Translate this page into:
Efficacy of a Benincasa hispida powdered drink in improving metabolic control in patients with type 2 diabetes: A placebo-controlled study
Address for correspondence: Dr. Wan Mohd Izani Wan Mohamed, Department of Internal Medicine, School of Medical Sciences, Universiti Sains Malaysia, Kubang Kerian, Kelantan, Malaysia. E-mail: izani@usm.my
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Qassim Uninversity and was migrated to Scientific Scholar after the change of Publisher.
Abstract
ABSTRACT
Objectives:
There is emerging evidence of the benefits of Benincasa hispida in improving metabolic profiles in people with diabetes. This study was conducted to analyze the effect of B. hispida aqueous extract on the metabolic control of patients with type 2 diabetes in Malaysia.
Methods:
A powdered drink formulated with 2.5 g of B. hispida extract was prepared as a test food. An intervention study was conducted with 50 participants randomly assigned to an intervention or a control group. Anthropometric, biochemical, and clinical variables were assessed at baseline and week 12 after intervention. Paired T-tests were applied to compare the mean differences between the baseline and post-intervention for each variable.
Results:
The intervention group presented a significant reduction in diastolic blood pressure (Δ −7.0 mmHg, 95% confidence interval [CI]: −11.4, −2.5). Mean fasting plasma glucose (Δ −0.8 mmol/L, 95% CI: −1.8, 0.2) showed a greater reduction in the intervention group compared to the control group (Δ −0.4 mmol/L, 95% CI: −1.2, 0.4). Mean lean body mass showed a favorable trend of increment at week 6 (Δ 0.05 kg, 95% CI: −0.40, 0.49) and week 12 (Δ 0.16 kg, 95% CI: −0.33, 0.64) as compared to baseline in the intervention group but not in the control group which manifested decreasing lean body mass.
Conclusion:
The use of B. hispida extract may potentially improve blood pressure and glycemic control in patients with type 2 diabetes and it may be an attractive candidate for the development of functional food products.
Keywords
Diabetes
glycemia
intervention
metabolic
winter melon
Introduction
Diabetes mellitus (DM) is a major public health issue affecting 537 million adults worldwide, a figure projected to escalate to 643 million by 2030 and 784 million by 2045.[1] DM, a rising epidemic over the previous century, has become more pressing in the past few decades in line with the exponential rise of obesity. It was responsible for 6.7 million mortalities in 2021 and is one of the top ten leading causes of death globally.[1,2] Type 2 DM (T2DM) is the most prevalent form of diabetes and accounts for more than 95% of all cases of the disease.[3] It is a chronic metabolic disorder characterized by persistent hyperglycemia caused by diminished sensitivity to the peripheral actions of insulin, decreased insulin secretion, or both.[4]
Metabolic control is an important element in the management of T2DM to reduce the risk of complications. Poorly controlled T2DM can progressively result in chronic microvascular, macrovascular, and neuropathic complications. This means patients should be targeted so that the values of key parameters such as fasting plasma glucose (FPG), glycated hemoglobin (HbA1c), insulin, triglycerides (TG), low-density lipoprotein (LDL-C), high-density lipoprotein (HDL-C), blood pressure, and body mass index (BMI) are maintained within the recommended range for people with diabetes.[5] Therefore, effective disease management is essential to minimize complications due to T2DM.
Medical nutrition therapy (MNT) is known as one of the main components in T2DM management. Besides MNT, the administration of functional foods has also been proposed for glycemic control in T2DM patients.[6] Foods that work beyond the basic nutritional functions have the potential to act as anti-diabetic agents through numerous complex mechanisms.[7] Both fruits and vegetables are gaining popularity and interest among researchers as alternative and emerging ingredients in the development of functional foods.
Winter melon (Benincasa hispida [Thunb.] Cogn.), a vegetable member of the family Cucurbitaceae, has enormous potential for functional food production. It is one of the most popular crops throughout the Asian region, including China, India, Philippines, Indonesia, Malaysia, and elsewhere in tropical Asia. It is grown primarily for its large fruit, which is often enjoyed as a vegetable in local cuisines.[8] B. hispida has been highlighted in recent decades due to its potential therapeutic anti-inflammatory, anti-hypertensive, anti-cancer, and anti-obesity effects, and it also has neuroprotective effects.[9] Moreover, previous findings have revealed that B. hispida fruit has a safe pharmacological profile for long-term oral consumption.[10]
Robust evidence exists of the promising role of B. hispida in treating T2DM and preventing related complications through the modulation of various cellular mechanisms due to the presence of polysaccharides, vitamins, minerals, sugars, organic acids, and other bioactive compounds.[8,9] Nagarajaiah and Prakash[11] suggested that B. hispida aqueous extract could inhibit the activity of alpha-amylase, an enzyme that hydrolyses carbohydrates, causing retardation of glucose absorption and eventually decreasing blood glucose levels. Moreover, recent evidence also showed that the consumption of B. hispida aqueous extract rich in gallic acid for 12 weeks reduced blood glucose, TG, and VLDL-C levels in rats.[12] To date, intervention studies involving the use of B. hispida extract supplementation in humans have been scarce, and the available data come predominantly from in vitro and in vivo studies.
Thus, the current study aimed to analyze the efficacy of a 12-week intervention using B. hispida aqueous extract supplementation in improving the metabolic parameters of patients with T2DM.
Methods
Study design
This was a parallel intervention study conducted in selected outpatient clinics at Hospital Universiti Sains Malaysia (Hospital USM) between June and December 2022. The study was conducted in accordance with the guidelines outlined in the Declaration of Helsinki, and all the procedures involving human participants were approved by the Human Research Ethics Committee of Universiti Sains Malaysia (protocol code USM/JEPeM/20090466 and date of approval September 13, 2021).
Population and sample
The study population consisted of adults with a medical diagnosis of T2DM, who had registered and been followed up by selected outpatient clinics at Hospital USM. Patients who attended the Hospital USM outpatient clinic were screened from the daily appointment list and their medical records were accessed thoroughly.
The fasting venous glycemia reported in the study by Neta et al.[5] was used as the parameter for determining the sample size, which totaled about 21 patients in each group. The sample in every group was increased by 10% to account for possible losses or withdrawals. Thus, 25 patients were assigned to the intervention group and 25 to the control group.
The inclusion criteria were a medical diagnosis of T2DM for at least 1 year, prescribed with oral hypoglycemic agents (OHAs), Malaysian citizen, literate, aged 18 years old or above, had a BMI between 18.5 and 39.9 kg/m2, baseline FPG <15 mmol/L, HbA1c between 7 and 12% and available to come to collect their laboratory test samples as scheduled. Meanwhile, the exclusion criteria were the use of insulin, glucocorticoids, psychotropic drugs, or chemotherapy; ongoing pregnancy or lactation; and advanced complications of diabetes, such as end-stage renal failure, blindness, limb amputation, congestive heart failure, stroke, or uncontrolled hypertension. Participants taking dietary supplements known to affect appetite, food intake, body fat distribution, or energy metabolism were also excluded from the study.
The study protocol was explained in detail and the participants voluntarily gave informed consent before participation. All the participants signed the consent form before being recruited for the study. Before initiating the intervention, all the participants were asked to refrain from unusual or intense physical activity, as well as restrict their intake of alcoholic and caffeinated beverages. Throughout the study, the patients continued their treatment regime, including the T2DM medications prescribed by physicians.
Randomization process
After consent had been obtained from the study participants, an initial interview was conducted using a sociodemographic data collection form. An additional interview was conducted to collect anthropometric, biochemical, and clinical data. The data taken at the first interview were considered the baseline of the study; the second meeting was scheduled after 6 weeks and the last meeting after 12 weeks.
A randomization plan was generated using online software (http://www.randomization.com). Participants were assigned to either the intervention or the control group following the sequence of the randomization plan. This stage was controlled by a staff member who had no interaction with the participants.
Intervention
All the participants were given powdered drink sachets weighing 5 g each, which were to be ingested once daily for 12 weeks. The samples were all coded with random numbers. The intervention group participants were given powdered drink sachets, each of which contained a 2.5 g extract of B. hispida. They consumed it once daily for 12 weeks. Meanwhile, the control group participants received powdered drink sachets containing the same ingredients as those of the intervention group but without the B. hispida extract; the sachets were to be ingested once a day for 12 weeks. Each powdered drink sachet needed to be mixed with 250 mL of warm water and stirred until it dissolved before drinking.
The participants in both groups received routine care in the outpatient clinics. They were also advised not to make any special changes to their diet or physical activity, as well as report any changes in medication during the intervention period. The only intervention was to be supplementation with the B. hispida extract or the placebo powdered drinks.
Powdered drink characteristics and preparation
The powdered drinks for the intervention study were prepared at the Institute of Bioproduct Development at Universiti Teknologi Malaysia, a facility with good manufacturing practice, hazard analysis and critical control point system, and Halal certifications. B. hispida fruit was acquired from a single plantation area in Kampung Sungai Lang, Sabak Bernam, Selangor, Malaysia. The plants were botanically authenticated by an expert from the School of Biological Sciences, Universiti Sains Malaysia (Voucher Number 11881).
The round B. hispida fruit was peeled, and the pulp was washed and cut into cubes. The pulp was mashed using a grinder and weighed. Distilled water was added at a 1:1 ratio to the pulp weight. Next, aqueous extraction of the B. hispida took place at 60°C for 30 min.[13] The extract was then mixed with resistant dextrin and gum Arabic as encapsulation materials before being brought for spray-drying. The extract powder produced was then homogenized with ingredients such as sweetener and flavoring to improve the taste of the powdered drink. Each powdered drink weighed 5 g, with the sachets used in the intervention sample containing 2.5 g of B. hispida extract and the rest of the weight contributed by 1 g of resistant dextrin, 1 g of gum Arabic, 0.4 g of sweetener, and 0.1 g of flavoring. Meanwhile, the placebo sample sachets also weighed 5 g and consisted of 2 g of resistant dextrin, 2 g of gum Arabic, 0.8 g of sweetener, and 0.2 g of flavoring only, without B. hispida extract. The sachets containing the powdered drink were neatly sealed to prevent ambient moisture absorption.
Measurements
Anthropometric measurements such as body weight, height, waist circumference, and hip circumference were assessed using a weighing scale, stadiometer, and measuring tape (Seca GmbH, Hamburg, Germany), respectively. Body compositions including body fat percentage, fat mass, lean body mass, visceral fat rating, and total body water were measured using a body composition analyzer (Tanita, Tokyo, Japan). Data for anthropometry were taken at baseline, after 6 weeks, and after 12 weeks.
Blood samples were collected for laboratory testing at baseline and after 12 weeks. However, FPG was also monitored after 6 weeks of intervention. Patients had 10 mL of blood samples collected after fasting for 10–12 h. The blood samples were immediately sent to the Chemical Pathology Laboratory and the Endocrinology Laboratory USM for further analysis.
Lipid profiles including specific variables LDL-C, HDL-C, and TGs were analyzed using a clinical chemistry analyzer (Abbott Architect analyzer, Illinois, USA).
Glycemic control consisted of the following variables: FPG and HbA1c, which were analyzed using a capillary electrophoresis instrument (Capillarys Octa Sebia automated electrophoresis capillary system, Lisses, France), as well as insulin (Roche Elecsys analyzer, Indiana, USA).
Insulin resistance (IR) was computed using the homeostatic model assessment (HOMA) method. The HOMA index is a mathematical calculation involving fasting blood glucose and fasting insulin concentrations. It is calculated to quantify HOMA-IR and pancreatic beta-cell activity in the pancreas (HOMA-β). The formulas used to determine these indices were as follows:
HOMA-β = (20 × fasting insulin (μIU/mL))/(fasting glucose (mmol/L) – 3.5)
HOMA-IR = insulin fasting (μIU/mL) × fasting glucose (mmol/L)/22.5
Insulin measurements in unit pmol/L were divided by 6.00 to convert from pmol/L to μIU/mL.[14] HOMA biomarkers have been widely used as an alternative for estimating insulin resistance because the method is easily obtainable, less invasive, safe, and cheaper than the gold standard hyperinsulinemic-euglycemic glucose clamp test, whereas it also produces well-correlated results.[15]
Data collection instruments
Anthropometry, clinical, and laboratory variables, i.e., weight, waist circumference, blood pressure, FPG, HbA1c, LDL-C, HDL-C, TG, and insulin, were gathered as primary data to represent clinical and laboratory disease control. Meanwhile, information on the patients’ characterization, disease-related data, and forms of treatment were obtained from their medical records and self-reports.
Data analysis
All the data were documented in a specific database and subsequently analyzed using the Statistical Package for the Social Sciences (SPSS) version 27.0 (SPSS Inc., Chicago, Illinois, USA) to calculate frequency, mean, and standard deviation.
The Chi-squared test was used to compare the participants’ categorical variables between groups, and a general normality test was performed to check the sample population distribution. Independent T-tests and paired T-tests were used to find the mean differences between groups and between baseline and week 12, respectively. The independent effects of the intervention on the FPG, insulin, and diastolic blood pressure outcomes were determined using analysis of covariance (ANCOVA). In all the analyses, the inferential statistical test with P < 0.05 was considered statistically significant. Missing data were handled by deleting the rows or columns with null values.
Results
Characteristics of the study sample
Initially, 200 patients with a medical diagnosis of T2DM were involved in the screening, but after eligibility checking, 50 participants were enlisted, leaving 25 patients in each of the two groups. Patients were excluded for the reasons summarized in Figure 1. Five patients withdrew from the study for several reasons. Finally, 45 patients – 24 in the intervention group and 21 in the control group – successfully completed the intervention in this study.
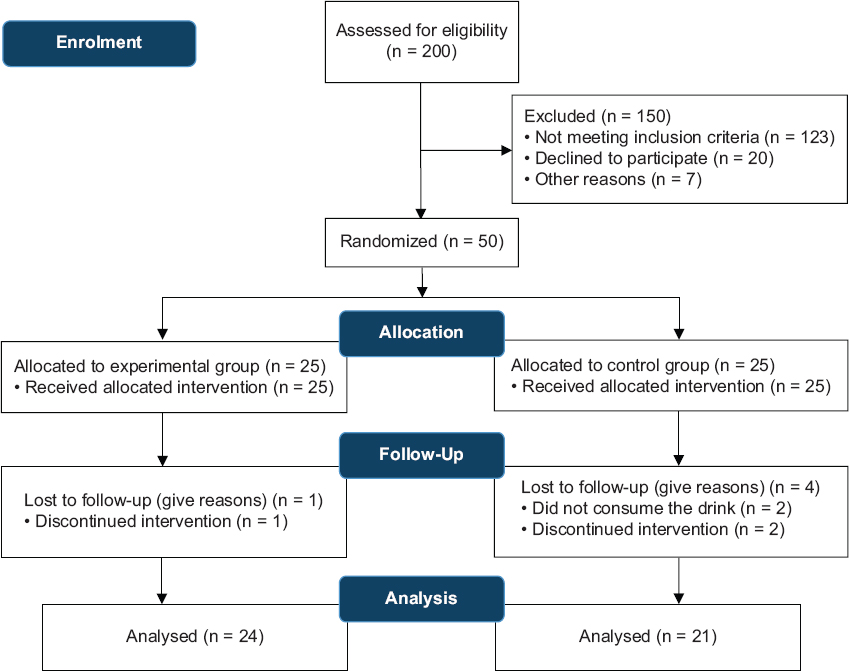
- Flowchart of selection and follow-up of the participants
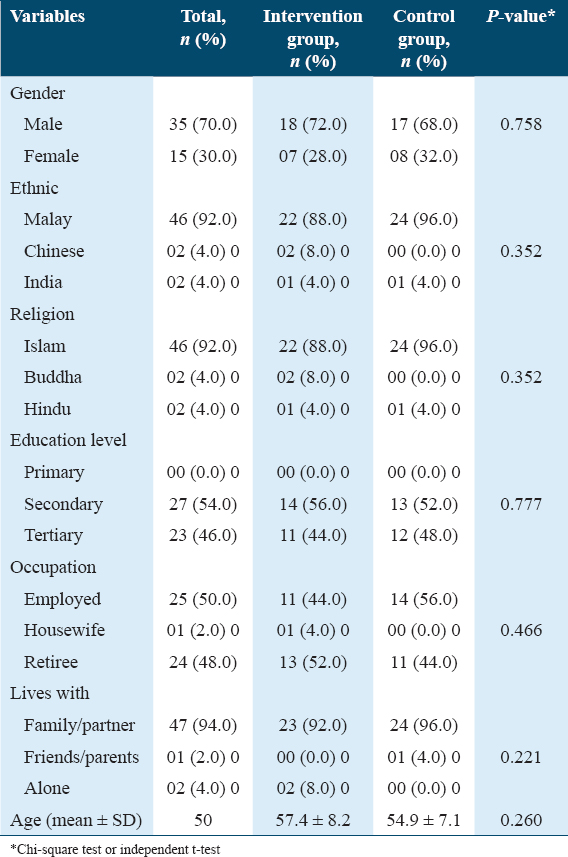
The majority of the participants consisted of men (70%) and those of Malay ethnicity (92%). The majority were married or living in a stable union (94%). The mean age was 56.2 (standard deviation [SD] ± 7.7) years. There was no statistically significant difference between the groups [Table 1], thus showing a homogeneity between the intervention and control groups for all the variables evaluated.
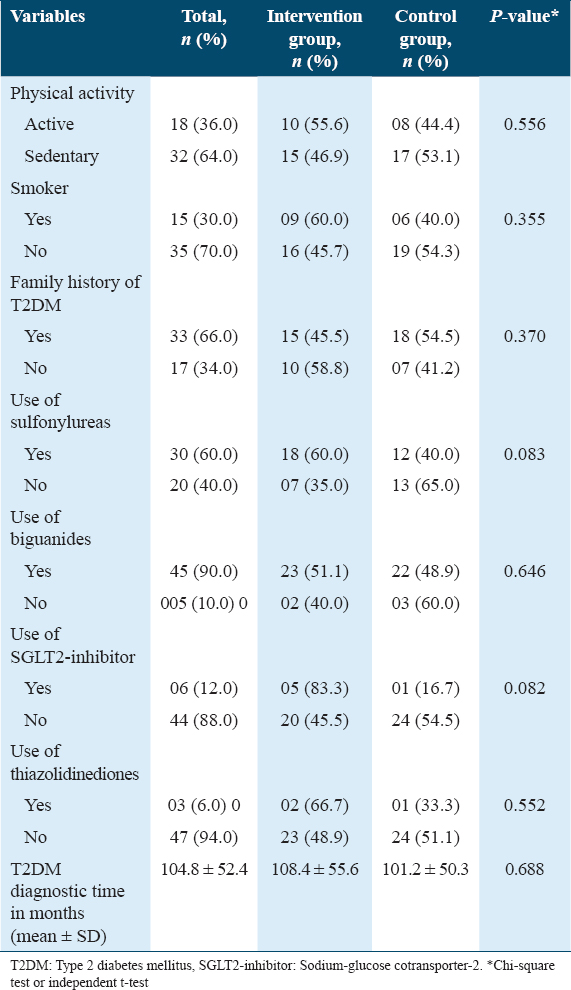
Most participants were self-declared as sedentary (64%). Smoking was reported by 30% of the participants; 96% of the T2DM patients were overweight or obese, and 66% reported a family history of T2DM. Most patients were taking sulfonylureas (60%) or biguanides (90%). Finally, the mean time of diagnosis was 104.8 (SD ± 52.4) months. The data are summarized in Table 2.
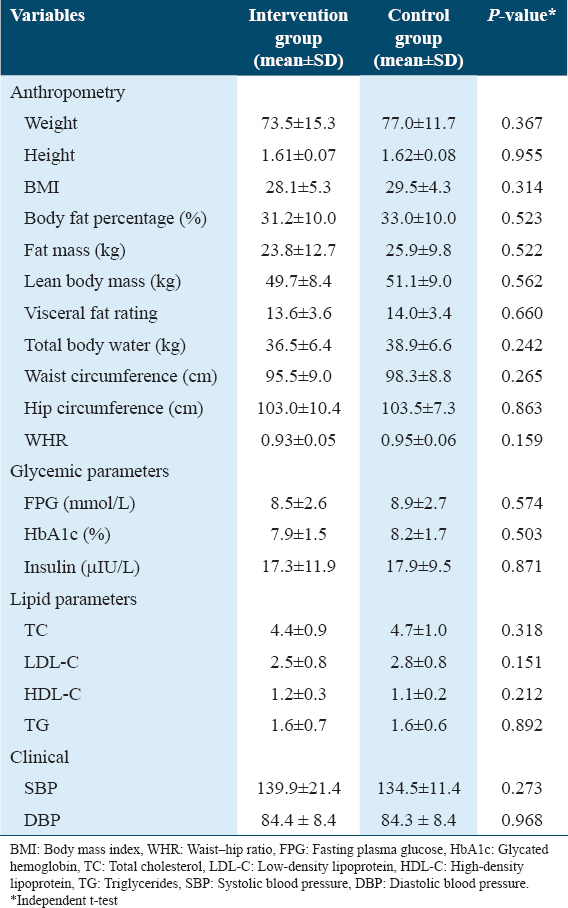
The mean and standard deviation of the main anthropometric, biochemical, and clinical variables at baseline were homogenous for the intervention and control groups for all the variables evaluated, as shown in Table 3.
Anthropometric measurements
The mean BMI of those in the intervention and control groups at baseline were 28.3 ± 5.3 and 29.6 ± 4.5 kg/m2, respectively [Table 4].
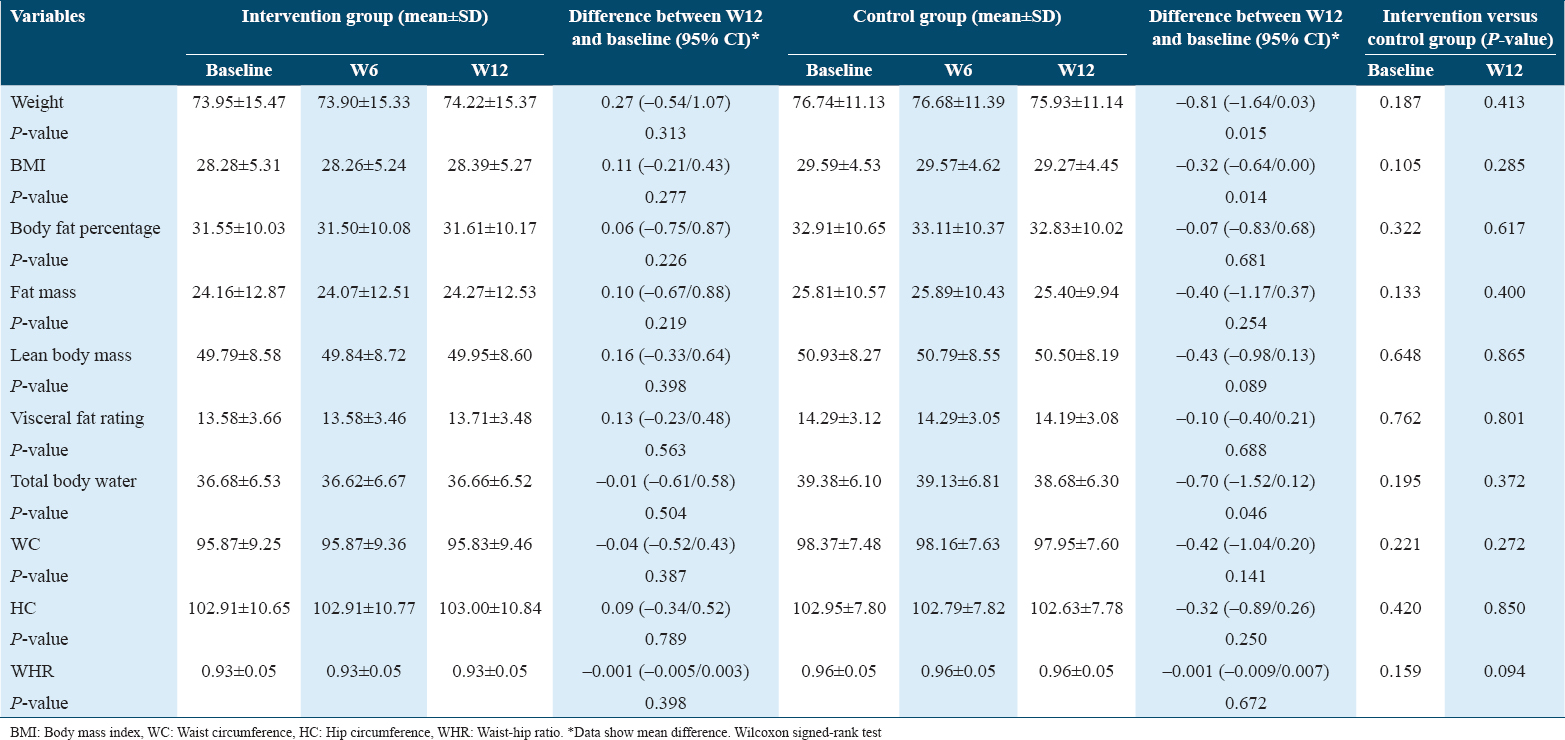
At week 12, the participants in the intervention group had experienced an average weight gain of 4.6%. However, no significant changes were recorded in fat mass or total body water. Conversely, the intervention with the powdered drink containing B. hispida led to an increase in lean body mass. On the other hand, participants in the control group experienced significantly decreased weight (P = 0.015) and total body water (P = 0.046). There was also a reduction in lean body mass within the control group. Decreased waist circumference was also identified in both groups, although this did not reach statistical significance.
Glycemic control
The intervention group showed a greater reduction in FPG between week 12 and baseline compared to the control group (Δ −0.8 mmol/L, 95% CI: −1.8, 0.2 vs. Δ −0.4 mmol/L, 95% CI: −1.2, 0.4, respectively). There were no significant changes in mean HbA1c between week 12 and baseline in both the intervention and control groups. Mean insulin in the intervention group had decreased from 17.3 to 16.0 μIU/mL at baseline and week 12, respectively.
Lipid profile
At baseline, the mean TG was 1.6 mmol/L, whereas it was 1.5 mmol/L (P = 0.390) at week 12; i.e., there was a reduction in TG levels of almost 4%. However, no changes in mean TG within the control group (P = 0.932) were detected after 12 weeks of intervention. Mean HDL-C was consistent in the intervention group yet reduced in the control group when compared to baseline.
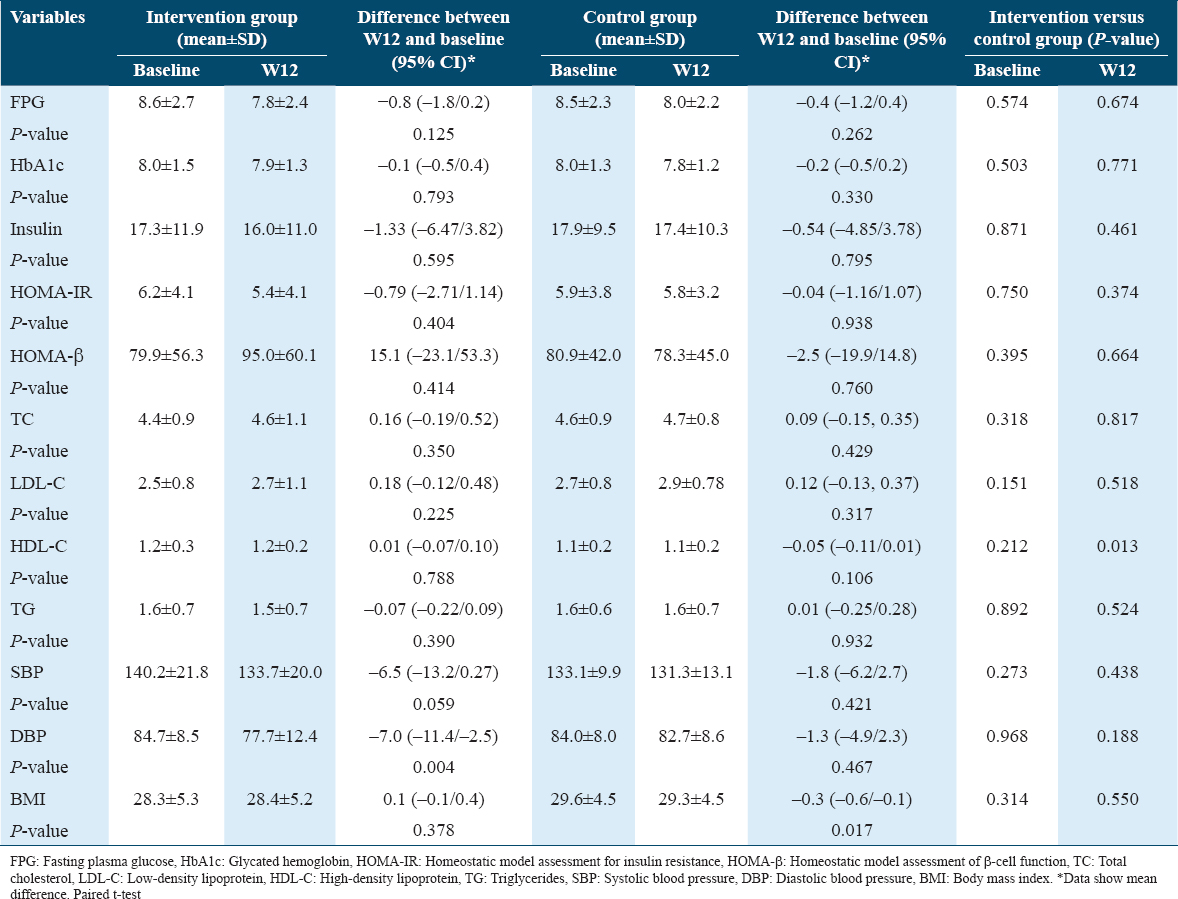
Table 5 shows the analysis of the efficacy of B. hispida extract at baseline and after 12 weeks of intervention. The results show that all the values, with the exceptions of HOMA-β, HDL-C, TC, and BMI, reduced, with the reduction being statistically significant for diastolic blood pressure (P = 0.004) in the intervention group. The intergroup analysis revealed that the FPG, insulin, HOMA-IR, and TG values had been reduced by more in the intervention group than in the control group. In addition, the reduction of FPG to 7.6 mmol/L at 6 weeks after the intervention began shows that more than 1 month was enough to demonstrate the efficacy of the B. hispida extract supplement in terms of controlling some variables in patients with T2DM.
Statistical analysis
In the model effect test, based on the general linear model, we observed no influence on the co-variables (gender, physical activity, smoking, age, weight, and T2DM time diagnosis) in relation to the outcome FPG (P > 0.05). Furthermore, we also found no significant influence on the co-variables (T2DM time diagnosis, use of sulfonylureas, age, and weight) in relation to the outcome insulin (P > 0.05).
The ANCOVA test revealed that the effect of the treatment based on B. hispida extract on the diastolic blood pressure variable remained significant (P = 0.004), even after controlling for the effects of the age (P = 0.189), physical activity (P = 0.173), smoking (P = 0.136), and weight (P = 0.209) variables.
Discussion
Our study showed a reduction in mean FPG from 8.6 mmol/L at baseline to 7.8 mmol/L (P = 0.125) after the intervention with a powdered drink consisting of B. hispida aqueous extract for a period of approximately 3 months. A greater reduction in mean FPG was seen in the intervention group compared to the control group at week 6. Corroborating these data, a previous in vivo study by Fatariah et al.[12] also proved the efficacy of B. hispida on blood glucose levels as early as 8 weeks after the supplementation was initiated. This effect of lowering the FPG could be attributed to the presence of phenolic compounds. Three important phenolic compounds in B. hispida – astilbin, catechin, and naringenin – were detected using high-speed counter-current chromatography (HSCCC), as documented by Du et al.[16] In an insulin-resistant state like T2DM, naringenin could modulate AMPK activation to promote glucose uptake in peripheral tissues, regardless of insulin stimulation.[17] AMPK signaling has become a current therapeutic target in the treatment of T2DM.[18]
Changes in the mean HOMA-IR index from 6.2 to 5.4 (P = 0.404) were observed in the intervention group in this study, indicating improved insulin resistance. Moreover, a mean HOMA-β augmentation was identified in the intervention group but not in the control group. This may have been due to the antioxidative activity of B. hispida aqueous extract.[19] In diabetes, oxidative stress resulting from the accumulation of reactive oxygen species can jeopardize insulin secretion, insulin sensitivity, and β-cell function through multiple signaling pathways. Dietary antioxidants can ameliorate diabetic status by regulating glucose metabolism, rectifying insulin secretion, and minimizing insulin resistance.[20]
Mean insulin decreased in both the intervention and control groups in line with the FPG reduction. The mean difference in insulin reduction was greater in the intervention group (−1.33 μIU/mL) than in the control group (−0.54 μIU/mL). Insulin is secreted by the pancreatic islet β-cells primarily in response to circulating glucose, whereas other nutrients such as amino acids and free fatty acids can augment the secretion of insulin.[21,22] In T2DM, insulin resistance occurs causing a large amount of insulin to be secreted by the pancreas to maintain the blood glucose levels at the normal range.[23] Patients with pathological obesity or T2DM who are insulin resistant have shown a two-fold increase in plasma insulin levels, indicating that the hallmark of insulin resistance is hyperinsulinemia.[24] Therefore, the reduction in insulin level suggested improved insulin resistance, as evidenced by the decreased HOMA-IR. This was unlikely to have been due to the destruction of β-cells because the HOMA-β index increased, as observed in this study. A substantial amount of gallic acid is found in B. hispida aqueous extract.[13] Gallic acid and its derivates can act as strong antioxidants and free radical scavengers, and they have the potential to modulate inflammation, apoptosis, or oxidative stress in various pathophysiological conditions. Regulation of caspase-9-related cellular apoptosis by gallic acid improves the function of the β-cells, whereas modulation of the Akt and AMPK signaling pathways by gallic acid increases insulin sensitivity.[25] AMPK signaling and its phosphorylation promote GLUT4 expression, thus enhancing glucose uptake by the muscle cells to reduce blood glucose levels.[18]
Baseline laboratory tests showed that the mean TC, LDL-C, HDL-C, and TG values were within the desirable targets in both groups, despite some participants presenting with dyslipidemia as comorbidity. Lipid abnormality manifested in 60–70% of T2DM patients.[26] At week 12, only TG in the intervention group showed a decrease of almost 4%, whereas no statistically significant changes were noted for the other lipid variables. A decrease in serum TG may be attributed to phytosterol in B. hispida, for example, β-sitosterol.[27] An earlier study demonstrated decreased serum TG with 8 weeks of β-sitosterol supplementation.[28] Phytosterol is believed to reduce TG through the alteration of intestinal fat metabolism by stimulating lipoprotein lipase activity and increasing fecal fatty acid excretion.[29] Moreover, the reduction in hepatic and plasma TG concentrations by phytosterol is also due to decreased mRNA expression of microsomal triglyceride transfer protein (MTP), a group of proteins essential in chylomicron and VLDL secretion in the intestine and liver, respectively. Downregulation of MTP caused physical interference to intestinal absorption and reduced chylomicrons incorporation, thus enhancing fecal fat loss.[30]
Glucose and lipid metabolism are interrelated in multiple ways. Diabetic dyslipidemia, for instance, is a clinical manifestation of this interaction. Insulin resistance in T2DM affects not only glycemia but also serum lipids. As observed in this study, the linear correlation between HbA1c and TG suggested a relationship between glycemia and serum lipids. A higher level of HbA1c is likely to represent higher TG.[5] Diabetes-related lipid changes occur because insulin resistance causes increased free fatty acid flux.[31] Besides insulin resistance, the relative insulin deficiency in patients with T2DM also leads to increased free fatty acid levels as well as hepatic VLDL-TG secretion.[32] Elevated concentrations of plasma triglycerides, low concentrations of HDL-C, and high concentrations of small dense LDL-C particles are attributes of diabetic dyslipidemia.[33,34] For this reason, it is paramount to focus on reducing glucose and lipid blood parameters in patients with T2DM after the initiation of an intervention.
The current study also found a reduction in blood pressure, with a statistically significant difference recorded in mean diastolic blood pressure after the B. hispida intervention. The mean differences in systolic and diastolic blood pressure were greater in the intervention group in comparison with the control group. A parallel finding was reported by Nakashima et al.[35] after intravenous injection of B. hispida juice, as a result of endothelium-dependent vasodilation due to nitric oxide (NO) as the key relaxing factor. The presence of amino acids such as aspartic acid, glycine, and malic acid in B. hispida serves as a precursor for the synthesis of NO, thus increasing the concentration of NO and decreasing the level of hydrogen peroxide, resulting in the attenuation of hypertension.[36] Optimal systolic and diastolic blood pressure control in T2DM patients can minimize the risk of cardiovascular and renal complications. This is vital as the risk of developing cardiovascular diseases in patients with T2DM was two- to four-fold higher than those without T2DM.[37]
We found a decreased lean body mass in the control group, in line with a reduction of total body water. According to Hirata et al.,[38] hyperglycemia and insufficient insulin action would suppress muscle cell growth and proliferation, which in turn would cause a decline in skeletal muscle mass. Furthermore, the oxidative stress that occurs in T2DM patients can also negatively affect body composition, particularly skeletal muscle, due to its involvement as an important organ in regulating glucose metabolism.[39] Meanwhile, the use of B. hispida in this study was not associated with decreased body weight or a lower BMI, although other studies have found a positive relationship between these aspects.[40,41] Body composition analysis revealed increased lean body mass with no changes in fat mass or total body water in the intervention group. This suggested that B. hispida has the potential to prevent the loss of muscle mass secondary to T2DM, perhaps by ameliorating glycemic control and insulin action. This relationship has not yet been highlighted in the preceding studies of B. hispida, but it could be analyzed in future studies.
Statistical analysis of measured metabolic parameters in our intervention study showed no significant differences in most of the outcomes with P > 0.05. Nonetheless, from a clinical point of view, the differences can be clinically relevant while statistically not significant. Clinically relevant results can contribute to a better quality of life in medical care for the targeted individuals by improving physical function, mental status, and ability to engage in social life.[42] The intervention with B. hispida among T2DM resulted in a reduction of mean FPG and improvement of insulin sensitivity which can help control and decrease the risk of complications of the disease that compromise quality of life.
Sociodemographic characteristics were balanced between the intervention and control groups [Table 1], showing that randomization has prevented selection bias; thus, the outcomes of the study were likely to be influenced by the intervention with B. hispida extract alone, rather than any other factors. Randomization has also been carried out to minimize the effect of confounders since they are sometimes inevitable.[43] In our study, participants’ dietary patterns and physical activities, for instance, could influence the outcomes. It is not possible to control these factors in human intervention studies with limited resources. Variations of dietary patterns and physical activities among participants and between days of a participant during the intervention period reflect real-world practices and may enhance generalizability when applied to a broader population.
The baseline metabolic control and body composition parameters of participants were not statistically significant between intervention and control groups. Yet, as there are differences in the values of baseline metabolic control and body composition parameters, the interaction between baseline parameters and efficacy of B. hispida extract may occur. Participants with good metabolic control at baseline may produce more desirable outcomes compared to those with poorer metabolic control at baseline.
One-way ANCOVA was employed to determine whether the effect of B. hispida extract on the mean difference in diastolic blood pressure remains statistically significant for differences in potential confounders, namely age, smoking status, physical activity, and body weight.[44-47] We applied ANCOVA with the assumption that the experimental treatment and the covariates are independent of each other and there is no interaction between them.
Consumption of B. hispida extract powdered drink in the long-term may produce greater efficacy than this 12-week study as more time is probably needed to alter the complex pathophysiology of T2DM. The current study indicates that B. hispida is safe for consumption for up to 12 weeks, but it should be monitored if consumed for more than 12 weeks. Adherence to supplementation of B. hispida extract for longer than 12 weeks is expected to be lower than the current study because dropout numbers will probably increase if the duration of the intervention is prolonged.
Dropout is a common issue in many intervention studies. This 12-week intervention study resulted in a dropout rate of 10% which is acceptable. Reasons for dropout in our study were discontinuation from the study due to a change in dose of OHAs during the intervention period (n = 3) and non-adherence to the intervention regimen (n = 2). Generally, a dropout rate >20% can compromise the internal validity of the study findings and reduce the statistical power.
Acceptable adherence rates were between 85% and 100% for optimal therapeutic efficacy.[48] The taste and palatability of the B. hispida aqueous extract powdered drink can have an impact on participants’ adherence. Low palatability may lead to poor adherence to a product. Therefore, improving the taste and palatability of newly developed intervention product samples is crucial in any study to ensure a higher adherence rate among participants as well as targeted populations.
Our study had certain limitations. First, it involved a small sample size, so it was probably difficult to find statistically significant differences in the data. Another limitation was the subjective methods like self-reporting used to evaluate the participants’ adherence to the powdered drinks. Although using direct measures of adherence is more reliable, this is more expensive and may pose more burdens to participants. Despite these limiting factors, the trends for some variables, especially the glycemic parameters, were seen to decrease within the intervention group throughout the 12-week intervention, thus proving the potential efficacy of B. hispida in the metabolic control of T2DM. The results obtained also have important clinical implications since they highlight new therapeutic possibilities in facing the challenges of a high prevalence of T2DM.
In individuals with T2DM, dietary and pharmacotherapeutic interventions work synergistically to produce beneficial effects.[49] In addition, a personalized approach in the management of T2DM can cut the cost and prevent failure associated with the algorithmic “one-size-fits-all” approach, resulting in optimum response to diabetes pharmacotherapy, thus lowering the incidence of complications associated with diabetes.[50] Diabetes education including dietary management is necessary in patients with T2DM, through health-care providers and health facilities to help them comprehend the disease management, for more appropriate self-care and better quality of life.[51]
Conclusion
T2DM patients using a powdered drink supplemented with B. hispida aqueous extract 2.5 grams once daily for 12 weeks showed a reduction in FPG, insulin, HOMA-IR, and both systolic and diastolic blood pressure compared to the control group when followed up in an outpatient clinic. B. hispida might be an attractive candidate for the development of functional food products. However, future research on mechanistic investigations of an identified active compound in B. hispida aqueous extract beneficial for the management of T2DM and studies involving participants from different centers across more diverse populations over a longer period of intervention may be needed to confirm findings from the present study.
Ethics Approval and Consent to Participate
The study was conducted in accordance with the guidelines outlined in the Declaration of Helsinki, and all the procedures involving human participants were approved by the Human Research Ethics Committee of Universiti Sains Malaysia (protocol code USM/JEPeM/20090466 and date of approval September 13, 2021). The study protocol was explained in detail, and the participants voluntarily gave informed consent before participation. All the participants signed the consent form before being recruited for the study.
Consent for Publication
Patients signed informed consent regarding publishing their data.
Availability of Data and Material
Data available on request from the authors.
Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding Statement
This work was funded by the Ministry of Higher Education (MOHE) Malaysia under Fundamental Research Grant Scheme.
Authors’ Contributions
CAJCMZ, WRWI, NAKK, and WMIWM contributed to the conception and design of the study. CAJCMZ acquired, analyzed, and interpreted the data under the supervision of WRWI, NAKK, and WMIWM.CAJCMZ drafted the article. WRWI, NAKK, and WMIWM revised the article critically for important intellectual content.
Acknowledgments
The authors would like to acknowledge Universiti Sains Malaysia, and the funding was provided by the Ministry of Higher Education Malaysia (FRGS/1/2019/STG05/USM/01/4 and FRGS/1/2021/SKK06/USM/02/18).
References
- 2021. Diabetes Atlas. (10th ed). International Diabetes Federation; Available from: https://diabetesatlas.org
- 2020. The Top 10 Causes of Death. Available from: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death
- Effectiveness of the piperine-supplemented Curcuma longa L in metabolic control of patients with type 2 diabetes:A randomised double-blind placebo-controlled clinical trial. Int J Food Sci Nutr. 2021;72:968-77.
- [Google Scholar]
- Functional foods-based diet as a novel dietary approach for management of type 2 diabetes and its complications:A review. World J Diabetes. 2014;5:267-81.
- [Google Scholar]
- The hypoglycemic potential of phenolics from functional foods and their mechanisms. Food Sci Hum Wellness. 2023;12:986-1007.
- [Google Scholar]
- Kundur Benincasa hispida (Thunb.), Cogn.:A potential source for valuable nutrients and functional foods. Food Res Int. 2011;44:2368-76.
- [Google Scholar]
- A literature-based update on Benincasa hispida (Thunb.), Cogn.:Traditional uses, nutraceutical, and phytopharmacological profiles. Oxid Med Cell Longev. 2021;2021:6349041.
- [Google Scholar]
- Acute and sub-chronic toxicity studies of Benincasa hispida (Thunb.), cogniaux fruit extract in rodents. Regul Toxicol Pharmacol. 2020;118:104785.
- [Google Scholar]
- Chemical composition and bioactive potential of dehydrated peels of Benincasa hispida, Luffa acutangula, and Sechium edule . J Herbs Spices Med Plants. 2015;21:193-202.
- [Google Scholar]
- Hypoglycaemic and protective effects of Benincasa hispida aqueous extract in streptozotocin-induced diabetic rats. Sains Malays. 2022;51:783-93.
- [Google Scholar]
- Quantitative HPLC analysis of gallic acid in Benincasa hispida prepared with different extraction techniques. Sains Malays. 2014;43:1181-7.
- [Google Scholar]
- Insulin units and conversion factors:A story of truth, boots, and faster half-truths. J Diabetes Sci Technol. 2019;13:597-600.
- [Google Scholar]
- Association between physical activity and insulin resistance using the homeostatic model assessment for insulin resistance independent of waist circumference. Sci Rep. 2022;12:6002.
- [Google Scholar]
- Isolation and identification of phenolic compounds in the fruit of Benincasa hispida by HSCCC. J Liq Chromatogr Relat Technol. 2005;28:137-44.
- [Google Scholar]
- The effects of naringenin and naringin on the glucose uptake and AMPK phosphorylation in high glucose treated HepG2 cells. J Vet Sci. 2021;22:e92.
- [Google Scholar]
- AMPK signaling in diabetes mellitus, insulin resistance and diabetic complications:A pre-clinical and clinical investigation. Biomed Pharmacother. 2022;146:112563.
- [Google Scholar]
- Effect of different solvent extracts of Benincasa hispida T. on experimental hypochlorhydria in rat. J Adv Pharm Technol Res. 2012;3:41-6.
- [Google Scholar]
- The role of dietary antioxidants in type 2 diabetes and neurodegenerative disorders:An assessment of the benefit profile. Heliyon. 2023;9:e12698.
- [Google Scholar]
- Regulation of insulin synthesis and secretion and pancreatic beta-cell dysfunction in diabetes. Curr Diabetes Rev. 2013;9:25-53.
- [Google Scholar]
- The association between physical activity and insulin level under different levels of lipid indices and serum uric acid. Front Physiol. 2022;13:809669.
- [Google Scholar]
- Hyperinsulinemia induces insulin resistance and immune suppression via Ptpn6/Shp1 in zebrafish. J Endocrinol. 2014;222:229-41.
- [Google Scholar]
- Gallic acid and diabetes mellitus:Its association with oxidative stress. Molecules. 2021;26:7115.
- [Google Scholar]
- Interaction between glucose and lipid metabolism:More than diabetic dyslipidemia. Diabetes Metab J. 2015;39:353-62.
- [Google Scholar]
- Neuroprotective effect of petroleum ether, methanolic and aqueous extracts of fruits of Benincasa hispida on lipofuscinogenesis and fluorescence product in brain of D-galactose induced aging accelerated mice. Indian J Pharm Educ Res. 2021;55:528-34.
- [Google Scholar]
- b-sitosterol attenuates high- fat diet-induced hepatic steatosis in rats by modulating lipid metabolism, inflammation and ER stress pathway. BMC Pharmacol Toxicol. 2023;24:31.
- [Google Scholar]
- Triglyceride-lowering response to plant sterol and stanol consumption. J AOAC Int. 2015;98:707-15.
- [Google Scholar]
- The lipid-lowering effects and associated mechanisms of dietary phytosterol supplementation. Curr Pharm Des. 2017;23:5077-85.
- [Google Scholar]
- Nitric oxide-dependent hypotensive effects of wax gourd juice. J Ethnopharmacol. 2011;138:404-7.
- [Google Scholar]
- Benincasa hispida extracts positively regulated high salt-induced hypertension in Dahl salt-sensitive rats:Impact on biochemical profile and metabolic patterns. J Food Biochem. 2022;46:e14497.
- [Google Scholar]
- Blood pressure control and impact on cardiovascular events in patients with type 2 diabetes mellitus:A critical analysis of the literature. Clin Investig Arterioscler. 2019;31:31-47.
- [Google Scholar]
- Hyperglycemia induces skeletal muscle atrophy via a WWP1/KLF15 axis. JCI Insight. 2019;4:e124952.
- [Google Scholar]
- Low muscle mass is associated with poorer glycemic control and higher oxidative stress in older patients with type 2 diabetes. Nutrients. 2023;15:3167.
- [Google Scholar]
- Possible anorectic effect of methanol extract of Benincasa hispida (Thunb), Cogn, fruit. Indian J Pharmacol. 2004;36:348-50.
- [Google Scholar]
- Antiobesity effect of Benincasa hispida fruit extract in high fat diet fed wistar albino rats. Int J Pharm Clin Res. 2016;8:1590-9.
- [Google Scholar]
- Statistical significance or clinical significance?A researcher's dilemma for appropriate interpretation of research results. Saudi J Anaesth. 2021;15:431-4.
- [Google Scholar]
- A simplified guide to randomized controlled trials. Acta Obstet Gynecol Scand. 2018;97:380-7.
- [Google Scholar]
- Effect of leisure-time physical activity on blood pressure in people with hypertension:A systematic review and meta-analysis. Sci Rep. 2023;13:10639.
- [Google Scholar]
- Age-related changes in the risk of high blood pressure. Front Cardiovasc Med. 2022;9:939103.
- [Google Scholar]
- Effects of changes in smoking status on blood pressure among adult males and females in Indonesia:A 15-year population-based cohort study. BMJ Open. 2020;10:e038021.
- [Google Scholar]
- Effect of weight loss on blood pressure changes in overweight patients:A systematic review and meta-analysis. J Clin Hypertens. 2023;25:404-15.
- [Google Scholar]
- Effect of multi-strain probiotics (multi-strain microbial cell preparation) on glycemic control and other diabetes-related outcomes in people with type 2 diabetes:A randomized controlled trial. Eur J Nutr. 2017;56:1535-50.
- [Google Scholar]
- Efficacy of dietary and supplementation interventions for individuals with type 2 diabetes. Nutrients. 2021;13:2378.
- [Google Scholar]
- Personalized type 2 diabetes management:An update on recent advances and recommendations. Diabetes Metab Syndr Obes. 2022;15:281-95.
- [Google Scholar]
- Effect of diet on type 2 diabetes mellitus:A review. Int J Health Sci (Qassim). 2017;11:65-7.
- [Google Scholar]







