Translate this page into:
Linking of oxidative stress and mitochondrial DNA damage to the pathophysiology of idiopathic intrauterine growth restriction
Address for correspondence: Dr. Shyam Pyari Jaiswar, Department of Obstetrics and Gynaecology, King George’s Medical University, Lucknow, Uttar Pradesh, India. Phone: +09415023358. E-mail: spjaiswar59@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Qassim Uninversity and was migrated to Scientific Scholar after the change of Publisher.
Abstract
ABSTRACT
Objective:
A common and serious pregnancy issue known as intrauterine growth restriction (IUGR) occurs when the fetus is unable to reach its full growth potential. Mitochondria are crucial to the development of the fetus and the placenta. We aimed to elucidate the role of oxidative stress parameters and markers of DNA damage. The integrity of the mitochondrial DNA (mtDNA) was studied.
Materials and Methods:
Blood samples were collected from 48 females (cases and controls, respectively). Oxidative stress parameters were analyzed. DNA was extracted followed by high-performance liquid chromatography to study 8-OH-dG and mt DNA by real-time polymerase chain reaction. Western blot analysis was performed for nuclear-encoded mitochondrial proteins and DNA damage markers.
Results:
When pregnant women were compared to non-pregnant women in their first, second, and third trimesters, a highly significant progressive drop in circulating mtDNA was found. In addition, mtDNA was considerably higher in mothers carrying IUGR fetuses than in healthy pregnancies. Sirtuin-3 protein expression was considerably suppressed in the IUGR placenta (P = 0.027), whereas Nrf1 expression was not statistically different from the control group in the IUGR. Increased oxidative stress led to greater DNA damage in IUGR. The highest concentrations of 8-OH-dG were found in IUGR with levels significantly higher than those in the non-pregnant group.
Conclusion:
Our research sets the path for further investigation into mitochondrial anomalies in IUGR pregnancies and offers evidence for disturbed mitochondrial homeostasis. The mtDNA might offer a fresh perspective on the processes involved in physiological gestation. In addition, the presence of mtDNA may aid in the diagnosis of IUGR during pregnancy.
Keywords
8-OH-dG
mtDNA
homeostasis
intrauterine growth restriction
oxidative stress
Introduction
Intrauterine growth restriction (IUGR) is a pathophysiological repression of fetal growth that results when a fetus fails to reach its full developmental potential. IUGR continues to rank among the world’s top causes of fetal morbidity and mortality and poses a larger risk to maternal health. A fetus’s inability to reach its full growth potential frequently causes pregnancies to be terminated early and increases the risk of both poor neonatal outcomes and long-term health effects. IUGR is pervasive everywhere, although it is especially common in underdeveloped nations.[1,2] Global statistics show that approximately 24% of neonates have IUGR, which results in 30 million growth-restricted infants each year. However, it is predicted that the rate of IUGR in poor nations is around 6 times higher than in industrialized nations.[2] Infants with growth restriction have been linked to an estimated 606,500 fatalities. According to reports, 21% of all live babies in India have IUGR.[3]
The 16.5 Kb-sized mitochondria, which are cellular organelles, produce ATP and are in charge of cellular respiration.[4] It has been established that a number of human diseases, including inherited disorders, is accompanied by mitochondrial dysfunction.[5] Because of decreased oxygen passage through the placenta in IUGR, cells experience hypoxia, which enhances the production of reactive oxygen species (ROS).[6] Previous research on the activity of the mitochondria during pregnancy and fetal development has paved the way for understanding how it contributes to the pathophysiology of IUGR.[7] It has long been understood that healthy mitochondrial function is necessary for pregnancy to provide the embryo with the necessary energy and metabolites for appropriate growth, a process that includes cell division, migration, and differentiation.[4]
Recent studies suggest that mitochondrial machinery, which is influenced by environmental factors such as temperature[8] or calorie restriction,[9] is involved in oxidative stress. Furthermore, the regulation of mitochondrial biogenesis can change in a variety of organs or tissues under hypoxic conditions.[10] In the context of oxidative stress, the participation of significant transcription factors that promote mitochondrial DNA (mtDNA) biogenesis and mtDNA maintenance has been proposed.[11] We proposed that this might be the result of either the mitochondria compensating for hypoxia or the metabolic placental mechanism adapting to the calorie restriction condition of the IUGR fetus.
Free radical attack on physiologically significant components, including DNA, lipids, and proteins, including enzymes, can result from increased oxidative stress.[12,13] As a result, purines and pyrimidines are damaged,[14] resulting in oxidative DNA alterations. The most prevalent of the oxidized bases is 8-hydroxy-20-deoxyguanosine (8-OH dG), which is regarded as a key indicator of oxidative DNA damage in tissues.
Endothelial cell dysfunction and an exacerbated maternal inflammatory response are characteristics of women with IUGR.[15] The stimulation of circulating neutrophils and monocytes is thought to be crucial for these alterations, while the precise processes are yet unclear.[13] In the peripheral circulation of women with pre-eclampsia and IUGR, there are more neutrophils and monocytes than in a typical pregnancy, and these cells are more active than usual.[16] Myeloperoxidase (MPO) synthesis and secretion are two of the several effects of neutrophil and monocyte activation. MPO can become trapped in the subendothelial region after entering the bloodstream and continue to build up in the cell matrix. There, it may be a significant source of reactive nitrogen and oxygen species and, through the action of hypochlorous acid, may consume antioxidants, causing oxidative stress and vascular dysfunction. Indeed, the likelihood of endothelial dysfunction is strongly correlated with serum MPO levels.[17,18] The precise function of MPO during pregnancy is yet unknown. It has been shown that MPO builds up on the surface of neutrophils[19] in pregnant women and that its movement during pregnancy increases the metabolic activity and oxidant generation of neutrophils. MPO levels have also been observed to be higher in the placenta and blood of pre-eclampsia-suffering women.[20]
The prevalence of IUGR, especially idiopathic, is high, and no absolute cure exists except to monitor and deliver. This increases the chance of preterm birth and delivery of hypoxic babies adding to perinatal mortality so this study is of vital importance. The present study sought to clarify the role of oxidative stress in IUGR by analyzing the levels of oxidative stress-related molecules and 8-OHdG (a hydroxyl product of deoxyguanosine that is generated by the DNA damage caused by oxidative stress). We examined the integrity of mitochondrial DNA (mtDNA) in maternal blood to suggest its association with the IUGR condition. We also looked into the placenta’s function in the synthesis and secretion of MPO.
Materials and Methods
Patients
The study population consisted of 48 healthy pregnant women with no previous history of IUGR (10, 15, and 23 women in the first, second, and third trimester, respectively), 48 women with pregnancy complicated by idiopathic IUGR, and 15 non-pregnant women in the follicular phase of the menstrual cycle and was conducted at Queen Mary’s Hospital, King George’s Medical University, Lucknow, UP, India. Women who smoked cigarettes or had any illness that influenced the count of endothelial progenitor cells (anemia, pre-eclampsia, heart disease, lung disease, tuberculosis, and carcinoma) were excluded from the study. Non-pregnant controls had regular menstrual cycles and were not using hormonal contraceptives. Normal pregnancies were illustrated by the non-existence of maternal or fetal pathologies, any obstetric complications, and not on any medication that directly has an effect on pregnancy outcome and fetal growth. Normal pregnancies were delivered at 37–42 weeks of gestation.[16] IUGR was characterized as having a gestational age in the 10th percentile measured by ultrasonography.[17] Women confirmed their participation by signing a consent form, and this study was approved by the Institutional Ethical Clearance Committee for Human Research (ECR/262/Inst/UP/2013/RR-16).
Blood sampling
Maternal blood samples of non-pregnant women of reproductive age were obtained. Maternal blood samples were collected by venipuncture in the first, second, and third trimesters of gestation in appropriate-for-gestational-age pregnancies at the time of their routine obstetric visit at the hospital. Samples of IUGR pregnancies were collected at the time of cesarean section. Blood was drawn from a brachial vein and was collected in tubes containing 1.0 mg/mL Na2 EDTA (disodium EDTA). The collected blood samples were left at room temperature for 2 h. After clotting, the blood was centrifuged at 2000 g, and the separated serum was placed in Eppendorf test tubes. The material thus obtained was placed in a nitrogen atmosphere and stored at −80°C.
Biochemical estimation
In blood, SOD, GSH-Px, CAT, ADA, LPO, and MPO analyses were performed as described, respectively.[21-26]
DNA isolation and chromatography
Using the Qiagen genomic DNA purification kit (cat. no. 51304) and adhering to the manufacturer’s recommended technique, total DNA was isolated from blood. Isolated DNA pellets were dried with a stream of N2, dissolved in 200 mL of 20 mmol/L sodium acetate buffer (pH 4.8) overnight, and then, digested with 20 mg of nuclease P1 before being added to 20 mL of Tris buffer (pH 7.4) containing 1.3 U of alkaline phosphatase and incubated for an additional 60 min at 30°C. Direct application of the DNA hydrolysate to a high-performance liquid chromatography column (8-OHdG) in micromoles per mole (20-deoxyguanosine) was used as a standard and to track native nucleosides.
Quantitative real-time polymerase chain reaction (PCR) analysis
The ABI Prism 7500 fast sequence detection system was used for real-time quantitative PCR analysis using the RNase P gene, as an endogenous control and AB Mitochondrial Gene7S, encoding D-loop, as the target gene. RNaseP is a single-copy nuclear gene that encodes the RNA moiety for the RNase P enzyme. D-loop is a replication start site of the mtDNA. Experiments were performed using the 1:2 diluted templates. Reaction conditions included 10L of 2X Taq Man fast universal PCR master mix, 1 L of primers and probes mixture, 50 ng of template DNA, and nuclease-free water in a 96-well reaction plate, with the total reaction volume being 20 L. PCR was carried out using the following conditions: 20 s at 95°C and 40 cycles of 3 s at 95°C, followed by 30 s at 60°C. Data were analyzed using the comparative Ct method, where Ct is the cycle number at which fluorescence first exceeds the threshold.
Western blot analysis
Western blot analysis was performed on protein extracted from the placental villi of IUGR and control specimens. Proteins from each sample (40 ug) were resolved in 12% SDS polyacrylamide gel electrophoresis. Following separation, the separated proteins were blotted on a nitrocellulose membrane (Sigma) and blocked with 5% skimmed milk (Sigma) powder in TBS-T (0.1% Tween 20 in TBS) for 2 h to prevent non-specific binding of the antibody and further washed with TBS-T for 30 min. Membranes were then incubated separately with primary antibodies (in 1% BSA-TBS-T) for Sirtuin-3 (Sirt3) (cell signaling) and Nrf1 (cell signaling) γ-H2AX and Nrf2 overnight at 4° under shaking conditions. Membranes were washed with TBS and subjected to incubation with a secondary antibody (antirabbit-HRP). All the blots were stripped and reprobed with β-actin to ensure equal loading of protein. Protein bands were detected using the enhanced chemiluminescence method (Bio-Rad) and visualized under ImageQuant LAS 4000 (GE Healthcare). All values were normalized against GAPDH (cell signaling), and a t-test was done for statistical analysis.
Statistical analysis
Data are presented as mean ± standard deviation. The Mann–Whitney U-test using SPSS version 11 (SPSS, Chicago, Ill., USA) was used for data analysis. The correlation between mtDNA copies and gestational age was assessed using the Pearson correlation coefficient. Differences were considered statistically significant at P < 0.05.
Results
Pregnant women in the second and third trimesters compared to non-pregnant women had considerably lower levels of mtDNA than non-pregnant women (P = 0.001). Furthermore, the amount of mtDNA in maternal blood reduced dramatically during the course of the three trimesters (first trimester values: 239 ± 64, second trimester values: 189 ± 45, and third trimester values: 144 ± 55; analysis of variance test, P < 0.001). When compared to healthy pregnancies of comparable gestational age, IUGR pregnancies exhibited considerably higher levels of maternal blood mtDNA (432 ± 186 and 144 ± 55, respectively; IUGR versus third trimester, P < 0.001). The individual mtDNA content values in IUGR-complicated pregnancies and in healthy third-trimester pregnancies are depicted in Figures 1a and b.
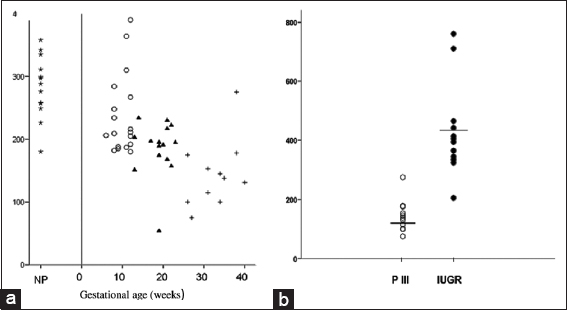
- (a) Mitochondrial DNA (mtDNA) content (relative quantification) of maternal blood in non-pregnant women (star) and normal pregnancies of the first (open circle), second (solid triangle), and third (cross) trimesters. (b). Relative quantification and mean levels of mtDNA content in the maternal blood of pregnant women in the third trimester (PIII) (open circle) and in pregnancies complicated by intrauterine growth restriction (solid circle)
Effect on Nrf2 levels and DNA integrity of cases and controls
It is well known that cells create a homeostatic balance between production and removal of ROS, and Nrf2 transcription factor has been identified as the master regulator for maintaining ROS balance. It was found that the expression of Nrf2 was decreased, thereby paralyzing the antioxidant defense machinery [Figure 2a].
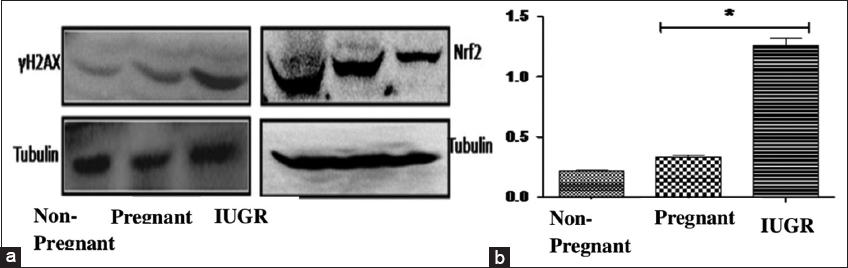
- (a) Western blot analysis of DNA damage (H2AX) and Nrf2. (b) Myeloperoxidase activity. The data represent mean ± SD *P < 0.05, which indicates a significant difference from the corresponding control
Since ROS generation has been known to cause DNA damage, the effect of BA on the integrity of DNA was studied. The western blot analysis revealed that the level of γ-H2AX protein, a well-established marker of DNA damage, was significantly elevated [Figure 2a].
Effect on MPO activity of cases and controls
MPO is secreted by inflammatory cells such as activated macrophages and neutrophils. It catalyzes the conversion of H2O2 into HOCl and tyrosine into a tyrosyl radical. HOCl and tyrosyl radicals are cytotoxic in nature and are key components of oxidative bursts and inflammatory responses. Increased MPO activity was observed in both pregnant and IUGR women. The MPO activity in pregnant women was upregulated by 157% ↑ and in IUGR by 571% ↑ [Figure 2b].
Oxidative stress parameters
Maternal plasma levels of CAT, GSH-Px, ADA, LPO, and protein were significantly higher in the IUGR group than in the non-pregnant and pregnant groups, while the SOD level was found to be lower in the IUGR group. The comparison of oxidative stress parameters for both cases and controls is summarized in Table 1.
Western blot analysis of nuclear-encoded mitochondrial proteins
The expression of mitochondrial Sirt3 (28 kDa), a member of the Sirtuin family necessary for regulating mitochondrial homeostasis, and Nrf1 (67 kDa), a transcriptional coactivator responsible for mitochondrial biogenesis, was examined by Western blot analysis [Figure 3a]. Comparing IUGR villus to controls, Nrf1 protein expression was not substantially different (P = 0.062) [Figure 3c]. However, Sirt3 expression in the IUGR villus increased significantly (P = 0.027) [Figure 3b]. Furthermore, Sirt3 protein expression and birth weight exhibited a significant positive connection (P = 0.013) in IUGR cases but not controls (0.509), according to Pearson’s correlation analysis [Figure 3d].
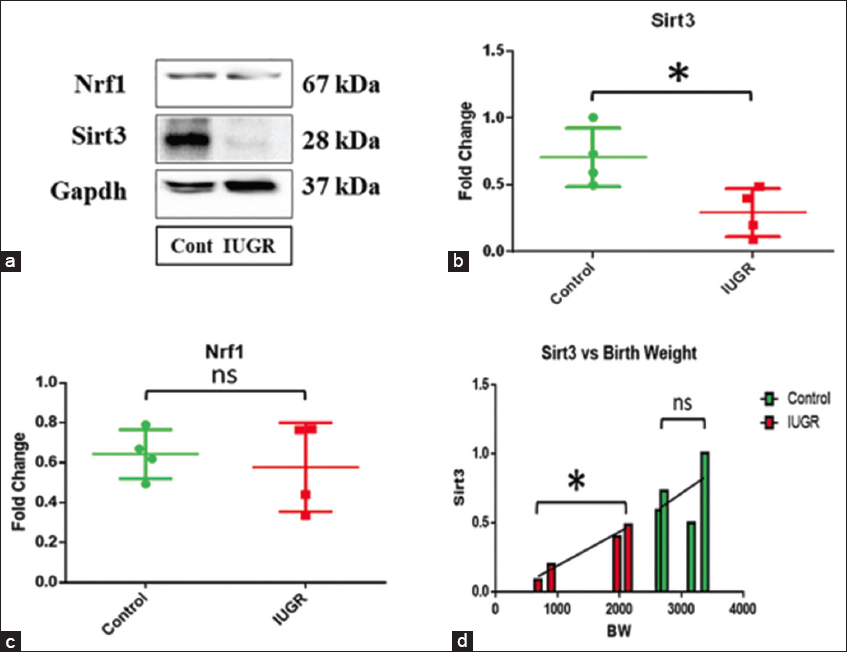
- (a) Western blot analysis of Sirt3, Nrf1, and Gapdh as endogenous control. (b) A significant decrease in Sirtuin-3 (Sirt3) protein expression was observed in the intrauterine growth restriction (IUGR) villus (P = 0.027). (c) No significant change in Nrf1 expression was found in IUGR. (d) A significant positive correlation of Sirt3 expression with birth weight was observed in IUGR (P = 0.013), but no significant correlation was established in controls (0.509)
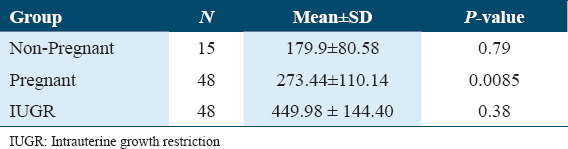
Concentration of 8-OH-dG
The lowest quantities of 8-OH-dG were found in samples from group non-pregnant, which had a mean value of 179.97 mmol/mol dG. This group was followed by group pregnant (mean 273.44 mmol/mol dG) and group IUGR (mean 449.98 mmol/mol dG). As a result, as compared to group NP, the mean values of 8-OH-dG concentrations in groups PE and PE-IUGR were found to be elevated by 52% and 138%, respectively. There were statistically significant differences in 8-OH-dG levels between non-pregnant and IUGR but not between non-pregnant and pregnant or pregnant and IUGR [Table 2]. Normal placental DNA hydrolysate chromatograms are shown in Figures 4a and b. The UV absorbance peaks of deoxycytosine (dC), deoxyguanosine (dG), deoxythymidine (dT), and deoxyadenosine (DA) are shown in Figure 4a. Since the minor peaks’ retention periods approximate those of RNA nucleoside standards, they most likely come from residual RNA contained in the DNA isolates. The matching electrochemical 8-OH-dG chromatogram is displayed in Figure 4b. There is also a chromatogram of an 8-OH-dG standard run. After several strong initial signals, 8-OH-dG elutes at around 9.8 min as a peak that is clearly distinguished from nearby signals.
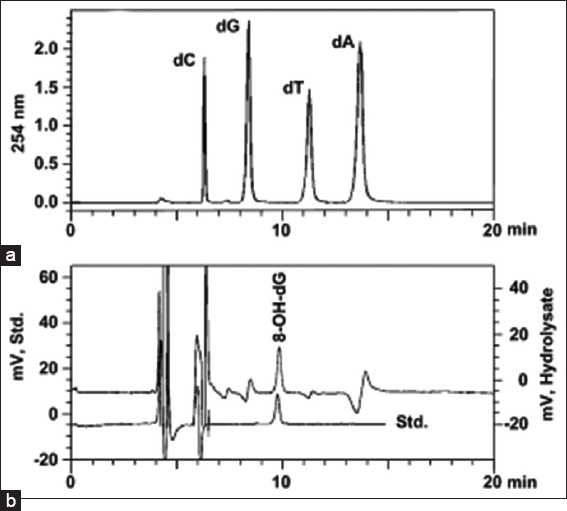
- Ultraviolet and electrochemical chromatograms of a DNA hydrolysate. The nucleosides deoxycytosine (6.22 min), deoxyguanosine (8.34 min), deoxythymidine (11.24 min), and deoxyadenosine (13.66 min) were monitored at 254 nm. (a) 8-hydroxy-20-deoxyguanosine (8-OH-dG) (9.87 min) using electrochemical detection. (b) during the same chromatographic run. Included is a chromatogram with 2 pmol of the 8-OH-dG standard (std: 9.78 min) dissolved in 20 μL of distilled water.
Discussion
This is the first investigation of the mtDNA content of maternal blood from normal and IUGR pregnancies, as far as we are aware. We assessed mtDNA consistency in physiological contexts, including healthy, non-pregnant women, women experiencing normal pregnancy at various gestational stages, and patients with IUGR. According to our findings, the amount of mtDNA in the blood of pregnant women in the first, second, and third trimesters is significantly and progressively lower than that of non-pregnant women.
It is interesting to note that mitochondria react to changes in the physiological environment of the cell and play a crucial role in the upkeep of cellular energy sources by mediating a variety of cellular signaling pathways.[27] Therefore, changes in stimuli like energy needs or caloric intake, including the molecular pathways of fetal and maternal tissues, may be reflected in the maternal decrease in mtDNA content in relation to gestation. In addition, we present findings in this study regarding a highly significant rise in the amount of mtDNA found in the blood of women carrying IUGR fetuses when compared to healthy pregnancies at a comparable gestational age. Abnormal fetomaternal cell flow is a hallmark of a number of placental diseases, including IUGR.[28] Recently, an increase in mtDNA content in the IUGR placenta was demonstrated, indicating that an increase in the number of mtDNA copies in maternal blood may be caused by an increase in the trophoblast cell releasing rate in the maternal circulation. There is an inverse relationship between mtDNA content in IUGR placenta sample oxygen levels in the umbilical vein.[29] Our findings indicate that the increase in mtDNA content could be an adaptive mechanism for the dysregulation that occurs in the altered placenta of IUGR fetuses.
The control of mitochondrial function is crucial for overall physiological adaptability when oxygen becomes scarce. Therefore, we could speculate that in IUGR pregnancies, the rise in mtDNA content may be a defense against hypoxia. Increased oxidative stress, a potent inducer of both apoptotic and necrotic alterations, is strongly correlated with IUGR. ROS activation has been found to harm proteins, lipids, and DNA in the IUGR placenta. In addition, ROS may alter the amount of mtDNA copies and the bulk of the mitochondria during mitochondria-to-nucleus signaling. The feedback reaction that corrects for defective mitochondria with a compromised respiratory chain or altered mtDNA, brought on by increasing levels of ROS, is thought to be the source of the rise in the number of mtDNA copies.[30]
According to our study, IUGR pregnancies had greater levels of oxidative stress markers than normal pregnancies did. This outcome agreed with that of Biri et al. These are in agreement with the finding that oxidative stress increases during pregnancy.[31,32] According to Little and Gladen,[33] this oxidative stress can be similar to or worse during the second and third trimesters of pregnancy. Due to the low quantity of syncytiotrophoblasts, placental tissues have a modest level of antioxidant activity, including SOD, CAT, and GPx, making the tissue susceptible to OS. Ismail et al. reported an increase in the level of oxidative stress markers and antioxidant activity in myocardial infections which was coherent with our findings.[34] Scholl and Stein[35] hypothesized that early in the third trimester of pregnancy, women with a poor pregnancy outcome (IUGR at the 10th percentile) have higher oxidative damage to their DNA.
The profiles of oxidative DNA adducts in human placentas have only been reported in a few studies. A higher concentration of 8-OH-dG was found in IUGR pregnancies. The mean concentration was higher than what Daube et al.[36] discovered in the placentas of non-smoking mothers, who found mean levels of about 10/106 dG. High inter-individual differences in 8-OH-dG concentration were not seen in our investigation, which is consistent with the findings of these authors.[35] The relatively high mean level of 8-OH-dG determined in this study in group NP is mostly the result of a single high concentration of 1413.36 mol 8-OH-dG/mol dG discovered in the placental sample of one person. Serum 8-OHdG levels were significantly (P < 0.05) high among healthy offspring of diabetic parents in comparison to healthy offspring of non-diabetic parents.[37]
The number of copies of mtDNA in maternal blood significantly increased, according to a study by Williams et al.[38], and this was associated with a higher risk of placental abruption. Furthermore, a prior study found a correlation between higher levels of mitochondrial DNA and a 24% rise in NRF1 gene expression, but no discernible change was seen,[39] indicating that mitochondrial biogenesis may not be the cause of the increase in mtDNA copy number. In this investigation, we discovered that the amount of mitochondrial Sirt3 protein decreased dramatically, whereas Nrf1 protein expression was not statistically different between control and IUGR samples. Sirt3 is in charge of controlling mitochondrial homeostasis by eliminating damaged mitochondria. Sirt3 is initially translated as a 44 kDa inactive protein, but once 142 amino acids are removed, it becomes an active 28 kDa protein that functions as a deacetylase for a number of mitochondrial maintenance proteins.[40] In IUGR placentas, the 44 kDa Sirt3 protein is increased.[41] However, in this work, we found a considerable downregulation of 28 kDa Sirt3, which may indicate that an active Sirt3 isoform is not present in the IUGR placenta. The buildup of faulty mitochondria in the IUGR placental villus is further suggested by this, which raises the possibility that the rise in mtDNA content is not the result of enhanced mitochondrial biogenesis.
Conclusion
IUGR is a severe and quite common complication that arises during pregnancy and is often confused with small for gestational age (SGA) due to the growth retardation of the fetus being similar in both conditions. Contrary to IUGR newborns, SGA infants do not have perinatal or postnatal problems. We sought to demonstrate a link in the current study between altered mitochondrial function and the pathophysiology of growth retardation related to IUGR. We noticed that maternal blood had less mtDNA and less Sirt3 expression. In addition, it demonstrates a notable rise in the amount of circulating mtDNA in pregnancies complicated by IUGR. We propose a connection between mitochondrial function and fetal development because an increase in mtDNA content is a sign of mitochondrial functional impairment. These results imply that severe fetal growth limits and poor perinatal outcomes, as found in IUGR pregnancies, might be linked to mitochondrial functional abnormalities. However, our study has its own limitations, such as a small sample size. To further understand the mitochondrial functional impairment in IUGR and develop an intervention approach to enhance the functions in growth-restricted pregnancies, more research must be done. Furthermore, prospective studies are required to assess the function of mitochondria in IUGR pathophysiology since maternal mtDNA content may aid in the early diagnosis of the condition and the creation of effective monitoring and treatment strategies.
Author’s Declaration Statements
Ethical approval and patient consent
The study was approved by the institutional ethical clearance committee for human research (ECR/262/Inst/UP/2013/RR-16). Written informed consent was obtained from all the participants.
Availability of data
All relevant data related to this manuscript are within this paper.
Competing interest
All the authors declared that there was no conflict of interest. All authors declared that the work is original and does not infringe copyright or other parties’ property rights. All authors have read and approved this submission and have given appropriate credit to everyone who participated in this work.
Funding statement
None.
Author’s contribution
Dr. S. P. Jaiswar supervised the work and writing of the manuscript. Apurva Singh performed the work, analyzed the data, and wrote the manuscript. Dr. Apala Priyadarshini revised the manuscript writing. Dr. Sujata Deo provided the critical feedback.
Acknowledgment
The authors would like to thank the Vice Chancellor, KGMU, and Director, CSIR-IITR, Lucknow, for their keen interest in the study.
References
- Intrauterine growth restriction:Implications for placental metabolism and transport. A review. Placenta. 2009;30(Suppl A):S77-82.
- [Google Scholar]
- Intrauterine growth restriction - part 1. J Matern Fetal Neonatal Med. 2016;29:3977-87.
- [Google Scholar]
- Intrauterine growth retardation--small events, big consequences. Ital J Pediatr. 2011;37:41.
- [Google Scholar]
- Mitochondrial toxicity in human pregnancy:An update on clinical and experimental approaches in the last 10 years. Int J Environ Res Public Health. 2014;11:9897-918.
- [Google Scholar]
- Oxidative stress, intrauterine growth restriction, and developmental programming of Type 2 diabetes. Physiology (Bethesda). 2018;33:348-59.
- [Google Scholar]
- The role of mitochondria from mature oocyte to viable blastocyst. Obstet Gynecol Int. 2013;2013:183024.
- [Google Scholar]
- Diet, energy metabolism and mitochondrial biogenesis. Curr Opin Clin Nutr Metab Care. 2007;10:679-87.
- [Google Scholar]
- Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314-7.
- [Google Scholar]
- Transient hypoxia stimulates mitochondrial biogenesis in brain subcortex by a neuronal nitric oxide synthase-dependent mechanism. J Neurosci. 2008;28:2015-24.
- [Google Scholar]
- Mitochondrial biogenesis and mitochondrial DNA maintenance of mammalian cells under oxidative stress. Int J Biochem Cell Biol. 2005;37:822-34.
- [Google Scholar]
- Misreading of DNA templates containing 8-hydroxydeoxyguanosine at the modified base and at adjacent residues. Nature. 1987;32:77-9.
- [Google Scholar]
- Placental stress and pre-eclampsia:A revised view. Placenta. 2009;30(Suppl A):S38-42.
- [Google Scholar]
- Neutrophil activation in preeclampsia and isolated intrauterine growth restriction. Am J Obstet Gynecol. 2000;183:1558-63.
- [Google Scholar]
- Emerging role of myeloperoxidase and oxidant stress markers in cardiovascular risk assessment. Curr Opin Lipidol. 2003;14:353-9.
- [Google Scholar]
- Serum myeloperoxidase levels independently predict endothelial dysfunction in humans. Circulation. 2004;1110:1134-9.
- [Google Scholar]
- Myeloperoxidase accumulates at the neutrophil surface and enhances cell metabolism and oxidant release during pregnancy. Eur J Immunol. 2006;36:1619-28.
- [Google Scholar]
- Increased myeloperoxidase in the placenta and circulation of women with preeclampsia. Hypertension. 2008;52:387-93.
- [Google Scholar]
- A new spectrophotometric assay method of xanthine oxidase in crude tissue homogenate. Anal Biochem. 1974;62:426-35.
- [Google Scholar]
- Activities of total, cytoplasmic, and mitochondrial superoxide dismutase enzyme in sera and pleural fluids from patients with lung cancer. J Clin Lab Anal. 1996;10:17-20.
- [Google Scholar]
- Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158-69.
- [Google Scholar]
- Acute effects of smoking of cigarettes with different tar content on plasma oxidant/antioxidant status. Inhal Toxicol. 2000;12:641-7.
- [Google Scholar]
- Regulation of placental efficiency for nutrient transport by imprinted genes. Placenta. 2006;27(Suppl A):S98-102.
- [Google Scholar]
- Higher mitochondrial DNA content in human IUGR placenta. Placenta. 2008;29:1029-33.
- [Google Scholar]
- The regulation, control, and consequences of mitochondrial oxygen utilization and disposition in the heart and skeletal muscle during hypoxia. Antioxid Redox Signal. 2007;9:1353-61.
- [Google Scholar]
- Role of Oxidative Stress in Intrauterine Growth Restriction. Gynecol Obstet Invest. 2007;64:187-92.
- [Google Scholar]
- Levels of lipid peroxides in uncomplicated pregnancy:A review of the literature. Reprod Toxicol. 1999;13:347-52.
- [Google Scholar]
- Oxidative stress markers and antioxidant activity in patients admitted to Intensive Care Unit with acute myocardial infarction. Int J Health Sci (Qassim). 2018;12:14-9.
- [Google Scholar]
- DNA adducts in human placenta in relation to tobacco smoke exposure and plasma antioxidant status. J Cancer Res Clin Oncol. 1997;123:141-51.
- [Google Scholar]
- Comparative study of serum 8-hydroxydeoxy-guanosine levels among healthy offspring of diabetic and non-diabetic parents. Int J Health Sci (Qassim). 2017;11:33-7.
- [Google Scholar]
- Maternal blood mitochondrial DNA copy number and placental abruption risk:Results from a preliminary study. Int J Mol Epidemiol Genet. 2013;4:120-7.
- [Google Scholar]
- Placental mitochondrial content and function in intrauterine growth restriction and preeclampsia. Am J Physiol Metab. 2014;306:E404-13.
- [Google Scholar]
- SIRT3 is a stress-responsive deacetylase in cardiomyocytes that protects cells from stress-mediated cell death by deacetylation of Ku70. Mol Cell Biol. 2008;28:6384-401.
- [Google Scholar]
- Mitochondrial implications in human pregnancies with intrauterine growth restriction and associated cardiac remodelling. J Cell Mol Med. 2019;23:3962-73.
- [Google Scholar]







