Another meta-analysis confirmed that fever and cough are the most frequent symptoms.[34]
A meta-analysis of 148 articles of 24,410 adults with confirmed COVID-19 from nine countries reported that fever 78% [95% CI 75–81%], a cough 57% [95% CI 54–60%], and fatigue 31% [95% CI 27–35%] are the most prevalent symptoms. The main concern of the authors is that a considerable percentage of patients have chronic diseases. These patients are more likely to have severe/critical disease conditions and more likely to die.
The CFR was 1.98% of 15,409 patients who were followed-up and included in this study. The CFR rate was significantly increased with increasing age, and having chronic diseases, including cardiovascular diseases, or type 2 DM. We found that asymptomatic patients had statistically higher CFR compared to symptomatic patients; 2.3% versus 1.6, respectively, except for the patients with SOB. The CFRs are different based on the coexisting chronic conditions, clinical features, and epidemiological factors. A meta-analysis of 148 articles reported a 7% mortality rate.[35]
The early findings reported from China revealed that CFR is 1.4% in laboratory-confirmed COVID-19 patients[24] and 24.0% in Italy.[22] The Italian study reported that patients aged older than 65 years (HR 3.17, 95% CI 1.84–5.44, P < 0.001), history of coronary artery disease (HR 2.93, 95% CI 1.77–4.86, P < 0.001), and active cancer (HR 2.32, 95% CI 1.15–4.67, P =0.001) as independent factors related to increased mortality risk. Zhou et al.[25] performed a multivariable regression and showed that in-hospital mortality is associated with older age (odds ratio 1·10, 95% CI 1·03–1·17), higher Sequential Organ Failure Assessment score (5·65, 2·61–12·23), and d-dimer greater than 1 mg/mL (18·42, 2·64–128·55) on admission.
In agreement with the literature, this study showed that being severe/critical or non-severe or being asymptomatic or symptomatic, or having chronic diseases are not the predictors for mortality and severity in patients with COVID-19. The older age was the only predictor of mortality and severity in COVID-19 patients. However, some other factors have been reported to be predictors for mortality. For example, Fabio et al.[22] reported that through multivariable analysis that older age, coronary artery disease, cancer, low lymphocyte count, and high Radiographic Assessment of Lung Edema score as factors independently associated with an increased risk of mortality. Other investigations have reported that older age and the presence of comorbidities are associated with an increased mortality rate in COVID-19 patients.[25,32,36]
We confirmed the previously published findings in patients from China,[37] United States,[26] and Italy.[22] They reported that older age, coexisting medical conditions such as coronary artery disease, history of hypertension, diabetes, chronic obstructive pulmonary disease, and chronic renal failure, and cancer are related to increased mortality. We back the effect of chronic diseases on increased mortality to their effect on immunity.[38] The current findings give us the utmost importance to reduce the burden of the general health system, targeting the efforts for sufficient screening of the patients at risk.
The role of age in immune system suppression must not be ignored in this disease. The countries with higher infection rates have an older population compared to the countries with low infection rates,[39] France and Italy compared to Iraq and Saudi Arabia, respectively. Adults aged 65 years and older and patients with coexisting medical conditions are more likely to have a severe-even deadly-coronavirus infection.[40] Therefore, the high infection and mortality rates in high-income countries could be due to aging and accordingly low immunity level.
The effects of aging on the immune system are presented at multiple levels. The production of B and T cells in bone marrow and thymus is decreased first. The functions of mature lymphocytes in secondary lymphoid tissues are diminished accordingly. Therefore, the aged populations unable to respond to the immune challenges as the young populations.[41]
Some other factors have been reported to associate with the mortality rate in the literature. For example, Abdulah and Hassan[42] in a global ecological study reported that the crude mortality rate is increased by raising consuming sugar-sweetened beverages and decreased by increasing fruit consumption and beans and legumes. The anti-inflammatory strategies inside foods, nutrients, or medicines are suggested as viable options for the management of COVID-19[43,44] since the coronavirus has serious inflammatory consequences for acute pneumonia in persons.[45] The human coronavirus infections cause mild to severe diseases,[3] systemic inflammation, high fever, cough, and acute respiratory tract infection, and dysfunction in internal organs leading to death.
Except for the insufficient age-related micronutrient, the nutritional status of the population has a role in the overall development of the SARS-CoV-2 infection, the clinical status, and outcomes. Therefore, the individuals need the maintenance of host macro- and micronutrient status to avoid the COVID-19 infection.[46]
The older individuals aged 60–65 years old have less ability to respond to the immune challenges and pathogens, antigens, and mitogens decreases due to immune dysregulation.[47] Characteristics of the immune system in older people are a reduction of circulating lymphocytes and loss of immune cells.[48] Besides, the older persons have reduced the production of T cells in the involved thymus. Accordingly, this decreases the function of mature lymphocytes in secondary lymphoid tissues.[41]
Implications and limitations
The main strong point of this study is that we tried to include as much as possible the patients with sufficient information that allowed us to present a clearer picture of clinical features of the COVID-19 in this region. The predictors reported in this study could assist clinicians to determine at an early stage patients with COVID-19 with poor prognosis. However, the study was not exempt from the limitations. We could not record the radiological and laboratory-based information of this large study. Anyhow, we suggest that a large study of radiological and laboratory-based information study be performed in a multi-center investigation.
The world requires serious investment in research and development to find out the current epidemics and prepare for possible future ones. Besides, we need to establish our healthcare system to develop new diagnostic and therapeutic solutions, invest in vaccines and broad-spectrum antivirals. Moreover, we need to take into account the social aspects.[49]
Conclusions
This study showed that patients with COVID-19 have a low incidence rate of severe/critical status in Iraqi Kurdistan. The incidence rate of severe/critical condition was significantly increased with increasing age and was more common in female and non-healthcare worker patients. We found that older COVID-19 patients and those with coexisting medical conditions were more likely to have severe/critical status and a high rate of CFR. Having older age was determined to be the only factor that predicted the severe/critical status and higher mortality.
Authors’ Declaration Statements
Availability of data and material
The data used in this study are available and will be provided by the corresponding author on a reasonable request.
Funding statement
The study was supported by the Duhok General Directorate of Health in Duhok/Iraqi Kurdistan.
Authors’ Contributions
The corresponding author claim that all authors whom their names were reported in this study had sufficient contribution to the concept, design, review, analysis, and final approval.
Acknowledgment
We would like to present our deep thanks to the persons who assist us in the Directorate of Preventive Health affairs and Duhok General Directorate of Health, three special COVID-19 treatment centers.
References
- Anovel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727-33.
- [Google Scholar]
- Response of the public to preventive measures of COVID-19 in Iraqi Kurdistan. Disaster Med Public Health Prep 2020:1-9.
- [Google Scholar]
- COVID-19 outbreak in Iraqi Kurdistan:The first report characterizing epidemiological, clinical, laboratory, and radiological findings of the disease. Diabet Metab Synd Clin Res Rev. 2020;14:547-54.
- [Google Scholar]
- 2020. WHO Coronavirus Disease (COVID-19) Dashboard. Geneva: World Health Organization; Available from: https://www.covid19.who.int
- At the epicenter of the Covid-19 pandemic and humanitarian crises in Italy:Changing perspectives on preparation and mitigation. NEJM Catal Innov Care Deliv. 2020;1:1-5.
- [Google Scholar]
- 2020. Situation Update Coronavirus (COVID-19), What the KRG is Doing. United States: Kurdistan Regional Govrnment; Available from: https://www.gov.krd/coronavirus-en/situation-update
- Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period:A scoping review. Infect Dis Poverty. 2020;9:1-12.
- [Google Scholar]
- 2020. Novel Coronavirus, Wuhan, China. United States: Centers for Disease Control Prevention; Available from: https://www.cdc.gov/coronavirus/2019-nCoV/summary.html
- Epidemiological and clinical aspects of COVID-19;a narrative review. AAEM. 2020;8:620.
- [Google Scholar]
- SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177-9.
- [Google Scholar]
- Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China:A descriptive study. Lancet. 2020;395:507-13.
- [Google Scholar]
- COVID-19:Utilizing local experience to suggest optimal global strategies to prevent and control the pandemic. IJHS. 2020;14:1.
- [Google Scholar]
- Role of precautionary measures in containing the natural course of novel coronavirus disease. JMDH. 2020;13:615.
- [Google Scholar]
- New variant of SARS-CoV-2 in UK causes surge of COVID-19. Lancet Respir Med. 2021;9:e20-1.
- [Google Scholar]
- 2021. About Variants of the Virus that Causes COVID-19. United States: Centers for Disease Control and Prevention; Available from: https://www.cdc.gov/coronavirus/2019-ncov/transmission/variant.html
- 2021. South African covid-19 Variant (B.1.351). United Kingdom: New Scientist; Available from: https://www.newscientist.com/definition/south-african-covid-19-variant
- Body composition phenotypes in pathways to obesity and the metabolic syndrome. Int J Obes. 2010;34:S4-17.
- [Google Scholar]
- Global Surveillance for COVID-19 Disease Caused by Human Infection with the 2019 Novel Coronavirus, Interim Guidance. Geneva: World Health Organization; 2020.
- Early predictors of clinical outcomes of COVID-19 outbreak in Milan, Italy. Clin Immunol. 2020;217:1-20.
- [Google Scholar]
- Diagnosis and treatment protocol for novel coronavirus pneumonia (Version 7) Chin Med J. 2020;133:1087-95.
- [Google Scholar]
- Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708-20.
- [Google Scholar]
- Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China:A retrospective cohort study. Lancet. 2020;395:1054-62.
- [Google Scholar]
- Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA. 2020;323:2052-9.
- [Google Scholar]
- 2020. Iraq Population 2020 (Live). India: World Population Review; Available from: https://www.worldpopulationreview.com/countries/iraq-population
- Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism. 2020;108:154262.
- [Google Scholar]
- Risk of COVID-19 among front-line health-care workers and the general community:A prospective cohort study. Lancet Public Health. 2020;5:e475-83.
- [Google Scholar]
- Covid-19:Risks to Healthcare Workers and Their Families. London, United Kingdom: BMJ Publishing Group; 2020.
- Epidemiology of and risk factors for coronavirus infection in health care workers:A living rapid review. Ann Intern Med. 2020;173:120-36.
- [Google Scholar]
- Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061-9.
- [Google Scholar]
- Clinical presentation of COVID-19:A systematic review focusing on upper airway symptoms. Ear Nose Throat J. 2020;99:569-76.
- [Google Scholar]
- Clinical characteristics of hospitalized patients with SARS-CoV-2 infection:A single arm meta-analysis. J Med Virol. 2020;92:612-17.
- [Google Scholar]
- The prevalence of symptoms in 24,410 adults infected by the novel coronavirus (SARS-CoV-2;COVID-19):A systematic review and meta-analysis of 148 studies from 9 countries. PLoS One. 2020;15:e0234765.
- [Google Scholar]
- Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China:A single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475-81.
- [Google Scholar]
- The novel coronavirus disease (COVID-19) threat for patients with cardiovascular disease and cancer. JACC CardioOncol. 2020;2:350-5.
- [Google Scholar]
- Recent patterns of multimorbidity among older adults in high-income countries. Popul Health Manage. 2019;22:127-37.
- [Google Scholar]
- Coronavirus Disease 2019 (COVID-19):Situation Report. Vol 72. Geneva: World Health Organization; 2020.
- Causes, consequences, and reversal of immune system aging. J Clin Invest. 2013;123:958-65.
- [Google Scholar]
- Relation of dietary factors with infection and mortality rates of COVID-19 across the world. J Nutr Health Aging. 2020;24:1011-8.
- [Google Scholar]
- Mast cells contribute to coronavirus-induced inflammation:New anti-inflammatory strategy. J Biol Regul Homeostat Agents. 2020;34:9-14.
- [Google Scholar]
- Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by coronavirus-19 (COVI-19 or SARS-CoV-2):Anti-inflammatory strategies. J Biol Regul Homeost Agents. 2020;34:327-31.
- [Google Scholar]
- COVID-19 Infection:The Perspectives on Immune Responses. Berlin, Germany: Nature Publishing Group; 2020.
- Individual risk management strategy and potential therapeutic options for the COVID-19 pandemic. Clin Immunol. 2020;215:108409.
- [Google Scholar]
- Nutrition and the immune system from birth to old age. Eur J Clin Nutr. 2002;56:S73-6.
- [Google Scholar]







