Translate this page into:
Factors affecting poor prognosis of COVID-19 in people living with human immunodeficiency virus: A systematic review and meta-analysis of co-infection
Address for correspondence: Sayed Abdulla Jami, Department of Spine Orthopaedic Surgery, General Hospital of Ningxia Medical University, 804 Shengli Street, Xingqing District, Yinchuan, 750004, Ningxia, People’s Republic of China. Phone: +8801966451073. E-mail: jami41@live.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Qassim Uninversity and was migrated to Scientific Scholar after the change of Publisher.
Abstract
ABSTRACT
Objectives:
This study aims to assess the correlation between clinical features and mortality in human immunodeficiency virus (HIV)-infected individuals with COVID-19.
Methods:
A systematic literature search was conducted for cohort, cross-sectional, and case series that reported co-infection with HIV and COVID-19 published from January to September 2020. Clinical features such as age, comorbidities, CD4+T lymphocyte counts, HIV RNA levels, and antiretroviral regimens were evaluated using meta-analyses and systematic reviews. Meta-analysis was performed using Stata 15.0 software.
Results:
A total of 24 articles with 939 cases of HIV/COVID-19 co-infection were included in this study. The overall mortality rate was 10.3% (97/939). Older age and comorbidities including hypertension, diabetes, renal insufficiency, chronic obstructive pulmonary disease/asthma, and tumors were significantly associated with increased mortality (95% confidence interval 0.005–0.050, 0.042–2.294, 0.390–2.754, 0.513–2.848, 0.348–3.743, and 1.943–7.101, respectively, P = 0.021, 0.043, 0.012, 0.008, 0.022, and 0.005). There was no significant correlation between mortality and CD4+T lymphocyte count <200/μL or >500/μL, HIV RNA level below the detection limit, or antiretroviral drugs (including tenofovir) (all P > 0.05). Improved HIV treatment, complex immune interactions, study population variability, and lack of direct SARS-CoV-2 targeting by ART likely obscure the correlation between CD4+ counts or ART and COVID-19 mortality in HIV patients.
Conclusion:
HIV-infected individuals with COVID-19 have a similar prognosis to the general population. However, older age, comorbidities (hypertension and diabetesetc.), and lower CD4+ T-cell counts are associated with increased mortality. Mainstream anti-HIV drugs do not offer significant protection against COVID-19.
Keywords
Co-infection
COVID-19
HIV
Meta-analysis
Review
Introduction
The COVID-19 pandemic has presented unprecedented challenges to global health, with particularly severe implications for populations already vulnerable due to underlying health conditions. People living with human immunodeficiency virus (PLWH) face compounded risks, as human immunodeficiency virus (HIV) infection compromises immune function and can be accompanied by comorbidities that heighten susceptibility to severe COVID-19 outcomes. Although antiretroviral therapy (ART) has significantly improved long-term health for PLWH, some immune dysfunction persists, increasing their vulnerability to infections like COVID-19.[1] Recent studies indicate a higher risk of severe COVID-19 complications, hospitalization, and mortality among HIV-positive individuals compared to the general population. Factors such as low CD4+ T-cell counts, advanced HIV disease, and HIV-related comorbidities, including hypertension, diabetes, and chronic respiratory issues, contribute to the worsened outcomes seen in this group. Despite this evidence, the extent to which HIV-related immunosuppression specifically exacerbates COVID-19 severity remains inconclusive. While some research suggests immunosuppression could lead to heightened COVID-19 complications, other studies report that immunosuppressed individuals may not experience significantly worse COVID-19 outcomes than the general population.[2]
In light of the challenges in treating COVID-19, attention has turned to repurposing antiviral drugs originally developed for other viral infections, including those used in ART. The researchers are also investigating potential COVID-19 treatments among existing anti-RNA virus drugs. Drugs like remdesivir and lopinavir, initially considered promising, have shown limited success against COVID-19, prompting further investigation into the potential protective role of long-term ART use for PLWH.[3] ART regimens, especially those involving nucleoside reverse transcriptase inhibitors (NRTIs) and integrase strand transfer inhibitors (INSTIs), are central to managing HIV by maintaining viral suppression and improving immune function. This potential interaction between ART and COVID-19 outcomes has led researchers to question whether ART might confer indirect protection against severe COVID-19 in PLWH.[4] However, the findings across studies remain mixed. While some research suggests that certain ART components, such as tenofovir, might reduce COVID-19 severity, other studies have not confirmed these protective effects, leaving questions regarding ART’s overall efficacy against COVID-19. In addition to ART, factors such as CD4+ T-cell counts and HIV RNA detectability further complicate our understanding of COVID-19 prognosis in co-infected patients. For instance, PLWH with CD4+ counts below 200 cells/μL indicative of significant immunosuppression are generally at risk for worse outcomes in many infections. Yet, their correlation with COVID-19 mortality is not definitively established.[5] Some studies posit that lower immune activation in these patients may provide protection against the excessive inflammatory response, or cytokine storm, which drives severe COVID-19 in the general population.[6]
Given the significant clinical implications, this systematic review and meta-analysis aim to identify the factors most strongly associated with severe COVID-19 outcomes in HIV-positive individuals. By focusing on clinical characteristics such as age, sex, comorbidities, CD4+ T cell counts, HIV RNA levels, and ART regimens, this study seeks to elucidate the specific factors influencing COVID-19 prognosis in PLWH. Synthesizing data from a wide range of studies across different regions, this research aims to offer insights into the underlying drivers of COVID-19 severity in co-infected patients, ultimately informing clinical guidelines and improving patient outcomes in this high-risk population. Through this comprehensive approach, the study endeavors to clarify whether ART regimens, immune status markers, to identify other promising antiviral options or other HIV-related factors significantly impact COVID-19 outcomes, thereby contributing valuable knowledge to managing co-infection cases in clinical practice.
Materials and Methods
Literature search
The retrieval time is from January 2020 to January 2021, including online published documents. The languages are Chinese and English. English documents were chosen from Embase, PubMed database, Wanfang, CNKI, and Weipu journal resource integration service platform in the Chinese language. The search includes three types of keywords: “coronavirus,” “human immunodeficiency virus” and “prognosis,” among which “coronavirus” includes the English search term “coronavirus” “nCoV” “2019-nCoV” “COVID” “SARS-CoV,” “HIV,” “AIDS” “acquired immunodeficiency syndrome.” Other English search terms are “outcome” “mortality” “death” “fatality” “severs” “ICU” “intensive care.”
Literature inclusion and exclusion criteria
Literature inclusion criteria
The research objects are confirmed patients of HIV co-infection with COVID-19, including cohort studies, case–control studies, case series, and cross-sectional studies. The article’s content includes demographic characteristics, clinical conditions, comorbidities, HIV control status, ART programs, and prognosis. The literature is published in a peer-reviewed journal.
Literature exclusion criteria
Literature that does not contain the original information of the case, such as reviews, comments, and correspondence, does not clearly explain the clinical information and prognosis of the case, the case has repeated inclusion relationships, and the number of cases is <4 cases.
Literature quality evaluation
Use the Joanna Briggs Institute Literature Quality Evaluation Scale.[7] The review of the literature cohort to establish comparability, inclusion or exclusion criteria, detailed description, exposure factor evaluation, variable measurement methods, confounding factor identification, confounding factor processing, outcome variable evaluation, use of statistical methods, and other methods were used to give scores. According to different literature types, the corresponding scale was used, in which the total score of the cohort study was 12, the total score of the case series was 10, and the total score of the cross-sectional study was 8. Two staff members independently completed the literature retrieval, screening, and evaluation.
Statistical analysis
Statistical analysis was performed using Stata 15.0 software. The meta-analysis calculates the case fatality rate of patients for each study, uses a random-effects model to calculate the combined value of the case fatality rate and its 95% CI, and performs a Z test. The heterogeneity test between studies adopts I2 to evaluate, and there may be substantial heterogeneity when I2 is 50–90%. The symmetry of the funnel chart evaluated the publication bias, and the Begg and Egger tests were performed simultaneously. P > 0.05 means that there is no publication bias. Heterogeneity in this study was managed using statistical techniques to account for variations across different studies. Meta-regression analysis is used to explore the reasons for the heterogeneity of mortality and its possible influencing factors. This study conducted a single-factor regression analysis. Regression analysis is required for at least 5 literature reports for each risk factor to avoid over-fitting. Since the case fatality rate of the patients in this study is skewed (the case fatality rate of 5 studies is 0), the case fatality rate is subjected to double arcsine transformation before meta-analysis and regression analysis and then obtained by the inverse change of double arcsine transformation. The composite value of the case fatality rate and its 95% CI. The count data is expressed as the number of cases, and the χ2 test compares groups. P < 0.05 is considered statistically significant.
Results
Search results
The review included a total of 2020 publications in Chinese and English (sourced from Embase: 618, Medline: 556, Cochrane: 278, PubMed: 548, Wanfang Data Resource System: 7, CNKI: 9, and VIP: 4). After removing 641 duplicate entries, 1312 studies were further excluded based on title and abstract, yielding 67 articles for preliminary review. Following a full-text assessment, 27 case reports, 10 correspondence articles, 3 review papers, and 3 studies not meeting inclusion criteria were excluded. Ultimately, 24 English-language studies were retained,[8-31] comprising 8 cohort studies, 14 case series, and 2 cross-sectional studies. Together, these studies encompassed 939 patients co-infected with HIV and COVID-19. Basic study details and quality assessment scores are presented in Table 1.
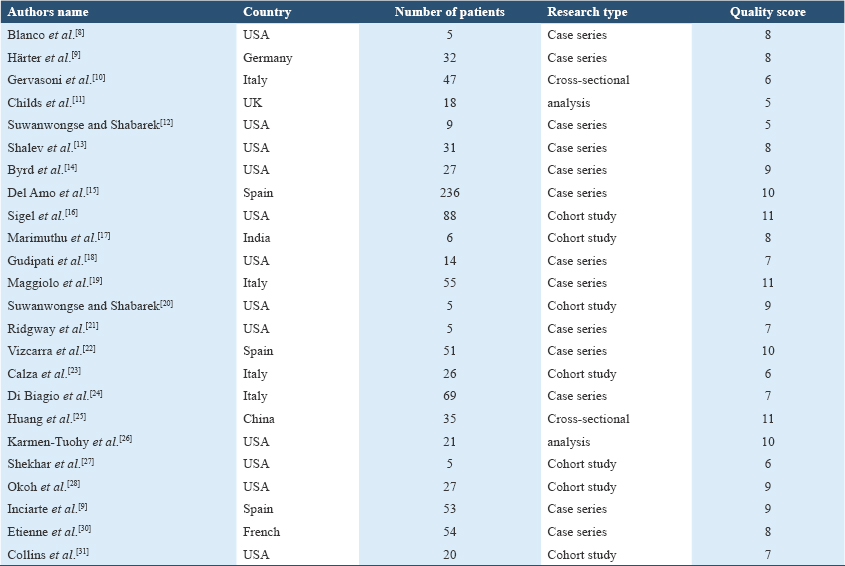
Among the 939 patients, the mean age was 53.3 years, with a demographic breakdown of 78.7% males (739), 20.8% females (195), and 0.5% transgender individuals (5). For studies reporting comorbidities, 62.8% (250/398) of the patients had at least one underlying health condition. The most prevalent comorbidities included hypertension (36.4%, 243/668), diabetes (18.0%, 120/668), obesity or hyperlipidemia (13.0%, 87/668), chronic obstructive pulmonary disease (COPD) or asthma (10.5%, 70/668), and renal insufficiency (9.9%, 66/668), with an average of 1.2 comorbid conditions per patient. Among studies documenting CD4+ T cell counts, 23.8% (74/311) of patients had CD4+ T-cell counts below 200/μL. In the subset of studies reporting HIV RNA levels, 9.2% (63/683) of patients had detectable viral loads. Regarding antiviral therapy, 54.0% (503/931) of patients were on an INSTI, 17.7% (165/931) were on a protease inhibitor (PI), 23.3% (217/931) were using non-NRTIs (NNRTI), and 77.9% (725/931) were on NRTI. In addition, 12.7% (119/939) of patients required mechanical ventilation, and the overall mortality rate was 10.3% (97/939).
Mortality rate of HIV/COVID-19 co-infected patients
24 included articles mortality of forest plot and funnel, in Figure 1; mortality Begg funnel plot test Pr > | z | = 0.274, Egger test P = 0.333, no publication bias, in Table 2.
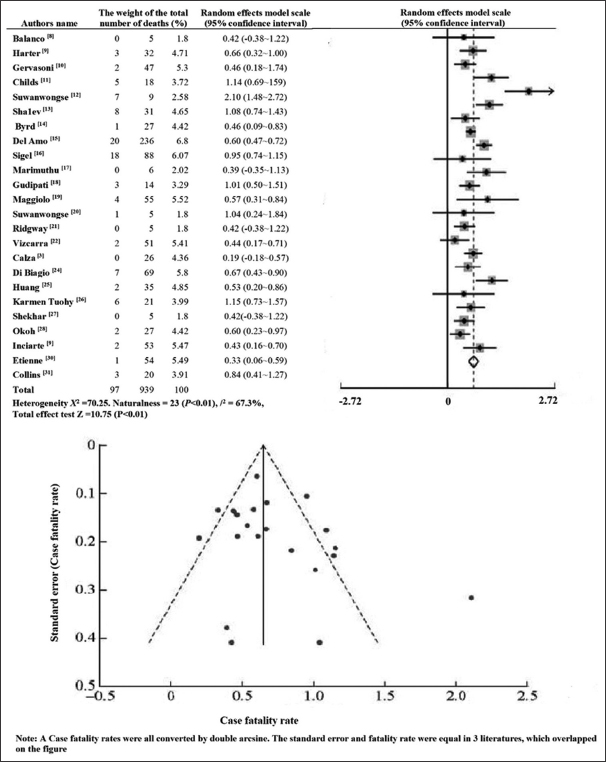
- HIV/COVID-19 co-infected patients with forest and funnel charts for the overall mortality rate
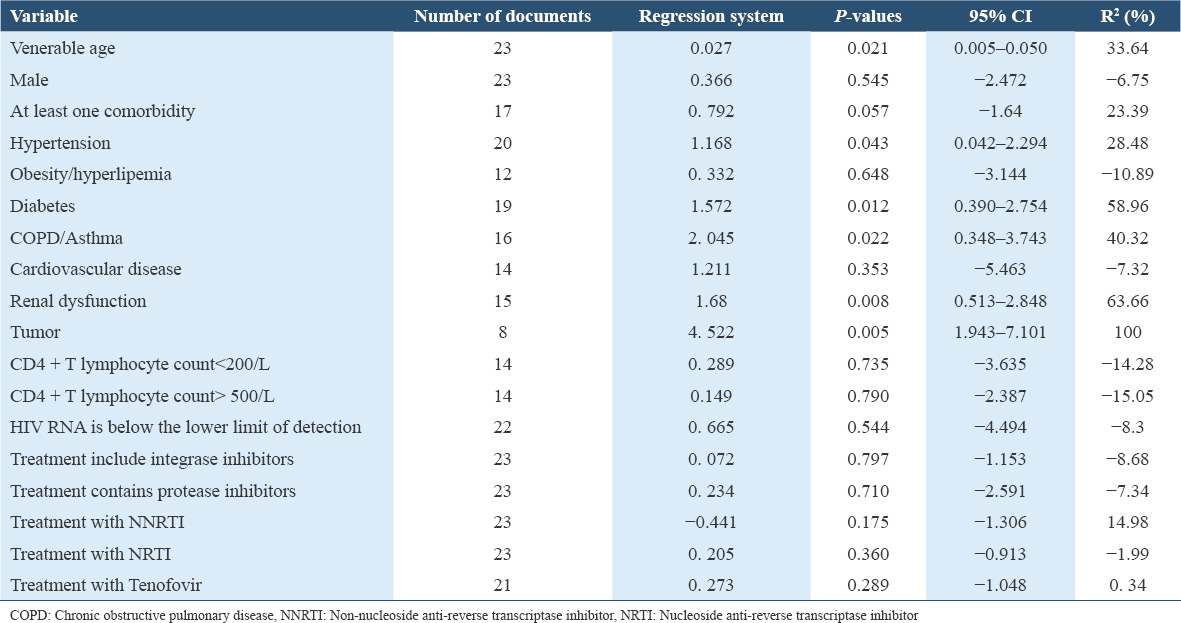
There is a correlation between the age of patients and the mortality rate, and the mortality rate of elderly patients increases (P < 0.05). Complicated with hypertension, diabetes, renal insufficiency, COPD/asthma, and tumors are all related to the increase in mortality (all P < 0.05), obesity/hyperlipidemia, cardiovascular disease, and mortality are not correlated (all P > 0.05). CD4+ T lymphocyte count <200/μL or HIV RNA below the detection limit were not correlated with mortality (P > 0.05). There was no correlation between antiviral treatment programs containing different drugs and mortality (all P > 0.05). Stratified analysis of CD4+ T lymphocyte counts in HIV/COVID-19 co-infected patients. Of 60 patients with CD4+ T lymphocyte count <200/μL, 6 died; 39 patients with CD4+ T lymphocyte count 200-500/μL, 5 died; CD4+ T lymphocyte count >500/μL of the 7 patients, 3 died. There was no correlation between CD4+ T lymphocyte count and mortality (χ2 = 5.936, P > 0.05).
Discussion
This study included data from various medical centers worldwide, encompassing both case reports and cohort studies. Significant heterogeneity existed among the studies (I2 = 67.3%) due to differences in patient comorbidities, HIV infection control levels, and long-term anti-HIV medication regimens. Meta-regression analysis revealed that advanced age and comorbidities, similar to the general population, were associated with poor prognosis in HIV/COVID-19 co-infected patients.[32] Studies have reported that HIV-infected or immunocompromised patients have a better prognosis for COVID-19,[2] and the subsequent review system prompts immunocompromised patients with a poor prognosis.[3] This study did not find that HIV infection control and CD4+ T lymphocyte count levels are associated with poor prognosis, especially patients with CD4+ T lymphocyte counts <200/μL did not show a significant increase in mortality.[33-35] Some studies believe that the death of COVID-19 is a manifestation of excessive activation of inflammation, whether the reduction of CD4+ T lymphocyte count itself plays a particular “protective” effect by reducing the formation of inflammatory storms, it may need further research to clarify.
Preliminary research has high hopes for lopinavir and tenofovir,[36] And even some agencies try to change HIV infection after infection COVID-19 basis ART programs to improve the prognosis.[8,11,12,22] According to the current evidence, various conventional anti-HIV ART drugs have not found adequate protection against COVID-19 infection, severe illness, and death.[37,38] Until further evidence is obtained, no attempt should be made to change the ART regimen to prevent and treat COVID-19.[39]
In this study, the overall mortality rate of HIV/COVID-19 co-infected patients reached 10.3%, which is a certain gap with the international understanding of the overall mortality rate of COVID-19 patients. The variation in overall case fatality rates, ranging from 0.1% to 25% across regions, is largely attributable to differences in calculation methods and indicators used to measure these rates.[40-42] This study focuses on patients diagnosed with COVID-19 after seeking medical treatment, aligning more closely with the concept of case fatality ratio, which typically yields higher values than the infection fatality ratio. The estimated case fatality rate remains the most practical clinical indicator for assessing mortality. However, the patients included in this study were ill during the early stages of the pandemic (January 2020–2021). As HIV-infected individuals constitute a significant portion of the population in certain regions, these areas often experienced more severe COVID-19 outbreaks. Consequently, medical centers in these regions reported a higher number of HIV/COVID-19 co-infection cases, potentially influencing the overall mortality rate.[43] These areas faced relatively insufficient medical resources in the epidemic’s early stages and insufficient respiratory and critical support treatments. Due to lack of experience, some centers even reported 7 deaths among 9 co-infected patients in May 2020;[12] until July 2020, due to the appropriate follow-up medical resources, the reduction of overall cases, again reported on new cases co-infected patients, only one died.[20] The results of the funnel chart of the fatality rate in this study showed no obvious publication bias in the included literature and the prospective cohort study. This study showed no significant difference in the fatality rate between HIV-infected people and the general population,[16] and it further shows that when other conditions are matched, HIV infection is not a high-risk factor for the death of COVID-19 patients.[44]
Despite its value, this study still has several limitations. This study, while valuable, has several limitations. First, due to ethical considerations and preventive measures, most current research on HIV/COVID-19 co-infection is observational. Compared to randomized controlled trials, observational studies are more prone to bias. However, this review mitigated some of these biases by including multiple study designs (cohort, cross-sectional, and case series) and excluding case reports. Conducting future studies with matched control groups could further reduce bias. Second, variations in testing rates, hospitalization criteria, and critical care availability across different settings can impact mortality rates. Large-scale single-center case-control studies should be interpreted cautiously due to these variations. Many individual studies included in this review had limited sample sizes, hindering the ability to draw definitive conclusions. Nevertheless, the systematic review and meta-analysis combined data from multiple studies to achieve statistically significant findings.
Conclusion
There has been no significant rise in the mortality rate of people with HIV who contract COVID-19 in comparison to the general population. Advanced age and comorbidities remain the primary risk factors for death from co-infection. The impact of HIV RNA and CD4+ T lymphocyte count on COVID-19 mortality requires additional investigation. The study found no conclusive evidence that anti-HIV treatments can prevent COVID-19 fatalities. Therefore, altering ART regimens due to COVID-19 infection is not recommended until more clinical data is available.
Ethics Approval and Consent to Participate
All the informed consents for publication from the patients did not need to be obtained. Because no human or animal trials are involved in this research by us. Also, no direct patient’s data are involved from our institution. So ethical approval consent is not required. All the data were collected from published articles.
Availability of Data and Materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
Not applicable.
Funding
Not applicable yet.
Authors’ Contributions
Substantial contributions to conception and design: Sayed Abdulla Jami
Data acquisition, data analysis, and interpretation: All authors
Drafting the article or critically revising it for important intellectual content: All authors
Final approval of the version to be published: All authors
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of the work are appropriately investigated and resolved.
Acknowledgments
Authors used A.I. tools to improve readability and language.
Declaration of Interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
- Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323:2052-9.
- [Google Scholar]
- How is immunosuppressive status affecting children and adults in SARS-CoV-2 infection?A systematic review. J Infect. 2020;81:e61-6.
- [Google Scholar]
- Impacts of immunosuppression and immunodeficiency on COVID-19:A systematic review and meta-analysis. J Infect. 2020;81:e93-5.
- [Google Scholar]
- Remdesivir in adults with severe COVID-19:A randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569-78.
- [Google Scholar]
- Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19:A randomized clinical trial. JAMA. 2020;324:1048-57.
- [Google Scholar]
- A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787-99.
- [Google Scholar]
- The revised JBI critical appraisal tool for the assessment of risk of bias for randomized controlled trials. JBI Evid Synth. 2023;21:494-506.
- [Google Scholar]
- COVID-19 in people living with human immunodeficiency virus:A case series of 33 patients. Infection. 2020;48:681-6.
- [Google Scholar]
- Clinical features and outcomes of patients with human immunodeficiency virus with COVID-19. Clin Infect Dis. 2020;71:2276-8.
- [Google Scholar]
- Hospitalized patients With COVID-19 and human immunodeficiency virus:A case series. Clin Infect Dis. 2020;71:2021-2.
- [Google Scholar]
- Clinical features and outcome of HIV/SARS-CoV-2 coinfected patients in the Bronx, New York city. J Med Virol. 2020;92:2387-9.
- [Google Scholar]
- Clinical characteristics and outcomes in people living with human immunodeficiency virus hospitalized for coronavirus disease 2019. Clin Infect Dis. 2020;71:2294-7.
- [Google Scholar]
- SARS-CoV-2 and HIV coinfection:Clinical experience from Rhode Island, United States. J Int AIDS Soc. 2020;23:e25573.
- [Google Scholar]
- Incidence and severity of COVID-19 in HIV-positive persons receiving antiretroviral therapy:A cohort study. Ann Intern Med. 2020;173:536-41.
- [Google Scholar]
- Coronavirus 2019 and people living with human immunodeficiency virus:Outcomes for hospitalized patients in New York City. Clin Infect Dis. 2020;71:2933-8.
- [Google Scholar]
- HIV and SARS CoV-2 coinfection:A retrospective, record-based, case series from South India. J Med Virol. 2021;93:163-5.
- [Google Scholar]
- Descriptive analysis of patients living with HIV affected by COVID-19. J Acquir Immune Defic Syndr. 2020;85:123-6.
- [Google Scholar]
- SARS-CoV-2 infection in persons living with HIV:A single center prospective cohort. J Med Virol. 2021;93:1145-9.
- [Google Scholar]
- Variation in mortality of HIV/SARS-CoV-2 coinfected patients in the Bronx, New York City. J Med Virol. 2021;93:603-5.
- [Google Scholar]
- A case series of five people living with HIV hospitalized with COVID-19 in Chicago, Illinois. AIDS Patient Care STDS. 2020;34:331-5.
- [Google Scholar]
- Description of COVID-19 in HIV-infected individuals:A single-centre, prospective cohort. Lancet HIV. 2020;7:e554-64.
- [Google Scholar]
- COVID-19 in patients with HIV-1 infection:A single-centre experience in northern Italy. Infection. 2021;49:333-7.
- [Google Scholar]
- Factors associated with hospital admission for COVID-19 in HIV patients. AIDS. 2020;34:1983-5.
- [Google Scholar]
- Epidemiological, virological and serological features of coronavirus disease 2019 (COVID-19) cases in people living with human immunodeficiency virus in Wuhan:A population-based cohort study. Clin Infect Dis. 2021;73:e2086-94.
- [Google Scholar]
- Outcomes among HIV-positive patients hospitalized with COVID-19. J Acquir Immune Defic Syndr. 2020;85:6-10.
- [Google Scholar]
- Coronavirus disease of 2019 in patients with well-controlled human immunodeficiency virus on antiretroviral therapy. J Acquir Immune Defic Syndr. 2020;85:e1-4.
- [Google Scholar]
- COVID-19 pneumonia in patients with HIV:A case series. J Acquir Immune Defic Syndr. 2020;85:e4-5.
- [Google Scholar]
- Clinical characteristics, risk factors, and incidence of symptomatic coronavirus disease 2019 in a large cohort of adults living with HIV:A single-center, prospective observational study. AIDS. 2020;34:1775-80.
- [Google Scholar]
- Clinical characteristics, comorbidities and outcomes among persons with HIV hospitalized with coronavirus disease 2019 in Atlanta, Georgia. AIDS. 2020;34:1789.
- [Google Scholar]
- Clinical characteristics and morbidity associated with coronavirus disease 2019 in a series of patients in Metropolitan Detroit. JAMA Netw Open. 2020;3:e2012270.
- [Google Scholar]
- People living with HIV have a prolonged virus shedding duration even with anti-SARS-CoV-2 treatment:A retrospective cohort study in Wuhan, China. Lancet 2024. 2024;395:1054-62.
- [Google Scholar]
- An online survey and review about the awareness, coping style, and exercise behavior during the “COVID-19 pandemic situation”by implementing the cloud-based medical treatment technology system in China among the public. Sci Prog.. 2021;104(2):368504211000889.
- [Google Scholar]
- Clinical and strategic outcomes of metastatic synovial sarcoma on limb. Int J Health Sci (Qassim). 2020;14:38-43.
- [Google Scholar]
- Ribavirin, remdesivir, sofosbuvir, galidesivir, and tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp):A molecular docking study. Life Sci. 2020;253:117592.
- [Google Scholar]
- Undesirable effects of COVID-19 vaccination on Saudi population:A descriptive study, Winter 2022. Int J Health Sci (Qassim). 2024;18:32-45.
- [Google Scholar]
- Comparison of chondrosarcoma cases:Current clinical situations among institutions. Int J Health Sci (Qassim). 2021;15:42-9.
- [Google Scholar]
- BHIVA guidelines on antiretroviral treatment for adults living with HIV-1 2022. HIV Med. 2022;23:3-115.
- [Google Scholar]
- Estimating excess mortality due to the COVID-19 pandemic:A systematic analysis of COVID-19-related mortality, 2020-21. Lancet. 2022;399:1513-36.
- [Google Scholar]
- Treatment of myasthenia gravis patients with Covid-19:Review of the literature. Acta Clin Croat. 2022;60:496-509.
- [Google Scholar]
- Evaluation of the relationship of MPV, RDW and PVI parameters with disease severity in Covid-19 patients. Acta Clin Croat. 2021;60:103-14.
- [Google Scholar]
- Synergy between psychological impact and biochemical manifestation of stress among the COVID-19 pandemic-affected population. Int J Health Sci (Qassim). 2024;18:46-57.
- [Google Scholar]
- A comparable risk of extensively drug-resistant typhoid fever in the pediatric cohort during the COVID-19 pandemic. Int J Health Sci (Qassim). 2024;18:24-8.
- [Google Scholar]







