Translate this page into:
Effect of non-surgical periodontal therapy on the serum and crevicular fluid interleukin-10 levels in chronic periodontitis - A systematic review and meta-analysis
Address for correspondence: Dr. Ashish Jain, Department of Periodontology, Dr. Harvansh Singh Judge Institute of Dental Sciences and Hospital, Panjab University, Chandigarh, India. E-mail: ajain.pu@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Qassim Uninversity and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective:
The objective of the study was to assess the effect of non-surgical periodontal therapy (NSPT) on the levels of anti-inflammatory cytokine interleukin 10 (IL-10) in serum, gingival crevicular fluid (GCF), and saliva of chronic periodontitis patients.
Methods:
A comprehensive search was made on PubMed, Embase, and Scopus databases from Jan 2000 to Sep 2020. Three focused questions were addressed: Do GCF, serum, saliva and IL-10 levels change significantly after NSPT? Randomized and Non-Randomized clinical trials were included in the study. Results of the meta-analysis are expressed as standardized mean differences and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines.
Results:
A total of 709 studies were retrieved and 17 met the inclusion criteria for systematic review, whereas subgroup meta-analysis was performed on seven studies (GCF-5, serum-2). All studies included had low risk of bias. IL-10levelin GCF showed an increase at 3 months after NSPT with subsequent decrease at 6 months. However, increased serum IL-10 level at 6 months was seen after therapy.
Conclusion:
Within the limitations, there is moderate evidence that NSPT significantly alters the IL-10 levels in body fluids. IL-10 levels increased in GCF at 3 months whereas decrease was seen at 6 months. Increased IL-10 levels were seen in serum at 6 months.
Keywords
Chronic periodontitis
Interleukin-10
Meta-analysis
Non-surgical periodontal therapy
Introduction
Chronic periodontitis is a long standing immune disease that causes destruction of periodontal tissue, including connective tissue, periodontal ligament, cementum, and alveolar bone.[1] At the outset and initiation of periodontitis, a biofilm is formed and microbial products from it, such as lipopolysaccharides and toxins interacts with Toll-like receptors and trigger intracellular signaling pathways that culminate in an inflammatory cytokine response leading to release of various inflammatory mediators (such as prostaglandin E2) and cytokines such as interleukins (ILs) and tumor necrosis factor alpha (TNF-α) as well as proteolytic enzymes including matrix metal loproteinases (MMPs). The recent paradigm states that relative levels of pro- and anti-inflammatory cytokines regulate the cytokine balance in the local environment of the disease lesion.[2,3]
IL-10 is a pleiotropic and an immunoregulatory cytokine that has been discovered recently. It is involved in the reduction of IL-1, IL-6, IL-8, TNF-α, and MMPs and inhibition of bone resorption.[4,5] This cytokine originates from immune cells such as monocytes, macrophages, and T and B-cells and acts by reducing the synthesis of the pro-inflammatory cytokines. It downregulates the macrophage activation and impacts the immune response by limiting the duration and extent of inflammation.[6] Therefore, IL-10 is documented to have a modulatory role on inflamed and progressive periodontal disease sites.[7]
Periodontal treatment consists of the mechanical scaling and root planning (SRP) of the surfaces to reduce the bacterial load and change the environmental conditions for microbial niches, reducing the host inflammatory response.[8] In patients with periodontitis, resolution of inflammation after non-surgical periodontal therapy (NSPT) along with improvement in clinical parameters has been reported in the previous investigation. For histological parameters, although a decrease was seen in the amount of inflammatory cell infiltrate some amount was still present in the connective tissue, together with the presence of pro-inflammatory cytokines, such as IL-1β. Moreover, when the concentration of IL-1β was evaluated in the gingival crevicular fluid (GCF) after the treatment, a slight increase was noticed.[9] Detection of molecular biomarkers in clinical practice may provide an insight into the degree of success of treatment outcomes after NSPT.[10]
Thus, the evaluation of cytokine levels before and after periodontal treatment may provide a mechanism to evaluate the treatment outcome. Hence, the present review was aimed to critically appraise the available literature on the impact of NSPT on IL-10 levels in GCF, saliva and serum of chronic periodontitis patients.
Methods
Criteria for standardization and study type
The present analysis was performed according to recommendations as per Cochrane collaboration guidelines for systematic review and meta-analysis.[11] To ensure the standardization of the data inclusion/exclusion criteria and analysis, Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) criteria were followed.
Protocol registration
The systematic review was registered as a protocol with PROSPERO at www.crd.york.ac.uk (CRD42020208097).
Research question
The analysis was designed based on the standard procedure adhering to PICO index as follows;
-
P(Population): Chronic periodontitis patients
-
I(Intervention): Non-surgical periodontal therapy
-
C(Comparison): Comparison between pre and post intervention levels of IL- 10 in GCF/Serum/Saliva
-
O(Outcome): level of IL-10.
Focused questions
-
Do GCF IL-10 levels change significantly after non-surgical periodontal treatment?
-
Do serum IL-10 levels change significantly after non-surgical periodontal treatment?
-
Do salivary IL-10 levels change significantly after non-surgical periodontal treatment?
Information sources and search
For the present meta-analysis, a comprehensive search was carried out by utilizing relevant key terms and search strategy in PubMed, Embase, and Scopus [Figure 1] supplemented by a grey literature search and manual search through libraries and cross references.The search words used were “Chronic Periodontitis or Chronic Periodontitides or Periodontitides, Chronic or Periodontitis, Chronic or Adult Periodontitis or Adult Periodontitides or Periodontitides, Adult or Periodontitis, Adult,” “Interleukin-10 or IL-10 or CSIF-10 or Cytokine Synthesis Inhibitory Factor,” “Non-surgical periodontal therapy.” During the search, the criteria for selection was based on full text articles, peer reviewed, randomized and non-randomized, controlled clinical trials. The studies measuring the levels of IL-10 before and after NSPT among patients with chronic periodontitis were selected.
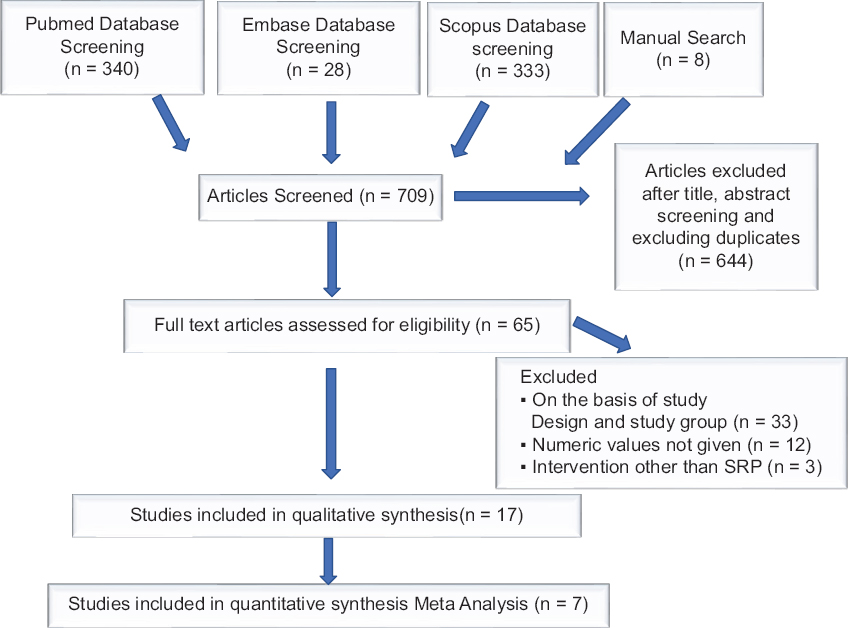
- Flow diagram of the literature search for the systematic review and meta-analysis
Inclusion and exclusion criteria
The inclusion criteria were: Studies discussing the effect of NSPT (SRP alone) on the levels of IL-10 in chronic periodontitis, studies with a minimum follow-up of 2 weeks, studies that showed actual numerical values forIL-10, studies that included patients (both males and females) who had no other systemic disease, studies published in English language from the year 2000 onward in human adult population were all included.
The exclusion criteria were: Studies estimating IL-10 levels in oral lesions/conditions other than periodontitis, unavailability of full text, abstracts, case reports, case series, narrative reviews, no author response to inquiry e-mail for data clarification, and all animal studies were excluded from the study.
Data collection and analysis
Data extraction and management
The data were extracted independently by three reviewers, strictly adhering to all the inclusion and exclusion criteria. Data were retrieved based on publication year, study design, publication language, total sample size, age at examination, inclusion/exclusion criteria of the study, actual numerical values for IL-10, and diagnostic criteria for periodontitis. If the required data were missing from the published article, the concerned authors were contacted for the provision of the same. All the data were tabulated in the Microsoft Excel sheet.
Data extraction
From the selected articles fulfilling the inclusion criteria, data addressing the numeric values of IL-10 in chronic periodontitis patients pre- and post-NSPT were extracted for qualitative and quantitative analysis.
Assessment of methodological quality
Risk of bias of the included studies was assessed with the Cochrane Collaboration’s tool (ROBINS 1 and Rob 2) for non-randomized and randomized interventional trials, respectively.
Data synthesis
Qualitative analysis
Data were extracted and collated for various study characteristics, that is, year, author name, type of study design, number of subjects, study groups, age groups, sample taken, clinical parameters assessed, and values of IL-10 at baseline and subsequent follow-up periods. Initial descriptive analysis was conducted and variations were assessed in studies relating to population, intervention, characteristics, outcomes and design, and also to evaluate possibility for pooling of data for potential meta-analysis.
Since adequate number of homogenous studies for saliva at any particular follow-up after NSPT was not found in the included literature; therefore, a pooled analysis was not performed.
Quantitative analysis
Meta-analyses were performed for separate subgroups based on follow-up intervals for evaluating levels of IL-10 after NSPT in chronic periodontitis patients in GCF and serum. A minimum of two studies at a particular interval in which actual numeric values for concentration was given for meta-analysis. The data were extracted as mean values and standard deviation. Standard deviations were estimated using appropriate formulas for the studies that provided data as range and mean values.[12] Data analysis was performed using statistical software (MedCalc for Windows, version 15.0 (MedCalc software, Ostend, Belgium). Random and Fixed effects models were used to estimate the effect size of the pooled studies. Summary effect measures, standardized mean difference, and 95% confidence intervals P-values were displayed in forest plots. The heterogeneity among studies was assessed by calculating I2.[13]
Results
Search and selection results
The initial search yielded 709 publications [Figure 1]. After exclusion of 644 studies based on their titles, abstracts, and the duplicates, 65 were selected for full text review. Of all these 59 studies, 33 were excluded on the basis of study design; and 12 were excluded because numeric values for IL-10 were not given; in further three studies, intervention other than SRP was given. Hence, a total of 17 clinical interventional trials were finally included based on the inclusion criteria. We found five studies for GCF and two for serum respectively for meta-analysis. Details have been included in the PRISMA chart [Figure 1].
An inter examiner Cohen’s kappa score of 0.749 was calculated according to the results from title and abstract screening of the included studies.
Quality assessment results
All randomized as well as non-randomized interventional studies were assessed by Cochrane’s Risk of bias Tool for Quality assessment [Figure 2a and b] and all the included studies had low risk of bias.
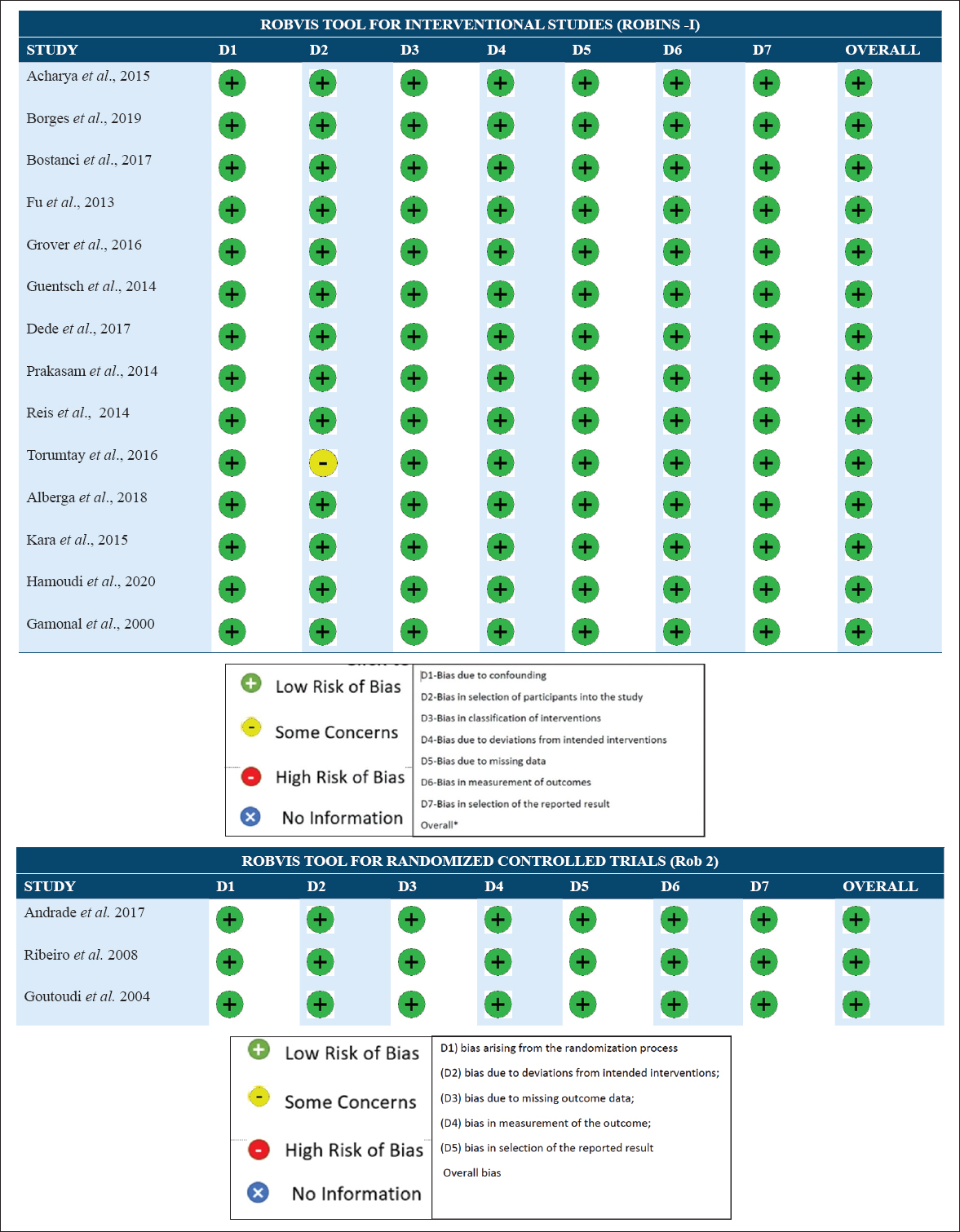
- (a) ROBVIS presentation for quality assessment results (interventional studies). (b) ROBVIS presentation for quality assessment results (Randomized controlled trials)
General study characteristics
Table 1 provides the descriptive analysis of all the studies included in systematic Review. 3 articles had randomized controlled trial design[4,8,14] while 14 articles were non-randomized interventional designs.[7,10,15-26]
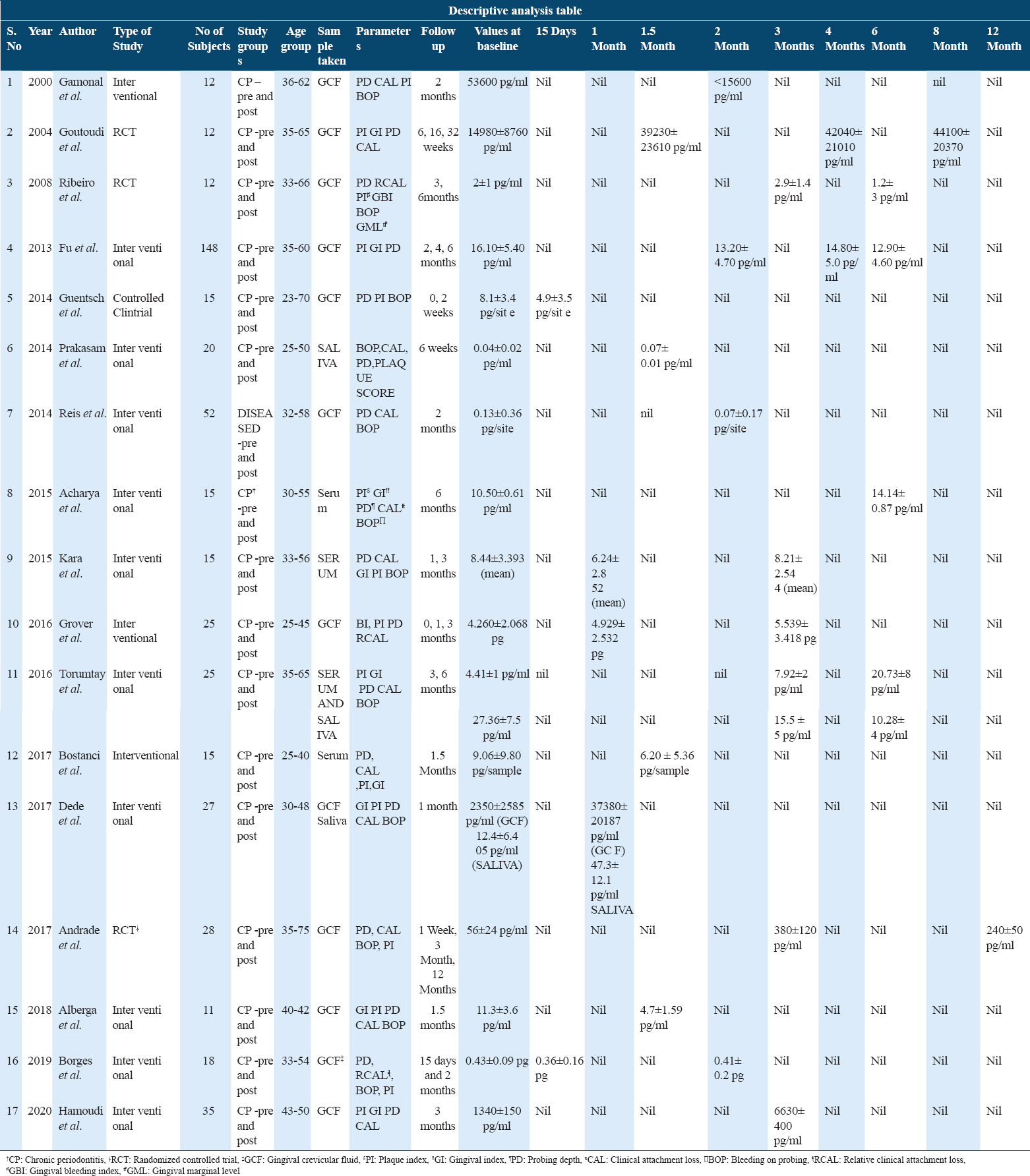
Of all the studies, 12 studies measured IL-10 in GCF, four used serum as a diagnostic fluid and three studies used saliva as the diagnostic fluid wherein we analyzed the change in levels of IL-10 pre- and post-NSPT in chronic periodontitis patients.
Age groups for all the studies fall under the range of 25–75 years with an average of 35 years.
There are two studies each for follow-up intervals at 15 days, 1, 1.5, 4, and 6 months in GCF. For follow-up at 2 and 3 months, four studies for GCF were available whereas at 8 and 12 months, one study could be found. For serum and saliva at 1 and 1.5 months, there was one study whereas at an interval of 3 and 6 months, two for serum and one for saliva were found.
There was observed great deal of variation in the included studies but in general most of them involved the recording of attachment loss, deep probing depth, and bleeding on probing.
From a total of 395 patients, the results showed that NSPT increases the value of IL-10 in GCF at 1, 3, and 4 months post-therapy but decrease was seen at 6 months. Some of the studies documented a decrease in levels of IL-10 at 15 days interval, 1.5 and 2 months post-therapy.
In serum, an opposite trend was seen as there was an increase in IL-10 levels at 6 months and at 1, 1.5 months a decrease in its level was noticed. For the interval of 3 months, both increase and decrease were seen in different studies.
The descriptive analysis of studies regarding saliva showed an increase was seen at 1 and 1.5 months post-NSPT whereas a subsequent decrease at 3 and 6 months.
Results of meta-analysis
The GCF samples for IL-10 level among patients suffering with periodontitis at baseline relative to their levels at 3 months following NSPT showed the standard mean difference of 6.994 pg/ml (0.922; 13.066). This is suggestive of an increase in IL-10 level in patients after NSPT which shows that the intervention was favorable, and the overall result was statistically significant (P = 0.024). A random effect model [Figure 3] was used to assess the effect size as heterogeneity of studies came out to be statistically significant (I2: 98.37%; P < 0.0001).
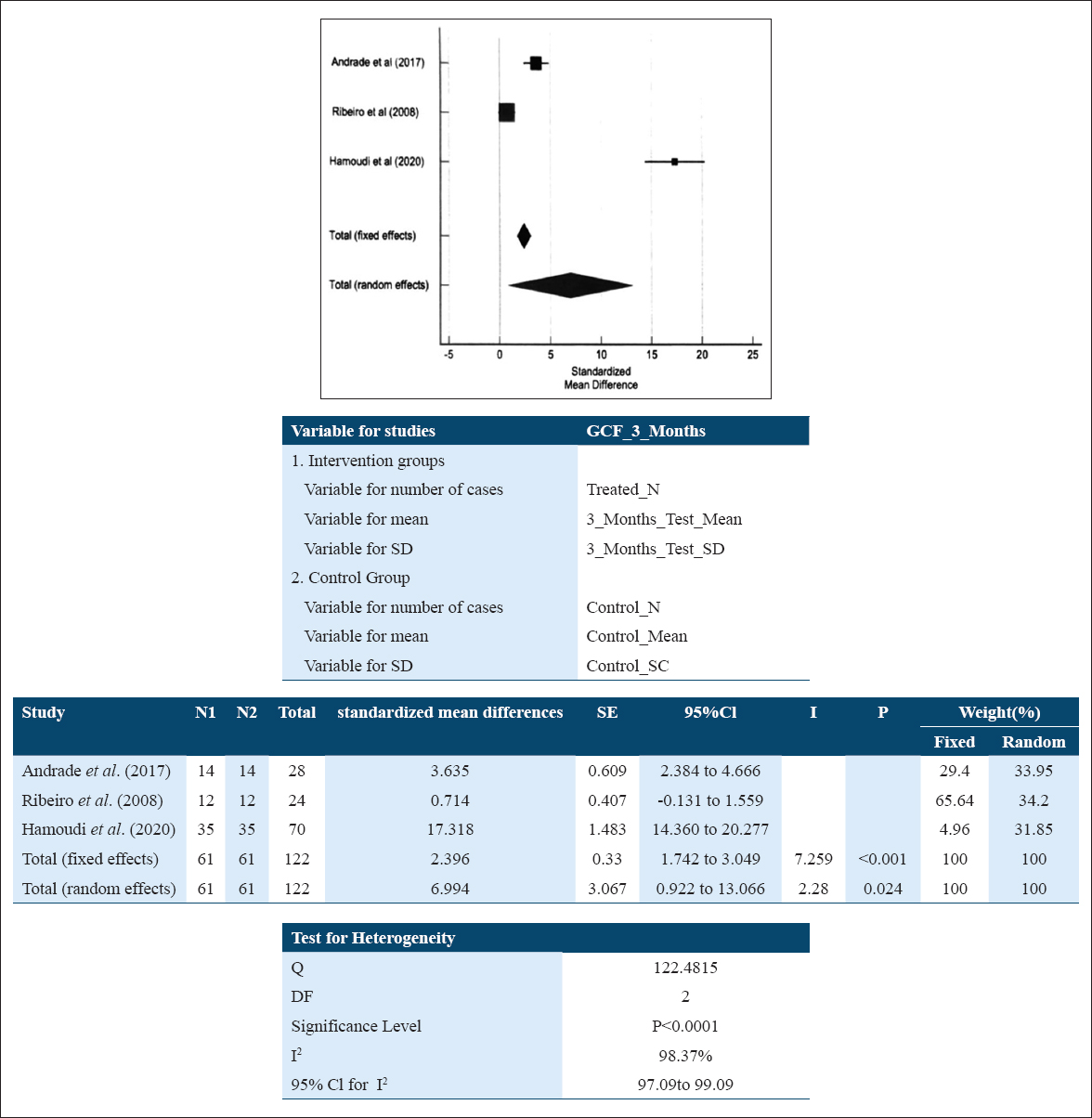
- Forest plot assessing the standardized mean difference of IL-10 levels pre and post NSPT in GCF at 3 months
The standard mean difference in GCF samples for IL-10 level among patients suffering with periodontitis at baseline relative to their levels at 4 months following NSPT was 0.634 pg/ml (–1.204; 2.473) suggestive of an increase in IL-10 level in patients after NSPT. This reflects that the intervention was favorable, but the overall result was found to be statistically non-significant (P = 0.498). A random effect model [Figure 4] was used for the existing high heterogeneity of studies (I2: 93.62 %; P = 0.0001).
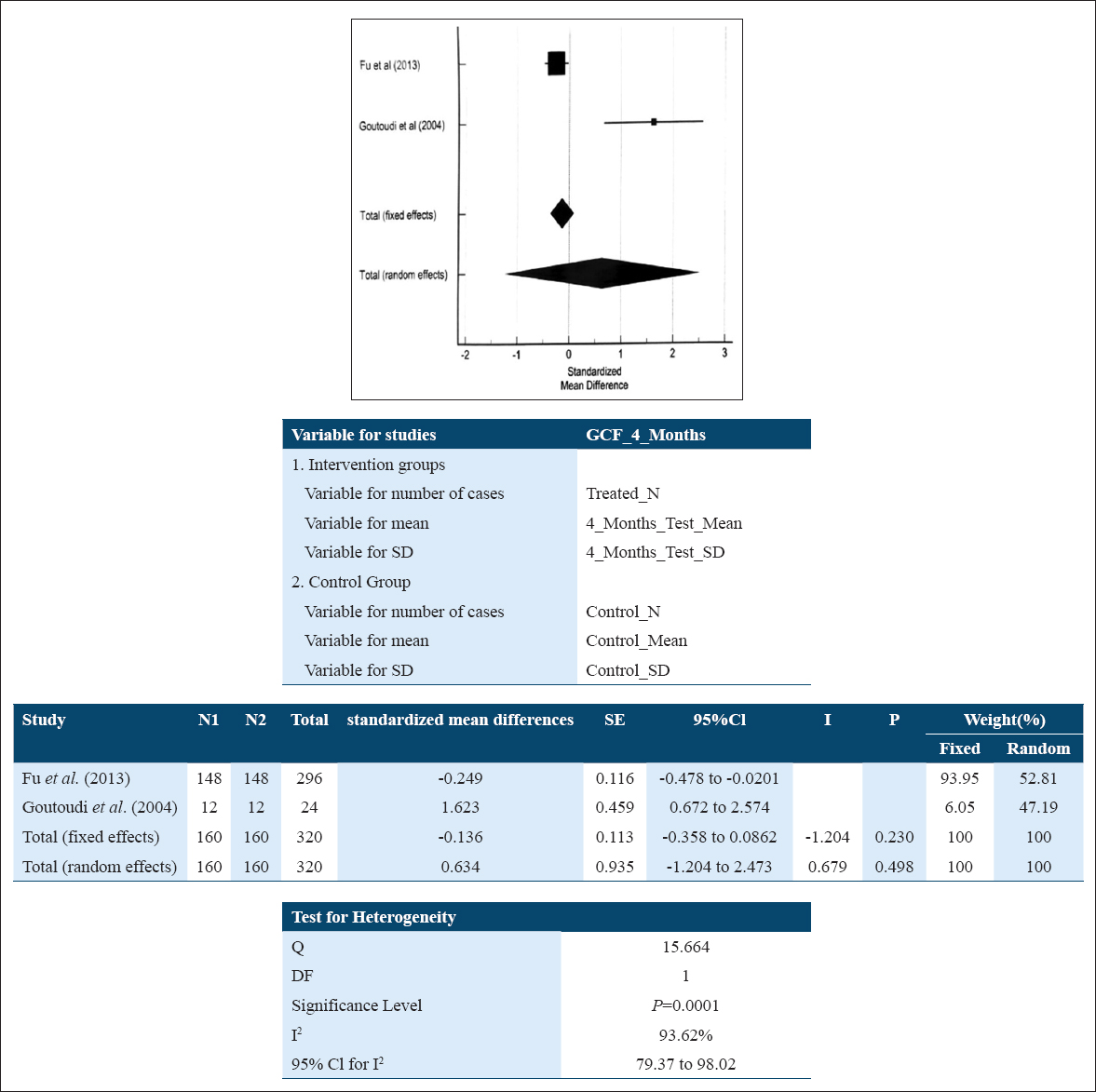
- Forest plot assessing standardized mean difference of IL-10 levels pre- and post-NSPT in GCF at 4 months
For GCF 6 month subgroup analysis, a fixed effect model [Figure 5] was used, as the heterogeneity of studies came out to be 0 (I2:0.00%; P = 0.4830) whereas the standard mean difference in GCF samples for IL-10 level among patients suffering with periodontitis at baseline relative to their levels at 6 months following NSPT was –0.612 pg/ml (–0.836; –0.388) which was found to be statistically significant. This suggests that the NSPT could have raised the levels of IL-10 post-therapy and the effect could not be sustained for longer duration (6 months).
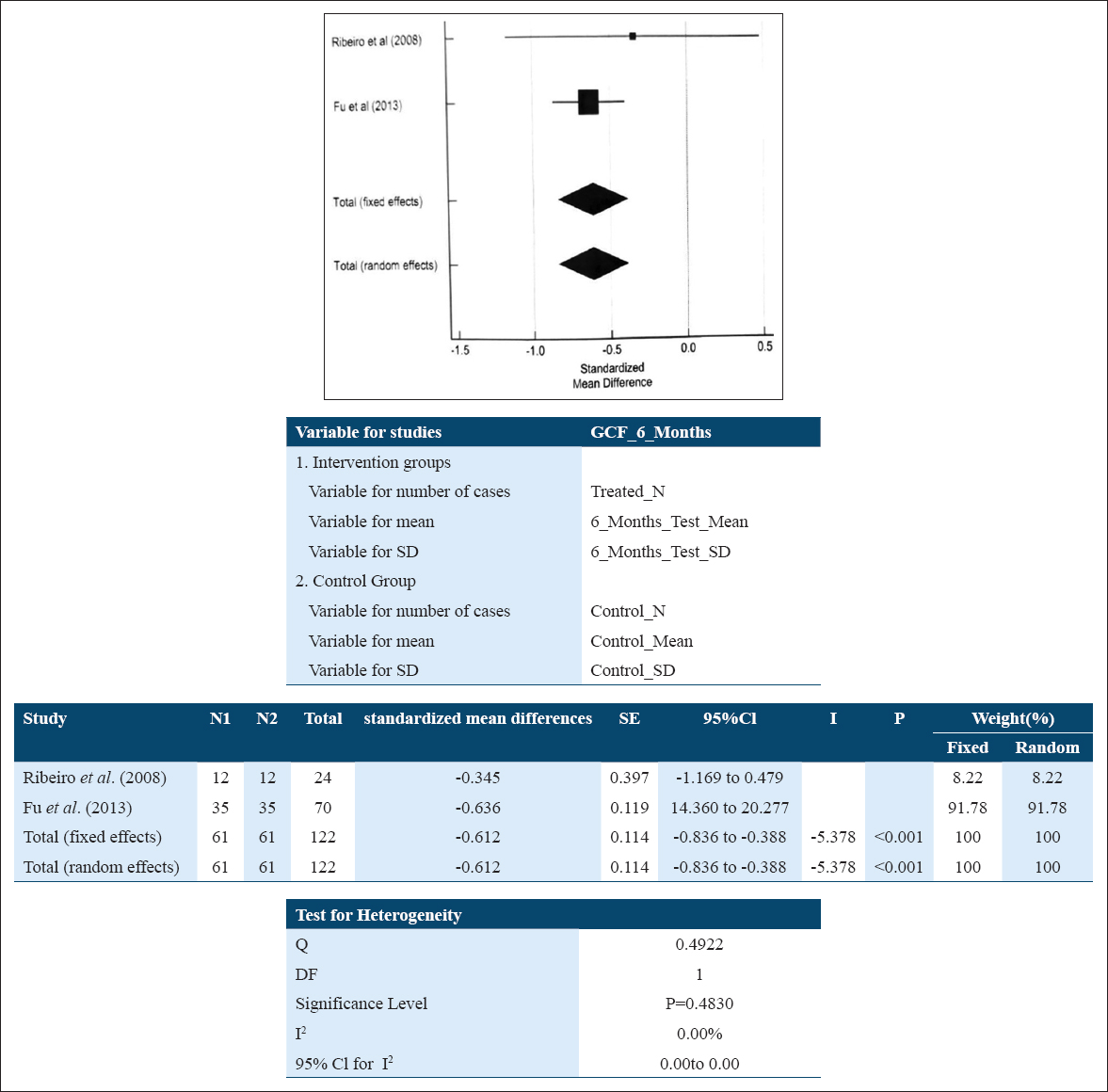
- Forest plot assessing standardized mean difference of IL-10 levels pre- and post-NSPT in GCF at 6 months
The subgroup analysis of IL-10 values in serum samples at baseline and at 6 months showed the standard mean difference of 3.676 pg/ml (1.797; 5.555) suggestive of an increase in IL-10 level in patients after NSPT which reflects that the intervention was favorable, and here the overall result was also found to be statistically significant (P < 0.001). A random effect model [Figure 6] was used due to high heterogeneity of studies (I2: 81.82%; P = 0.0190). Based on the small number of studies in the meta-analysis (i.e., <10), funnel plots were not included to display publication bias.
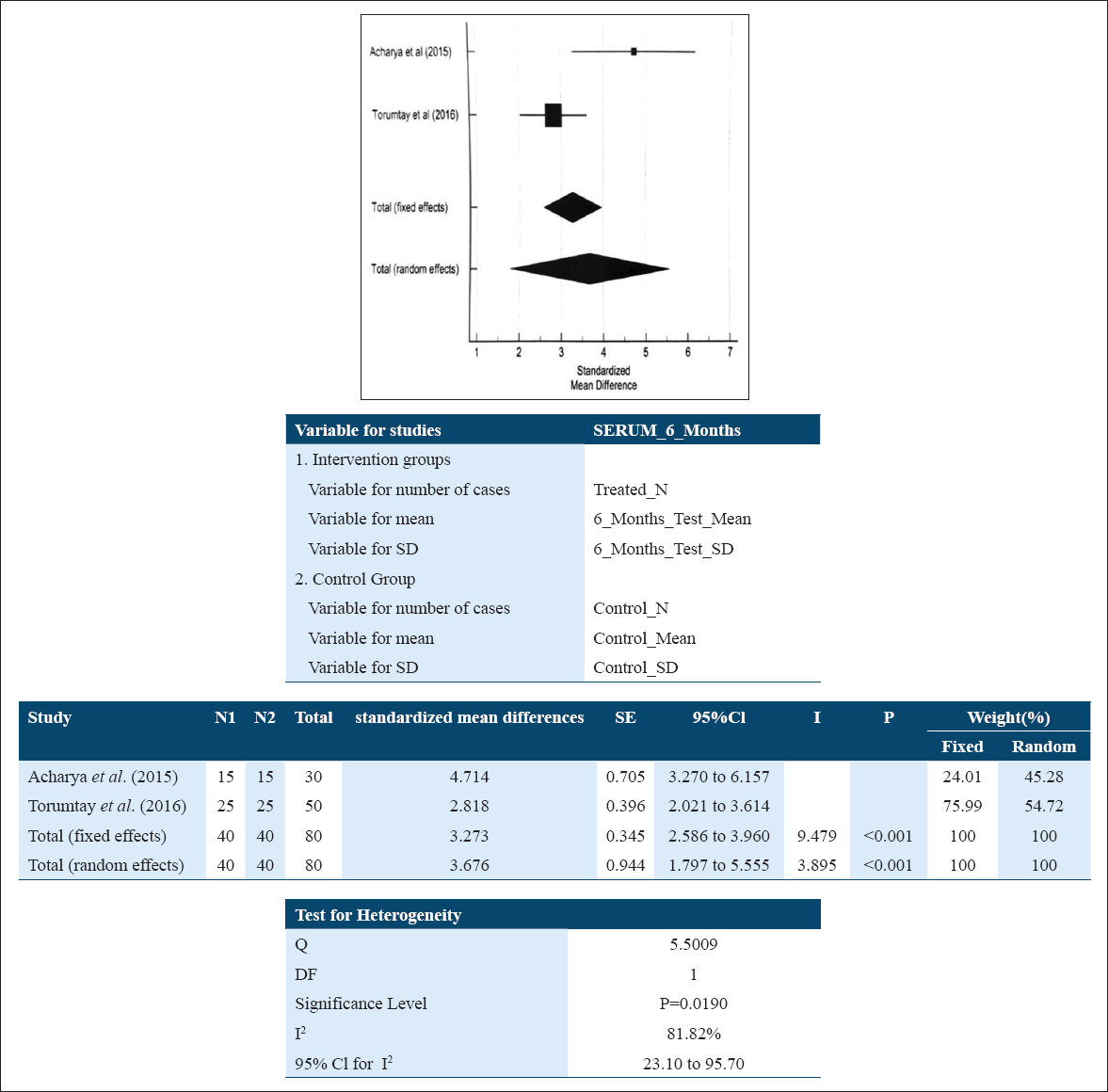
- Forest plot assessing standardized mean difference IL-10 levels pre- and post-NSPT in serum at 6 months
Discussion
As periodontal diseases are prevalent both in developed and developing countries and affect about 20–50% of global population, thus high prevalence of periodontal disease in adolescents, adults, and older individuals makes it a public health concern.[27]
IL-10 is a pluripotent cytokine with effects on numerous cell populations, in particular circulating and resident immune cells as well as epithelial cells. With its potent immunoregulatory capacities, its main biological function seems to be the limitation and termination of inflammatory responses.[28]
The primary aim of the systematic review was to evaluate the effect of Non-Surgical Periodontal therapy on the level of anti-inflammatory cytokine IL-10 in GCF, serum and saliva samples in Chronic Periodontitis patients. The systematic review identified 17 controlled trials assessing the use of SRP for management of chronic periodontitis with follow-ups ranging from 15 days to 12 months. The results of meta-analysis including five studies for GCF and 2 for serum could be pooled for analysis, whereas there were relatively inadequate and heterogeneous studies of small duration that analyzed the levels of IL-10 in saliva so that was not pooled.
To the best of our knowledge, this is the first systematic review and meta-analysis exploring the GCF, serum and saliva IL-10 cytokine levels in chronic periodontitis disease pre- and post-NSPT.
The results from the systematic review demonstrated that NSPT significantly affects the level of IL-10 in GCF and increases the level of this cytokine post-therapy. The effect was consistent at three and 4 months; however, there was observed a decrease at 6 months of duration.
To compensate the small number of studies available, separate clinical procedure employed in the particular RCTs has been considered as separate treatment units. This could be a cause of weakness because it reduced the independent inclusion of clinical studies not designed to answer the direct research question. Nevertheless, the strict inclusion criteria and absence of significant differences between treatment arms and methods of evaluation, the approach adopted was deemed reasonable. The same approach had been utilized previously by other authors conducting analysis for similar situations.[29] Very stringently, clinical trials reporting the concentration of IL-10 only were included in the meta-analysis, rather than the total activity pre- and post-treatment as reported in the original studies were excluded.[7,10,16,18,19,24] Further the method of estimation of the cytokine was by ELISA technique in majority of the studies which added to the methodological similarity of analysis. As there is only one reported systematic review and meta-analysis by Stadler et al., 2016, which has evaluated GCF levels of cytokines and chemokines in chronic periodontitis after NSPT and non-significant increase has been revealed regarding IL-10, the findings of the studies have been compared to RCTs published earlier.
Studies by Andrade et al., 2017, Ribeiro et al., 2008, Grover et al., 2016; and Hamoudi et al., 2020; have reported an increased level of GCF IL-10 at 3 months after non-surgical periodontal therapy which are in agreement with the results of current meta-analysis also. Grover et al., 2016, Dede et al., 2017, and Goutoudi et al., 2004 depicted an increase in GCF levels of IL-10 following NSPT at 1, 1.5.4 months, respectively. The increase in concentration of anti-inflammatory cytokines seems plausible as IL-10 might have an important regulatory role in limiting the duration and extent of the acute inflammatory response. In addition, it has been suggested that IL-10 increases the synthesis of tissue inhibitors of metal loproteinases in macrophages which, in turn, inhibit MMP production thus representing itself as an important matrix protective cytokine during inflammation.[30,31]
Studies mentioning values of IL-10 in GCF at 6 months by Ribeiro et al., 2008, and Fu et al., 2013, showed a decrease in its value comparable to baseline significantly.
IL-10 levels in serum samples that showed increased IL-10 levels in chronic periodontitis after NSPT at 3 and 6 months stated that NSPT has been regulating the overall circulating inflammatory status. And this increased level of IL10 is indicative of its antiinflammatory role that has been implicated in the suppression of tissue destruction. Moreover it inhibits the synthesis of monocyte-derived pro-inflammatory cytokines such as IL-1, IL-6, IL-8, and TNF-α thus enhancing the production of the IL-1 receptor antagonist in polymorphonuclear leukocytes stimulated with lipopolysaccharide.[32,33]
A key observation from the identified studies showed that there is a clear-cut lack of defined value or range of IL-10 levels in GCF, as only a few studies reporting on the levels of the cytokines were found in literature. Further the range observed by Fenol et al., 2014, in a total of 91 subjects was: 260.0 ± 1728.0 pg/ml (periodontitis group), 1128 ± 532.9 (Gingivitis group); 648 ± 505.75 (healthy group), wherein pocket depth ≥5 mm and CAL ≥3 mm was taken as the diagnostic criteria for periodontitis group and in another study by Gamonal et al., 2000: Range was 23.1–241 pg/ml which showed that the levels of IL-10 was usually variable among the studies.[26,34]
The strengths of our review include it’s novelty and potential clinical relevance as IL-10 is a primary anti-inflammatory cytokine in the local milieu determinant of the clinical status and response of periodontal tissues to therapy. IL-1β and IL-10 ratios have been documented to be significantly altered in response to therapy in cases of chronic and aggressive periodontitis.[35] Further we have limited our meta-analysis to clinical trials at specific time point durations of 3 and 6 months of observations which are clinically relevant as supportive periodontal therapy intervals to provide clinically meaningful answers.
Furthermore, a number of limitations are there in our reviewed and identified literature as there is significant heterogeneity among studies (statistical, clinical, and methodological) which results from multiple factors associated with the study design. These include aspects related to patient population and characteristics, variation in the SRP procedures, oral hygiene instructions, the baseline gingivitis, and plaque levels.
This could not be controlled and contributed to the heterogeneity that was observed. The follow-up durations for the pooled analysis in the included studies were limited to 6 months which may be considered short. It should be remembered that there could be differences in the self-performed infection control and distribution of modifying factors too.
While the overall risk of bias for included studies was found to be low, most were institutional and were performed in well-controlled environment in patient samples. Hence, the present review probably describes the efficacy rather than the effectiveness of intervention.
In conclusion and within the limitations of the present review a comprehensive search and analysis of the available literature based upon controlled trials within 15 days, 1, 1.5, 2, 3, 4, 6, 8, 9, 12 months follow-up duration demonstrated that non-surgical periodontal therapy significantly alters the levels of anti-inflammatory cytokines IL-10 in GCF and serum and there is inconclusive data regarding the same when evaluated in saliva. Reporting of results has been described on the basis of GRADE guidelines and suggests a moderate evidence of recommendation for the study results owing to low imprecision, indirectness, and inconsistency found in the literature.
Future RCTs providing insight into IL-10 levels must be registered on the publicly accessible database before commencement and be appropriately designed with longer duration of follow-ups and defined outcome measures. It is important that the baseline clinical characteristics and diagnosis should be recorded based on uniform disease definition criteria to provide best available evidence based on clinically relevant answers to improve the patient outcomes.
Conclusion
Within the limitations of the current analysis, there is moderate evidence to suggest that the NSPT affects significantly the levels of IL-10 in GCF, serum and saliva in chronic periodontitis patients. Increased levels of IL-10 at 3 months for GCF, 6 months for serum, was seen whereas a decrease was evident in its level at 6 months in GCF, 3 and 6 months for saliva. Due to much variable heterogenous data available, more well-designed randomized trials on this cytokine needs to be conducted in the future.
Clinical Relevance
Clinical significance
An increase in the level of anti-inflammatory cytokine IL-10 in GCF at 3 months indicates the effectiveness of NSPT in the management of chronic periodontitis. The levels were raised in serum till 6 months for the anti-inflammatory cytokine which also depicts the impact of NSPT on systemic status.
Scientific rationale for review
The aim of the systematic review was to assess the effect of NSPT on the levels of anti-inflammatory cytokine IL-10 in chronic periodontitis patients.
Principal findings
NSPT significantly increases the levels of IL-10 in GCF at 3 months and serum at 3 and 6 months whereas decrease was seen at 6 months in GCF. There were relatively inadequate and heterogeneous studies for saliva.
Practical implications
There is only moderate evidence for IL-10 to be considered as a marker for response to NSPT, so further well-designed clinical trials are needed in future.
Authors’ Declaration Statements
Ethics approval
NA.
Consent for publication
None.
Availability of data and material
The data used in this study are available and will be provided by the corresponding author on a reasonable request.
Competing Interests
The authors do not report any conflicts of interest.
Funding Statement
None.
Authors’ Contributions
Vishakha Grover: Conceptualization; Methodology; validation, visualization, data curation, Formal analysis and investigation: Writing - original draft preparation: Jyoti Gupta: Conceptualization; Methodology; validation, visualization, Formal analysis and investigation: Writing - original draft preparation: Suhani Maheshwari; data curation, writing, (pre liminary and original draft preparation), protocol writing; Narinder Kumar: statistical analysis; Ashish Jain: Conceptualization; Methodology; validation, visualization.
Acknowledgment
None.
ORCID link of the submitting author: 0000-0003-1010-0294.
References
- Associations of cytokine levels in gingival crevicular fluid of mobile teeth with clinical improvement after initial periodontal treatment. J Oral Sci. 2020;62:189-96.
- [Google Scholar]
- Expression of suppressors of cytokine signaling in diseased periodontal tissues:A stop signal for disease progression? J Periodontal Res. 2006;41:580-4.
- [Google Scholar]
- Gingival crevicular fluid levels of cytokines/chemokines in chronic periodontitis:A meta-analysis. J Clin Periodontol. 2016;43:727-45.
- [Google Scholar]
- Effect of periodontal therapy on crevicular fluid interleukin-1beta and interleukin-10 levels in chronic periodontitis. J Dent. 2004;32:511-20.
- [Google Scholar]
- Proinflammatory and anti-inflammatory cytokines in gingival crevicular fluid and serum of patients with rheumatoid arthritis and patients with chronic periodontitis. J Periodontol. 2013;84:84-93.
- [Google Scholar]
- Cytokine and metalloproteinases in gingival fluid from patients with chronic periodontitis. Invest Clin. 2016;57:131-42.
- [Google Scholar]
- Clinical attachment loss and molecular profile of inflamed sites before treatment. J Appl Oral Sci. 2019;27:671.
- [Google Scholar]
- Photodynamic therapy decrease immune-inflammatory mediators levels during periodontal maintenance. Lasers Med Sci. 2017;32:1-9.
- [Google Scholar]
- Immunohistochemical, histomorphometric, and gingival crevicular fluid analysis of residual and shallow periodontal pockets in patients with periodontitis stages III and IV. J Periodontol. 2020;91:870-9.
- [Google Scholar]
- Clinical improvement following therapy for periodontitis:Association with a decrease in IL-1 and IL-6. Exp Ther Med. 2014;8:323-7.
- [Google Scholar]
- 2011. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. London, UK: The Cochrane Collaboration; Available from: http://www.cochrane-handbook.org
- Dealing with missing standard deviation and mean values in meta-analysis of continuous outcomes:A systematic review. BMC Med Res Methodol. 2018;18:25.
- [Google Scholar]
- Periodontal debridement as a therapeutic approach for severe chronic periodontitis:A clinical, microbiological and immunological study. J Clin Periodontol. 2008;35:789-98.
- [Google Scholar]
- Effect of scaling and root planing on serum interleukin-10 levels and glycemic control in chronic periodontitis and Type 2 diabetes mellitus. J Indian Soc Periodontol. 2015;19:188.
- [Google Scholar]
- Evaluation of IL-1β, IL-1ra, and IL-10 levels and outcome of periodontal therapy in chronic periodontitis with familial Mediterranean fever. Clin Oral Investig. 2017;21:469-75.
- [Google Scholar]
- Correlation of chronic periodontitis in tropical area and IFN-gamma, IL-10, IL-17 levels. Asian Pac J Trop Med. 2013;6:489-92.
- [Google Scholar]
- Effect of topical simvastatin (1.2 mg) on gingival crevicular fluid interleukin-6, interleukin-8 and interleukin-10 levels in chronic periodontitis-a clinicobiochemical study. J Oral Biol Craniofac Res. 2016;6:85-92.
- [Google Scholar]
- Oral prophylaxis and its effects on halitosis-associated and inflammatory parameters in patients with chronic periodontitis. Int J Dent Hyg. 2014;12:199-207.
- [Google Scholar]
- Interleukin-32 levels in gingival crevicular fluid and saliva of patients with chronic periodontitis after periodontal treatment. J Period Res. 2016;52:397-407.
- [Google Scholar]
- Evaluation of salivary biomarker profiles following non-surgical management of chronic periodontitis. Oral Dis. 2014;20:171-7.
- [Google Scholar]
- Effects of periodontal treatment on inflammation and oxidative stress markers in patients with metabolic syndrome. J Period Res. 2016;51:489-98.
- [Google Scholar]
- Cytokines and MMPs levels in gingival crevicular fluid from patients with chronic periodontitis before and after non-surgical periodontal therapy. J Oral Res. 2018;7:24.
- [Google Scholar]
- Short term effects of periodontal therapy on inflammatory markers in patients with Type-2 diabetes. Saudi Med J. 2015;36:469-76.
- [Google Scholar]
- Effect of scaling and root planing on the expression of anti-inflammatory cytokines (IL-4, IL-9, IL-10, and IL-13) in the gingival crevicular fluid of electronic cigarette users and non-smokers with moderate chronic periodontitis. J Period Implant Sci. 2020;50:74-82.
- [Google Scholar]
- Levels of interleukin-1 beta, -8, and -10 and RANTES in gingival crevicular fluid and cell populations in adult periodontitis patients and the effect of periodontal treatment. J Periodontol. 2000;71:1535-45.
- [Google Scholar]
- Prevalence of periodontal disease, its association with systemic diseases and prevention. Int J Health Sci (Qassim). 2017;11:72-80.
- [Google Scholar]
- A systematic review on the clinical efficacy of subgingival debridement in the treatment of chronic periodontitis. J Clin Periodontol. 2002;29:55-71.
- [Google Scholar]
- IL-10 inhibits metalloproteinase and stimulates TIMP-1 production in human mononuclear phagocytes. J Clin Invest. 1995;96:2304-10.
- [Google Scholar]
- IL-10 modulates formation of osteoclasts in murine hemopoietic cultures. J Immunol. 1996;157:936-40.
- [Google Scholar]
- Interleukin 10 (IL-10) upregulates IL-1 receptor antagonist production from lipopolysaccharide-stimulated human polymorphonuclear leukocytes by delaying mRNA degradation. J Exp Med. 1994;179:1695-9.
- [Google Scholar]
- Interleukin 10 (IL-10) inhibits the release of proinflammatory cytokines from human polymorphonuclear leukocytes. Evidence for an autocrine role of tumor necrosis factor and IL-1 beta in mediating the production of IL-8 triggered by lipopolysaccharide. J Exp Med. 1993;178:2207-11.
- [Google Scholar]
- Levels of interleukin-10 in gingival crevicular fluid and its role in the initiation and progression of gingivitis to periodontitis. J Oral Hyg Health. 2014;2:135.
- [Google Scholar]
- Cytokine thresholds in gingival crevicular fluid with potential diagnosis of chronic periodontitis diferentiating by smoking status. Sci Reports. 2018;8:18003.
- [Google Scholar]







