Translate this page into:
Evaluating dasatinib nanocarrier: Physicochemical properties and cytotoxicity activity on cancer cells
Address for correspondence: Alaa S. Tulbah, Department of Pharmaceutical Sciences, College of Pharmacy, Umm Al-Qura University, Makkah, Saudi Arabia. E-mail: astulbah@uqu.edu.sa
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Qassim Uninversity and was migrated to Scientific Scholar after the change of Publisher.
Abstract
ABSTRACT
Objective:
Dasatinib-(DAS) is a tyrosine kinase inhibitor usually used to treat leukemia. However, DAS is a poorly water-soluble drug. Therefore, oil-in-water emulsions were used for DAS to enhance its solubility and cancer treatment efficacy. This study aims to develop an appropriate DAS nanoemulsion (NE) that can overcome the issue of DAS solubility and provide an effective anticancer effect.
Methods:
Spherical particles dispersed in an aqueous media approach within an oily phase (oleic acid, Kolliphor RH40, and dipropylene glycol) were used to formulate DAS-NE using high-energy methods. Different formulas were developed and an appropriate formula was analyzed to identify its physicochemical properties. Raw DAS and nonformula cytotoxicity were evaluated through MTT assay against three cancer cell lines, MCF7 (human breast adenocarcinoma), HT29, and SW480 (human colorectal carcinomas), in addition to MRC5 (Normal human fetal lung fibroblast).
Results:
Different DAS-NEs (1–7) have been developed successfully. Formulas had a droplet size of a diameter ranging from 84.167 ± 10.178 nm to 273.433 ± 45.267 nm. The drug content of the appropriate formula (DAS-NE3) was found to be 83.2%. The drug release result of DAS-NE3 when compared to raw DAS was about 58%, falling to 13% after 24 h. The DAS-NE3 showed cytotoxicity against the three cancer cells below 26.11 μM but showed 30-fold significantly increased selectivity against MRC5 normal cells compared to that of raw DAS.
Conclusion:
This study shows that the DAS-NE3 formula may provide a potentially effective and sustained drug delivery for cancer treatment. This provides valuable information to the scientific community and the pharmaceutical industry.
Keywords
Cytotoxicity
dasatinib nanocarrier
drug release
entrapment
selectivity
Introduction
The World Health Organization states that cancer is one of the most widespread causes of death around the globe.[1] In 2022, it was estimated that there would be 1.9 million new cancer cases in the United States, and 609,360 cancer-related fatalities, or 1,670 deaths each day. In Saudi Arabia (total population = 33,554,333), there were 10,518 cancer deaths and 24,485 new cancer cases in 2018.[2] An increase in cancer cases is expected over the years. Typically, cancer is viewed as a collection of tumor cells and is considered a global disease.[1] A thorough comprehension of complicated occurrences is essential for developing accurate and effective treatment regimens.[1] Cancer is treated with a combination of chemotherapy, radiation therapy, and surgery.[3] Unfortunately, these approaches are not selective, which means that they might harm good tissue as well as tumors, resulting in harmful side effects.[1,4] Moreover, most chemotherapeutics that are offered on the market are administered orally or intravenously.[5] Improving chemotherapy outcomes using nanotechnology in the design of drug delivery systems, particularly those for therapeutic administration to the cancer cell, could help prevent severe adverse effects.[6,7]
Liposomes, Dendrimers, carbon nanotubes, nanoparticles, polymeric or lipid micelles, and nanoemulsions (NE) are widely used in drug development[7] They can be between 1 nm and 1000 nm in diameter.[6,7] There are several advantages to using nanotechnology, compared to conventional chemotherapeutic agents, including the ability to encapsulate hydrophobic molecules, increase their solubility/biocompatibility and retention time in tumoral leaky vessels, as well as the ability to conjugate targeting ligands for diagnostic and therapeutic purposes, enhancing intracellular penetration and specificity, and enhancing the efficacy and selectivity of chemotaxis.[8]
A NE is a combination of emulsifiers and lipids that significantly improves the absorption of an active pharmaceutical ingredient (API), which is influenced by the carrier’s solubility. A NE is an isotopically transparent dispersion of two immiscible liquids, such as dispersed phase (oils) and continuous phase (water), stabilized by an interfacial coating of surfactant molecules with stable thermodynamic properties and droplet sizes ranging from 100 to 200 nm.[9] There are different methods to develop NE formulation based on their energy demands including high- and low-energy methods.[10] These methods have been an effective drug delivery technique, improving the solubility and bioavailability of many poorly soluble drugs.[11] For example, a study by Tulbah et al., found that eucalyptol nanoemulsion formulation had greater efficacy and cytotoxicity activity.[12] Another study found that the high-energy ultrasonication method of preparing piperine NEs using oleic acid, Tween 80, and Cremophore EL had the ability to improve the formulation in comparison to the conventional treatment.[13] In addition, the NE formulation of thyme[14] or Origanum majorana[15] has been found to improve the anticancer activities of essential oils.[15]
Dasatinib (DAS) is an oral, once-daily tyrosine kinase inhibitor used to treat chronic myeloid leukemia (CML) and acute lymphoblastic leukemia with the Philadelphia chromosome.[16] It prevents the activation of the intracellular signal transduction pathway in tumor cells by blocking the ATP binding site and preventing the autophosphorylation of tyrosine residues on several proto-oncogenes.[17] DAS is also a first-line treatment for chronic myelogenous leukemia (CML) that inhibits BCR/ABL activity 300 times more effectively than imatinib.[17] Recently, the drug has been found to reduce cancer cell proliferation and migration, as well as invasion and mortality.[18] Previous studies found that DAS had a potential for treatment of prostate, breast, liver, and colon cancers.[19,20] It has been approved for clinical use by the Food and Drug Administration (FDA); SPRYCEL® (Bristol–Myers Squibb-BMS) and YINISHU® (CHIA TAI TIANQING) were approved by the FDA in 2006 and 2013, respectively.[21] However, DAS is a biopharmaceutical class II medication with high permeability and low solubility.[21] DAS has limited solubility in the small intestine, a significant first-pass effect, and low bioavailability in mammals at only 14–34%.[18,21] Many side effects are caused, such as bone marrow suppression, diarrhea, dermatitis, and pleural/pericardial effusions, and it needs to be corrected for heart rate (QT) prolongation.[18] DAS also demonstrated pH sensitivity, and short half-life (3–4 h).[18] Using a nanocarrier to deal with these side effects could help to develop a novel, safe, and effective therapeutic drug dispersion, improve drug performance, and reduce unwanted responses.[22]
The goal of this study is to create a novel and ideal DAS NE that can overcome the shortcomings of the drug’s solubility and provide a powerful anticancer effect.
Materials and Methods
Materials
Oleic acid was bought from Riedel-de Haën- Honeywell Research Chemicals; Germany), and DAS (purity >90%) was bought from Xian Lukee Bio-Tech Co., Ltd., XI’AN, China. Dipropylene glycol was bought from Fluka Chemie GmbH, Switzerland, and Kolliphor RH 40, Glycerol and deionized water (DW) obtained through Direct-Q® Water Purification System also was bought from Sigma-Aldrich (Germany).
Cell lines
MCF7 (human breast adenocarcinoma), HT29 and SW420 (human colorectal carcinomas), and MRC5 (Normal human fetal lung fibroblast) were obtained from the American Type Culture Collection, ATCC (Rockville, USA). The three cancer cells were sub-cultured in Roswell Park Memorial Institute -RPMI 1640 media (10% Fetal bovine serum [FBS]), while MRC5 was maintained in Eagle’s minimum essential medium (EMEM, 10% FBS); all at 37°C, 5% CO2, and 100% relative humidity, for a maximum of 5–10 passages.
Determination of maximum absorption of DAS
Different samples of DAS-NEs and raw drugs were scanned using a UV-VIS Spectrophotometer (Cary 60 UV-Vis; Aglient Technology) in the range of 200–800 nm, and the wavelength corresponded to maximum absorbance (λ max).[23,24] At 324 nm, the absorbance of the resultant solution was compared to a blank. The absorbance was plotted against the concentration of DAS to draw the calibration curve.
DAS-NE preparation
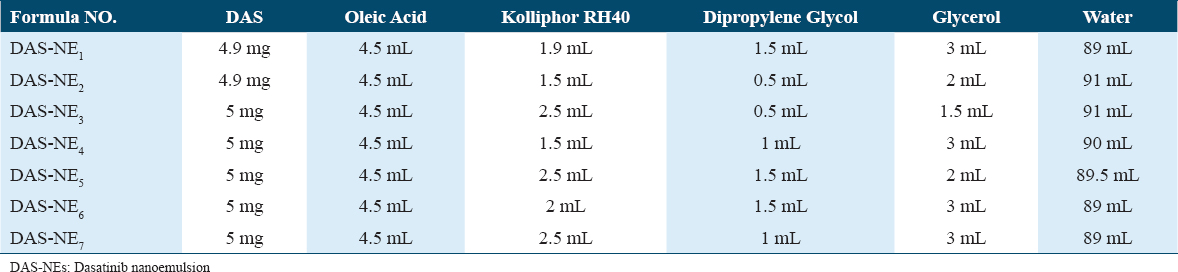
Serial formulations of DAS-NEs were prepared (DAS-NE1-7) to choose an appropriate one after evaluating the best oil/water (o/w) mixtures using the drug entrapment test and particle size distribution. A high-energy method was used to prepare the NE.[10] Different NEs composed of various amounts of DAS, oleic acid, Kolliphor RH40, dipropylene glycol, glycerol, and water were prepared,[25] as shown in Table 1. An aliquot of these NE formulations was used to directly dissolve DAS in oleic acid weighed at 40°C. After the sample was completely dissolved, RH 40 and dipropylene glycol were added to the mixture. Then, the oil phase was gradually mixed with the aqueous phase (glycerol and water) using an overhead stirrer (IKA@ RW 20 Digital, Nara, Japan) for 30 min at a speed of 300 rpm. After that, the emulsion was homogenized in a high-shear homogenizer at 10,000 rpm for 15–20 min. This was based on a literature review to prepare the formula with good flow, optimum size, and EE.[26] At room temperature, all the steps were performed, and a control formula was prepared, free of DAS. In the preparation of the NEs, oleic acid was used as the oil phase because of its significant ability to dissolve DAS (25°C, 26.66 ± 4.20 mg/mL)[21] RH 40 was selected because of the crucial function of amphiphilic surfactants in decreasing interfacial tension and promoting emulsion stability.[21] Dipropylene glycol can also function as a cosurfactant by adsorbing at the oil-water interface and further lowering the interfacial tension.[27]
Physicochemical characterization of prepared NE
Drug entrapment efficiency (EE) experiment
In the pharmaceutical sciences, drug EE is a metric that compares the total amount of drug added during the formulation process to the amount of drug that is successfully entrapped or encapsulated within a drug delivery system. It is a crucial metric since it shows how well the formulation holds the medication.[28] To assess this, DAS-NE samples were diluted in a Buffer of pH 3 and stirred for 30 min before being centrifuged at 5500 rpm for 10 min to determine the amount of drug entrapped in the formula.[29] UV-VIS spectroscopy was used to measure the absorbance of the supernatant at 324 nm, which corresponds to the maximum absorption wavelength of DAS. After centrifugation, a standard curve was created using DAS and nanoparticle supernatant, as described previously.[30]
Calculations were performed using the formula below:

Zeta potential, droplet size, and polydispersity index (PDI)
The parameters needed in the formulation required small particle size and good size distribution. Therefore, dynamic light scattering was used to determine the optimal DAS-loaded NE droplet size, PDI value, and zeta potential using the Zetasizer (Nano ZS, Malvern Instruments Ltd., UK). DW (1:100) was used to dilute the samples before injecting for analysis. The mean average (z-average) droplet size was calculated using the intensity distribution.[12]
In vitro drug release study
An in vitro drug release experiment is a laboratory experiment designed to replicate physiological settings and assess and comprehend the release behavior of a medication or API from a dosage form. The drug’s release kinetics, dissolution profile, and possible physiological effects are evaluated with the aid of this investigation.[31] For this purpose, using the dialysis bag diffusion technique, the diffusion of an appropriate DAS-NE3 and raw drug across a cellulose acetate membrane (cutoff molecular weight of 10,000 Da) was investigated, as described previously.[12] Overnight, cellulose membranes were immersed in the release media. The cellulose membrane was injected with three milliliters of the sample, and both ends of the bags were sealed. Afterward, the dialysis bags were immersed with care in beakers containing buffer solution (pH: 7.4). The mixture was utilized to simulate the physiology of the body fluid. The elution medium was mixed at 100 rpm using a magnetic bar. To maintain the sink conditions, three milliliters of the release medium were taken at various time intervals from 15 min to 24 h and replaced with the same volume of fresh media. The amount of medication released was determined by analyzing these samples using a UV spectrophotometer at 324 nm.
Biological characterization of the NE
Cytotoxicity assay
An assessment of a drug’s toxicity on living cells is done in a lab setting using a cytotoxicity assay. It assesses how this drug affects the viability, morphology, and health of cells. When evaluating the safety of a substance on biological systems, this assay is crucial in the pharmaceutical industry, chemical testing, and biomedical research. MCF7 cells (human breast adenocarcinoma), HT29 (human colorectal carcinomas), SW420 (human colorectal carcinomas), and MRC5 (Normal human fetal lung fibroblast) lines were used in this study. A maximum of 5–10 passages was allowed for each of the three cancer cells in RPMI-1640 media (10% FBS), while MRC5 was kept in EMEM (10% FBS) at 37°C, 5% CO2, and 100% relative humidity.[32]
MTT assay was used to assess the cytotoxicity of compounds, as reported previously.[12,33,34] Each cell line was cultured separately in 96-well plates (3 × 103/well) and incubated with DAS-NE3 at a final concentration of 0–5 μM for 72 h at 37°C overnight (DMSO 0.1%; n = 3 of three separate experiments). MTT was added to each well at a concentration of 0.5 mg/mL and incubated for 3 h at 37°C. DMSO was used to dissolve the formazan granules after the MTT solution was removed. The absorbance was measured using a multi-plate reader (BIORAD, PR 4100, Hercules, CA, USA) at A550 optical density, which is proportional to the number of viable cells. GraphPad Prism was used to calculate the compound concentration that inhibited cell growth by 50% (IC50) compared to control cell growth (100%).
Statistical analysis
For every outcome, the mean ± standard deviation (S.D.) is expressed for at least three distinct determinants. The results of “Normalize” and “Transform” demonstrate a nonlinear fit, as demonstrated by the IC50 and selectivity index (SI) plot. The data value was determined to be the “best fit value” using the GraphPad Prism 9 program.
Results
DAS analytical technique
Determining the maximum absorption
Different DAS-NEs and raw DAS were analyzed successfully using a UV-VIS spectrophotometer over the wavelength range of 200–400 nm, and the wavelength corresponding to the maximum absorbance (λ max) was noted. The absorbance of the sample was at its highest at a wavelength of 324 nm.
Physicochemical Characterization of prepared NE
Droplet size, PDI, and zeta potential
All formulations of DAS-NEs were evaluated using a Zetasizer, and they demonstrated a small range of droplet size (diameter), ranging from 76.6 ± 3.8 nm to 427 ± 35.3 nm. Regarding the droplet size [Table 2], DAS-NE3 with the smallest particle size was engineered with an average size of 76.6 ± 3.8 nm and a narrow PI value of 0.241 ± 0.022. The PDI value serves to characterize the consistency of a particle distribution in an emulsion system. In addition, it was observed that the size of DAS-NE1 and DAS-NE6, prepared using 5 mg of DAS [Table 1], were 120 ± 9.3 nm and 141 ± 19.5 nm, respectively.
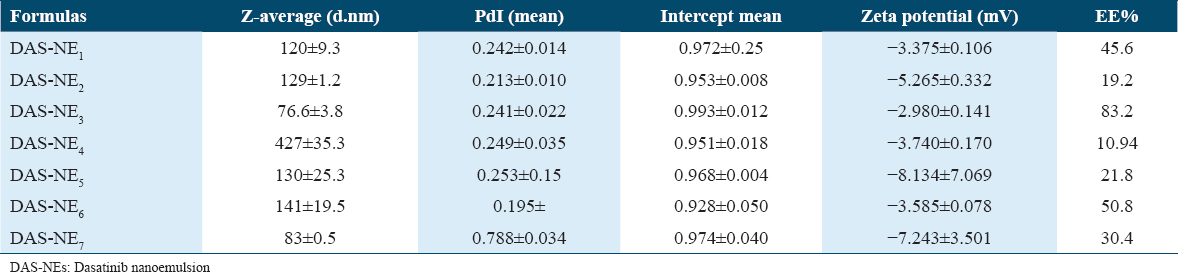
All designed formulations had a negative charge, and formulas with a charge, such as −3.375 ± 0.106, −2.99 ± 0.141, and −3.585 ± 0.078 mV, were DAS-NE1, DAS-NE3, and DAS-NE6, respectively [Figure 1 and Table 2].
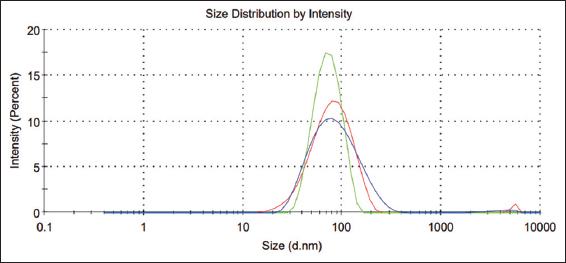
- The particle size distribution of the Dasatinib-nanoemulsion formula
Drug EE test
The EE of the DAS drug is a measure for quantifying the concentration of free drug in the medium containing the dispersion. To guarantee that the encapsulated drug remains contained within the droplet, it is necessary for the NE system to have a high EE. Due to the limited aqueous solubility and lipophilic properties of DAS, which contributed to its retention time in the disperse phase of the formulation.
The efficiency of DAS encapsulation was evaluated on all DAS-NE1-7 formulations. Table 2 shows the DAS concentrations (4.9 and 5 mg/mL) prepared in aliquots of these formulations to optimize the loading efficiency of DAS into the NEs [Table 1]. The encapsulation efficiencies for the engineered 120 ± 9.3, 76.6 ± 3.8, and 141 ± 19.5 DAS-NEs were 45% (DAS-NE1), 83% (DAS-NE3), and 50% (DAS-NE6), respectively.
In vitro drug release study
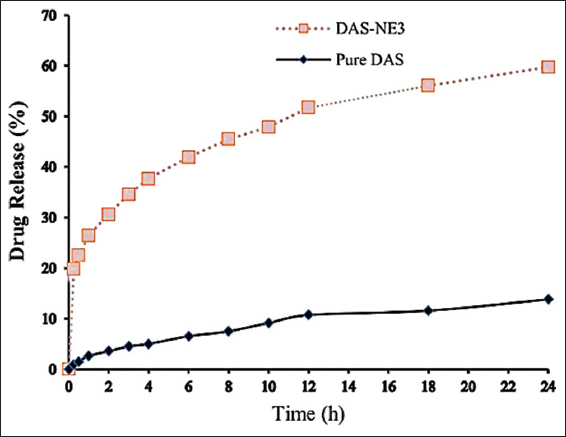
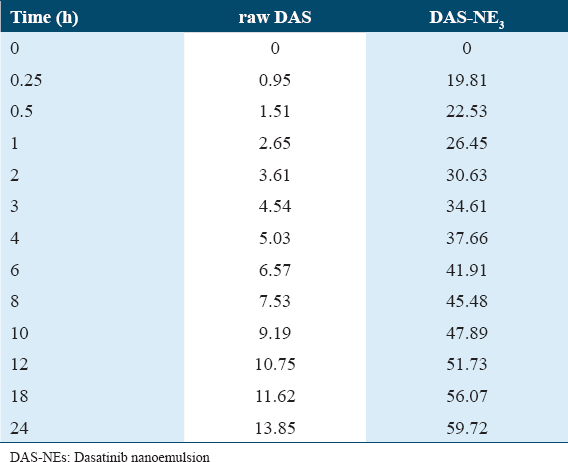
The results of an in vitro release study provide information regarding the efficiency of the drug delivery method that is being considered for use. It is useful to verify the distribution of the DAS in the NE system with the help of a drug release profile. Figure 2 and Table 3 both display the findings of the in vitro drug release research that was conducted in this study. The graph demonstrates that the release of the DAS-NE3 reached 59.72% after cumulative release for 24 h, whereas the dissolution of raw DAS was only 13.85%. The developed formulation confirmed DAS diffusion from the dialysis bag, with 41% and 51% of the drug released at the 6th and 12th h, respectively. In contrast, approximately 4% and 10% of the drug was released at the 6th and 12th h of the raw drug. Biological examination of DAS-NE3 formula.
-
In vitro drug release (%) of dasatinib (DAS) nanoemulsion formula and raw DAS
Cytotoxicity assay
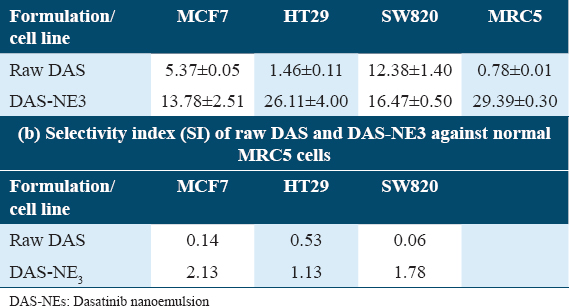
The determinations of the cytotoxic activity of raw DAS and DAS-NE3 against each of the three cancer cell lines (MCF7, HT29, and SW820) are shown in [Table 4a and Figure 3]. The IC50 of raw DAS ranges from 1.46 to 12.38 μM, with HT29 colon cancer cells being the most sensitive cell line. However, the cytotoxicity of raw DAS against MRC5 cells showed significantly low selectivity below 1 [SI: 0.06, Table 4a]. On the other hand, the cytotoxicity of DAS-NE3 against the same cancer cell lines ranges between 13.78 and 26.11 μM, with MCF7 being the most sensitive cell line.
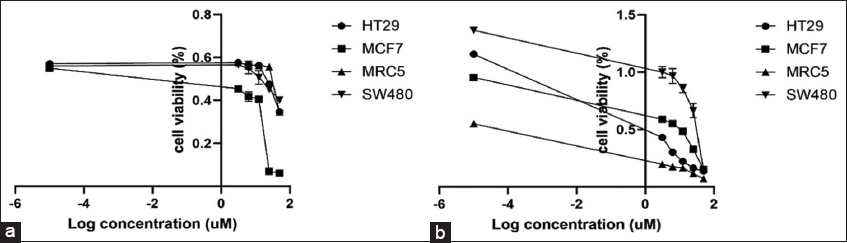
- Cytotoxicity of dasatinib (DAS) nanoemulsion formulation (a) and raw DAS (b) on three different cancer cell lines: MCF7, HT29, and SW420 in addition to MRC5 (normal fibroblast)
Discussion
DAS is a well-known anticancer compound, acting as a multiple tyrosine kinase inhibitor, but has poor water solubility.[35] These features have posed significant obstacles to the development of DAS as a cancer therapy agent. Several appropriate nanocarrier formulations have been created, including NEs, micelles, liposomes, and polymeric nanoparticles, because DAS is nearly insoluble in water. Before being administered, these formulations should ideally simultaneously solubilize. Since the surface energy of dispersed oil and water phases of NEs is often high, their separation is straightforward. In response, surfactants and co-surfactants have been used in the development of certain NE formulations. In this study, using a high-energy method,[26] we developed different DAS NEs (numbered from 1 to 7) that are soluble, readily dispersed in water, and demonstrate enhanced drug release, encapsulation efficiency, and cytotoxicity against cancer cells. To determine the maximum absorption of DAS-NEs and raw DAS, a UV-VIS spectrophotometer was used with a range of 200–400 nm wavelength. The absorbance of the samples was found to be at 324 nm compared to a blank. This absorbance was plotted against the concentration of DAS to create the calibration curve with an R2 value of 0.99970. The standard concentrations of DAS demonstrated good linearity.
As indicated in Table 1, several NEs were created using varying concentrations of DAS, oleic acid, Kolliphor RH40, dipropylene glycol, glycerol, and water. The developed formulas were successfully prepared and all steps were performed at room temperature to select an appropriate one in terms of drug entrapment and particle size distribution of the optimum o/w mixes of DAS-NE (DAS-NE1–7) formulations. Similar observations were reported wherein the NEs were prepared using oleic acid, Kolliphor RH40, and dipropylene glycol to get a nanosized droplet.[36,37] Interestingly, DAS-NE1–7 formulations demonstrated a good particle size distribution [Table 2]. We found that DAS-NE3 was the appropriate formula to carry on in this study, with the smallest engineered particle size of 76.6 ± 3.8 nm size. In addition, it had a narrow PDI value of 0.241 ± 0.022 and a negative charge of the zeta potential. The PDI value serves to characterize the consistency of a particle distribution in an emulsion system. When an optimized DAS-NEs PDI is small, this indicates a narrow droplet size distribution in the developed formula. In addition, the formula revealed a negative charge of the zeta potential values and the formation of monodisperse systems, similar results to those mentioned by Wang et al.[38] The zeta potential, according to Asmawati et al.[27] characterizes the electrostatic interactions between particles.[27] For electrostatically stabilized dispersions, the higher the zeta potential, the more stable the dispersion is expected to be. The lower the zeta potential, the less likely it is that flocculation will occur. In addition, NEs with zeta potentials greater or < ±30 mV have significantly increased stability.[27,39] In the drug delivery literature, guidelines defining NP-dispersions with ZP values of ± 0–10 mV, ±10–20 mV and ±20–30 mV, and > ±30 mV, described as very unstable, slightly stable, moderately stable, and highly stable, are frequent.[27,40]
In addition, besides the sample nanosize particle distribution of DAS-NE3, among other formulas the drug entrapment experiment was also used to determine the amount of free drug in the medium containing the dispersion to look at the DAS drug’s EE. High entrapment effectiveness of the NE system is required to ensure that the medicine encapsulated stays inside the droplet. DAS’s poor aqueous solubility and lipophilic characteristics lengthened its retention period during the formulation’s disperse phase: The DAS-NE3EE percentage was at 83%. This was the highest encapsulation efficiency obtained when the concentration of DAS used was 5 mg/m due to the optimization of the NE. The results of EE% inspection of DAS could also improve drug solubility in different concentrations of oil to optimize the efficiency of the NE. A similar finding was found and reported in another study, which demonstrated that NE formulation was capable of improving drug encapsulation with a high degree of effectiveness.[41,42] Therefore, based on the results of the EE and small particle size, the DAS-NE3 formula was chosen for further investigation in the project.
The release profile of encapsulated DAS in NE revealed that the release of the DAS-NE3 reached 59.72% after cumulative release for 24 h, whereas the dissolution of raw DAS was only 13.85%. This result could be due to the improved dissolution rate of the DAS-NE3 and its increased solubility caused by particle size reduction [Table 3].[43] Even though DAS is a type of BCS II medication that has strong permeability, it has poor water solubility, which makes it difficult for it to pass through the epithelial cells that line the small intestine.[21] This results in limited absorption. Because of its smaller size and greater solubility, the nanoformulation makes it feasible to improve cell permeability and, as a result, achieve greater bioavailability. The incorporation of surfactants and the production of extremely small oil-water droplets led to an increase in the NE’s permeability, which is a desirable property.[21,41]
The in vitro cytotoxicity of DAS-NE3 (about 70 nm) was tested to prove that the nanodroplets are safe on cells. According to published research, apoptosis, manifested as chromatin condensation and nuclei blebbing, is one of the toxicity indicators brought on by polymeric NPs.[44] Regarding the biological examination of the DAS-NE3 formula, [Table 4a and Figure 3] demonstrate our evaluation of the cytotoxic efficacy of raw DAS and DAS-NE3 against the three cancer cell lines (MCF7, HT29, and SW820). The most sensitive cell line is HT29 colon cancer cells, with an IC50 of 1.46–12.38 μM for raw DAS. On the other hand, raw DAS’s cytotoxicity against MRC5 cells demonstrated noticeably poor selectivity below 1 [SI: 0.06, Table 6]. The cytotoxicity of DAS-NE3 denotes 2.5–17-fold decreased activity compared to raw DAS. However, DAS-NE3 induced a SI over 1 [SI: 1.78, Table 4b], which is 30-fold better than the selectivity of raw DAS against MRC5 normal cells. Thus, it is concluded that DAS-NE3 showed cytotoxicity against the three cancer cells below 26.11 μM but showed 30-fold significantly increased selectivity against MRC5 normal cells compared to that of raw DAS.
This valuable result of DAS cytotoxicity will have a potential impact on both industrial processes and human health, because the incidence of cancer has increased and become a particular concern, putting people’s health at risk. It not only interferes with people’s health but also with hospital systems’ ability to function effectively due to increasing case numbers. It is obvious that the novel approach of a new cancer treatment could also be used to study its effect on other cancer diseases. This could be done to better understand cancer mechanisms, as well as to create and assess the efficacy of the novel DAS-NE3 formula. Ultimately, this will directly lessen the social and economic burden that diseases, infestation, and biofouling have on industry and healthcare, which would otherwise cost money and claim countless lives each year.
Conclusion
In this study, DAS-NEs were successfully developed using a high-energy method to produce NEs of DAS, enhancing its solubility and anticancer effect. The DAS-NE3 formulation had an appropriate particle size of about 70 nm, with a uniform distribution of particles, and good EE. Interestingly, DAS-NE3 showed both cytotoxicity against MCF7 breast, HT29, and SW480 colorectal cancer cells, in addition to a non-toxic selective effect against MC5 cells. Considering these findings, it was determined that the DAS-NE3 could have the potential to be utilized in the treatment of cancer in a manner that allows for the regulated delivery of medication. This novel approach to a new cancer treatment could lead to a better understanding of cancer mechanisms, as well as the treatment’s creation and assessment of its efficacy.
For future studies, DAS nanoemulsion treatment in vivo trials will provide more confirmation of its efficacy and advantages and will aid in the establishment of an optimal delivery system.
Ethical Approval
The authors declare that no ethical approval is needed, and no animals or patients are included in the study. This type of study is non-human subject research and there was not any kind of individual participation, so ethical approval and consent are exempt.
Consent for Publication
None.
Availability of Data and Material
Data and materials are available upon request.
Competing Interests
The authors declare that there are no conflicts of interest.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, and not-for-profit sectors.
Authors’ Contributions
AT, AA, and MA- designed and conceived the study, conducted research, provided research materials, and organized and collected data. AT analyzed and interpreted data. JA and AT wrote the initial and final drafts of the article and provided logistic support. The final draft of the manuscript has been critically reviewed and approved by all authors, who also bear responsibility for its content and similarity index.
References
- Epidemiology of cancer in Saudi Arabia thru 2010-2019:A systematic review with constrained meta-analysis. AIMS Public Health. 2020;7:679-96.
- [Google Scholar]
- Innovative approaches for cancer treatment:Current perspectives and new challenges. Ecancermedicalscience. 2019;13:961.
- [Google Scholar]
- A concise review on cancer treatment methods and delivery systems. J Drug Deliv Sci Technol. 2019;54:101350.
- [Google Scholar]
- Inhaled nano-and microparticles for drug delivery. Global Cardiol Sci Pract. 2015;2015:2.
- [Google Scholar]
- A brief review on solid lipid nanoparticles:Part and parcel of contemporary drug delivery systems. RSC Adv. 2020;10:26777-91.
- [Google Scholar]
- Passive and active targeting in cancer therapy by liposomes and lipid nanoparticles. Drug Metab Personal Ther. 2019;34:20180032.
- [Google Scholar]
- Nanoemulsion:A brief review on development and application in Parenteral Drug Delivery. Adv Pharm J. 2018;3:43-54.
- [Google Scholar]
- Nanoemulsion formulation for enhancing the aqueous solubility and systemic bioavailability of poorly soluble drugs. Records Pharm Biomed Sci. 2023;7:103-8.
- [Google Scholar]
- Nanoparticles and cancer therapy:Perspectives for application of nanoparticles in the treatment of cancers. J Cell Physiol. 2020;235:1962-72.
- [Google Scholar]
- In vitro evaluation of nebulized eucalyptol nano-emulsion formulation as a potential COVID-19 treatment. Saudi Pharm J. 2022;30:1691-9.
- [Google Scholar]
- Formulation of piperine-loaded nanoemulsion:In vitro characterization, ex vivo evaluation, and cell viability assessment. ACS Omega. 2023;8:22406-13.
- [Google Scholar]
- Thymus vulgaris oil nanoemulsion:Synthesis, characterization, antimicrobial and anticancer activities. Molecules. 2023;28:6910.
- [Google Scholar]
- Anticancer, antioxidant, and antibacterial effects of nanoemulsion of Origanum majorana essential oil. Iran J Microbiol. 2023;15:565-73.
- [Google Scholar]
- Imatinib, dasatinib and nilotinib:A review of adverse cutaneous reactions with emphasis on our clinical experience. J Eur Acad Dermatol Venereol. 2013;27:1471-80.
- [Google Scholar]
- Stable fatty acid solvates of dasatinib, a tyrosine kinase inhibitor:Prediction, process, and physicochemical properties. ACS Omega. 2022;7:7032-44.
- [Google Scholar]
- Dasatinib reduces 5-Fu-triggered apoptosis in colon carcinoma by directly modulating Src-dependent caspase-9 phosphorylation. Cell Death Discov. 2018;4:61.
- [Google Scholar]
- Dasatinib inhibits proliferation of liver cancer cells, but activation of Akt/mTOR compromises dasatinib as a cancer drug. Acta Biochim Biophys Sin. 2021;53:823-36.
- [Google Scholar]
- Synergistic anti leukemia effect of a novel Hsp90 and a Pan cyclin dependent kinase inhibitors. Molecules. 2020;25:2220.
- [Google Scholar]
- Dasatinib self-assembled nanoparticles decorated with hyaluronic acid for targeted treatment of tumors to overcome multidrug resistance. Drug Deliv. 2021;28:670-9.
- [Google Scholar]
- Design, optimization and in vitro characterization of dasatinib loaded PLGA nano carrier for targeted cancer therapy:A preliminary evaluation. Res J Pharm Technol. 2021;14:2095-100.
- [Google Scholar]
- UV spectrophotometric method development and validation of dasatinib in bulk and formulation. Asian J Pharm Anal. 2021;11:203-6.
- [Google Scholar]
- Redox mechanism, spectrophotometrical characterisation and voltammetric determination in serum samples of kinases inhibitor and anticancer drug dasatinib. J Electroanal Chem. 2015;752:47-53.
- [Google Scholar]
- Nano-emulsion formulation using spontaneous emulsification:Solvent, oil and surfactant optimisation. Int J Pharm. 2004;280:241-51.
- [Google Scholar]
- Characteristics of Cinnamaldehyde Nanoemulsion Prepared Using APV-high Pressure Homogenizer and Ultra Turrax. In: In:AIP Conference Proceedings. United States: American Institute of Physics; 2014.
- [Google Scholar]
- Loading hydrophilic drug in solid lipid media as nanoparticles:Statistical modeling of entrapment efficiency and particle size. Int J Pharm. 2012;424:128-37.
- [Google Scholar]
- Formulation and optimization of quercetin nanoemulsion for enhancing its dissolution rate, bioavailability and cardioprotective activity. J Cluster Sci. 2023;34:1893-906.
- [Google Scholar]
- Inhaled atorvastatin nanoparticles for lung cancer. Curr Drug Deliv. 2022;19:1073-82.
- [Google Scholar]
- First-principles and empirical approaches to predicting in vitro dissolution for pharmaceutical formulation and process development and for product release testing. The AAPS J. 2019;21:1-20.
- [Google Scholar]
- Significance of targeting VEGFR-2 and cyclin D1 in luminal-A breast cancer. Molecules. 2020;25:4606.
- [Google Scholar]
- Smart Dispersant Formulations for Reduced Environmental Impact of Crude Oil Spills [Dissertation] 2015
- Chemosensitization of HT29 and HT29-5FU cell lines by a combination of a multi-tyrosine kinase inhibitor and 5FU downregulates ABCC1 and inhibits PIK3CA in light of their importance in Saudi colorectal cancer. Molecules. 2021;26:334.
- [Google Scholar]
- Dasatinib. In: In:Small Molecules in Hematology. Germany: Springer; 2018. p. :29-68.
- [Google Scholar]
- Self-nanoemulsifying drug delivery systems (SNEDDS) containing rice bran oil for enhanced fenofibrate oral delivery:In vitro digestion, ex vivo permeability, and in vivo bioavailability studies. AAPS PharmSciTech. 2020;21:1-10.
- [Google Scholar]
- Optimization of ultrasound induced emulsification on the formulation of palm-olein based nanoemulsions for the incorporation of antioxidant β-d-glucan polysaccharides. Ultrason Sonochem. 2016;31:71-84.
- [Google Scholar]
- Dasatinib nanoemulsion and nanocrystal for enhanced oral drug delivery. Pharmaceutics. 2022;14:197.
- [Google Scholar]
- DLS and zeta potential-what they are and what they are not? J Control Release. 2016;235:337-51.
- [Google Scholar]
- Development of Dasatinib Loaded Nano Carrier for Solubility and Bioavailabity Enhancement to Treat Leukemia. Coimbatore: KMCH College of Pharmacy; 2020.
- Amorphous nanoparticulate formulation of sirolimus and its tablets. Pharmaceutics. 2018;10:155.
- [Google Scholar]
- Dasatinib-loaded topical nano-emulgel for rheumatoid arthritis:Formulation design and optimization by QbD, in vitro, ex vivo, and in vivo Evaluation. Pharmaceutics. 2023;15:736.
- [Google Scholar]
- A novel interfacial thermodynamic model for predicting solubility of nanoparticles coated by stabilizers. Chin J Chem Eng. 2021;31:103-12.
- [Google Scholar]







