Translate this page into:
Measuring cognitive performance in older adults through completion time and accuracy in brain games
*Corresponding author: Maria Justine, Centre for Physiotherapy Studies, Faculty of Health Sciences, Universiti Teknologi MARA, Selangor, Malaysia. maria205@uitm.edu.my
-
Received: ,
Accepted: ,
How to cite this article: Sufian L, Arman LN, Siriphorn A, Suriyaamarit D, Justine M. Measuring cognitive performance in older adults through completion time and accuracy in brain games. Inter J Health Sci. 2025;19:20-8. doi: 10.25259/OA03_8810
Abstract
Objectives
This study aimed to compare the cognitive performance of brain games in older adults with different sociodemographic, cognitive function, and mental health and determine their associated factors.
Methods
This observational study recruited 101 participants (mean age = 72.3 ± 6.83 years) from institutions for older adults. All participants provided data on sociodemographic, cognitive function (Montreal Cognitive Assessment [MoCA]), anxiety (Beck Anxiety Inventory), and depression levels (Beck Depression Inventory). The Jenga, as a brain game, was used to indicate cognitive performance by measuring the completion time and accuracy in building a 10-level Jenga tower. Data were analyzed using the Chi-square test and binary logistic regression.
Results
Completion time and accuracy of Jenga games were significantly different among age groups, education levels, and cognitive function (All P < 0.05) but not in gender, marital status, anxiety, and depression levels (All P > 0.05). Age is significantly associated with both time and accuracy, with older individuals (≥75) showing a greater likelihood of taking longer time and lower accuracy to complete the task, while a higher education level is associated with significantly higher odds of shorter completion time and higher accuracy; normal cognitive function (MoCA ≥26) is associated with both shorter completion time and better accuracy in the task (All P < 0.05).
Conclusion
The findings show that age, education, and cognitive function may affect completion time and accuracy, suggesting the potential for Jenga to be used as an assessment tool or cognitive training. However, the generalization of this study may be limited to the institutionalized population.
Keywords
Aged
Brain games
Cognitive function
Cognitive training
Dementia
Older people
INTRODUCTION
Good cognitive ability is essential for older adults for independence in activities of daily living (ADL), social relationships, physical well-being, and overall quality of life. However, a systematic review reported a globally high incidence of cognitive impairment among the older population, ranging from 22 to 76.8/1000 person-years.[1] As people age, the brain may gradually undergo atrophy, especially in the hippocampus and prefrontal cortex, affecting executive function, memory, and information processing.[2,3] Even mental disorders such as anxiety and depression have been shown in many studies to affect cognitive performance in older adults.[4-8] Ultimately, older adults with mental disorders may engage in less physical activity,[9] disrupting antioxidant defenses and accumulating free radicals, leading to oxidative damage to the brain.[10,11]
A previous study showed that a high level of participation in leisure activities was linked to a 41% reduction in the risk of cognitive impairment compared to low-level engagement after controlling for age, sex, education, and other confounders.[12] Leisure activities such as brain games are believed to stimulate brain functions and effectively prevent dementia,[13] while brain games have potential benefits for sustained attention and mental engagement.[14] This is further supported by a systematic review and meta-analysis of 15 trials among 759 older adults that were noted to enhance several brain functions such as processing speed, selective attention, and short-term memory.[15] Jenga, for instance, which is a type of brain game, promotes critical thinking, speed, and accuracy,[16] while also enhancing decision-making, problem-solving, and multitasking skills.[17] In addition, brain games may provide a higher satisfaction level and encourage social interaction among players.[18] However, Murman also argued that the ability to continuously perform unfamiliar, complex, and timed activities tends to decline, particularly in older adults of advanced age.[17] Hence, exploring the factors associated with time and accuracy in completing brain games can provide insight into the evaluation of cognitive performance in older adults. There have been limited studies on how the cognitive performance of older adults is affected by playing brain games. For example, one study examined how eye-hand coordination and anxiety are linked in nursing professionals.[19] Another study discovered that brain exercises involving Jenga games improved abilities as measured by the Montreal Cognitive Assessment (MoCA).[18] A study has also explored the reaction time and accuracy in adults using brain games such as Whack-a-mole and Hit-the-ball.[20] Hence, investigating time and accuracy with Jenga games could offer insights into the clinical application of brain games such as detecting cognitive changes in older adults.
Understanding the role of brain games in cognitive functions can be explained by the cognitive reserve theory (CRT), which proposes that the ability to maintain cognitive functions relatively well at a given level of pathology can be influenced by early experiences such as the level of education that could protect against cognitive decline.[21] Accordingly, the CRT advocates that lifestyle factors result in individual differences in the flexibility and adaptability of brain networks that may allow some people to cope better than others with cognitive changes.[22] The CRT can be used to hypothesize that brain functions can be influenced by several individuals’ characteristics. Cognitive reserve may be essential for the enhancement of executive function by challenging cognitive skills.[23] A recent study has shown that engaging in novel, active, and productive intellectual tasks could mitigate cognitive decline in older adults’ cognitive reserve.[24] Therefore, the CRT can be used to guide further studies in elucidating the roles of brain games, such as Jenga, in enhancing the executive function of older people. So far, there are limited studies supporting how characteristics such as sociodemographics, cognitive function, and mental health impact intellectual or challenging tasks at a later age, such as playing Jenga games that demand efficiency.
To fill the gap in the current literature, we proposed the use of the CRT to develop the theoretical model of this study [Figure 1] that explains the interrelationship of study variables influencing brain performance in older adults while playing brain games. We hypothesize that brain performance while playing games that demand accuracy and timely completion can be distinguished and influenced by several factors related to lifestyles or sociodemographic factors, the current level of cognitive and mental functions. In the model, sociodemographic factors (such as education level, age, marital status), mental health, and cognitive function may impact brain performance while performing challenging tasks.
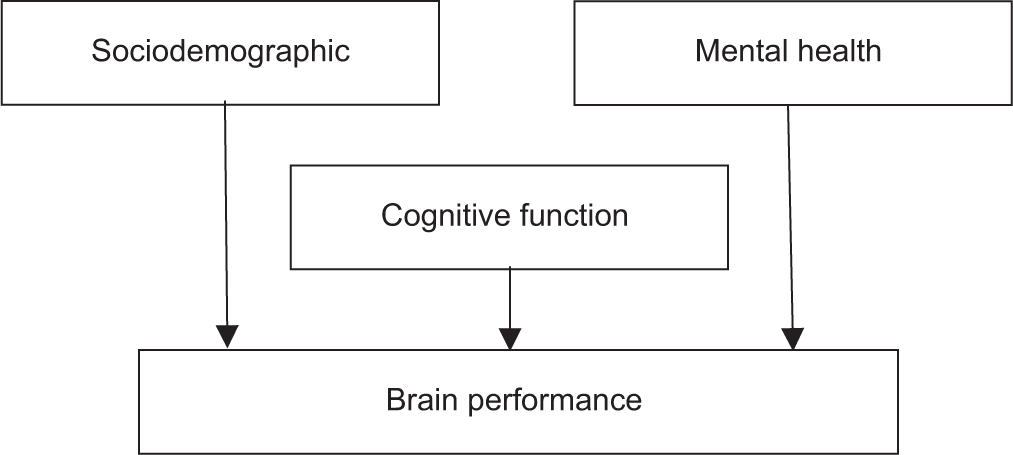
- The theoretical model of the influence of cognitive reserve (sociodemographic, cognitive function, and mental health) on brain performance.
Ultimately, this study aimed to compare older adults with varying sociodemographic, cognitive function, and mental health (anxiety and depression levels) regarding the time and accuracy of completing the Jenga games. This study also aimed to determine whether sociodemographic factors, cognitive functions, and mental health (anxiety and depression levels) influence the time and accuracy that represent cognitive functions in completing the Jenga games. Using logistic regression analysis, we hypothesized that cognitive function, mental health, and sociodemographic factors influence game performance among older adults playing Jenga. Participants with higher cognitive function, lower anxiety and depression levels, and greater educational attainment are expected to perform better, while older age may reduce performance due to age-related cognitive decline.
MATERIALS AND METHODS
Study design
This observational study was conducted between October 2022 and April 2023 at 11 institutions for older adults. The university’s Research Ethics Committee approved the study protocol (FERC/FSK/MR/2021/0186). All participants provided written informed consent before data collection.
The sample size was determined using G*Power software. Given the focus on categorical comparisons, Pearson Chi-square was selected to examine comparisons between categorical variables. For this study, an effect size (w) of 0.25, an alpha level of 0.05, and a power of 0.85 were applied, resulting in a required sample size of approximately 105 participants. However, only 101 participants met the inclusion criteria.
All questionnaires used in this study were administered through one-to-one interviews conducted by the researchers (first and second authors), who are trained physiotherapists. This method ensured accurate data collection by allowing clarification of questions and minimizing potential misunderstandings among participants. Each participant was guided through the questionnaires in a quiet and comfortable environment to reduce distractions and promote honest responses.
Study participants
The participants included in the study were (1) aged 60 years and above, (2) able to walk and perform basic ADL, and (3) able to follow and understand verbal instructions. Participants who were excluded from the study presented with (1) Mini-Mental State Examination (MMSE) score below 18; (2) poor vision, color-blindness, and hearing problems; (3) underlying conditions such as neurological conditions (e.g., epilepsy, stroke, Parkinson’s disease); and (4) major psychiatric disorders (e.g., major depression, schizophrenia).
Sociodemographic data
The sociodemographic data gathered included age, gender, education level, and marital status. The participants’ medical history, such as underlying diseases, vision, and hearing problems, was also documented to meet the criteria for inclusion in the study.
Cognitive function
The MoCA was used to assess the cognitive function of the participants. According to Hobson, MoCA assesses a more challenging task and, hence, maybe more accurate in detecting mild cognitive impairment.[25] The MoCA consists of 30 questions on multiple cognitive domains such as attention and concentration, executive function, memory, language, visuo-constructional skills, conceptual thinking, calculation, and orientation. The score indicators were normal (26 and above), mild cognitive impairment (MCI) (19–25), and Alzheimer’s disease (11–21). The sensitivity and specificity of MoCA in detecting MCI among Malaysians were 68.2% and 61.3%, respectively.[26]
Anxiety and depression levels
Anxiety levels were measured with the Beck Anxiety Inventory (BAI). The BAI consists of 21 questions to indicate the extent to which participants had been bothered by anxiety symptoms for the past month. The score was rated from 0 to 3 (0: Not anxious at all, 1: Mildly but did not bother much, 2: Moderately but was not pleasant at times, and 3: Severely bothered a lot), and the total score for the 21 questions was calculated (0–21: Low anxiety; 22–35: Moderate anxiety; ≥36: Potentially concerning anxiety level). The BAI has high test-retest correlation and internal consistency.[27] It also shows excellent concurrent validity with correlations between 0.78 and 0.81 on the Hamilton Anxiety Scale (HAM-A), Symptom Checklist-90 (SCL-90) anxiety subscale, and State-Trait Anxiety Inventory.[27]
Depression was measured using the Beck Depression Inventory (BDI-II). The BDI-II consists of 21 items and is a self-rated scale that evaluates symptoms of depression, including mood, pessimism, and a sense of failure.[28] The BDI-II scores are normal (0–9), mild to moderate (10–18), moderate to severe (19–29), and severe depression (30–63). The BDI-II has high validity between the questionnaire and other measurement tools for depression, such as the Minnesota Multiphasic Personality Inventory-D (r = 0.77). The BDI-II was also positively correlated with the Hamilton Depression Rating Scale (r = 0.71) with a 1-week test-retest reliability (r = 0.93).[28]
The Jenga games procedure
In this study, colored Jenga was used to test the time and accuracy in completing the Jenga tower. Jenga consisted of 54 wooden blocks that comprised six colors: red, pink, yellow, green, blue, and purple. Each color is represented by nine wooden blocks [Figure 2]. The following are the procedures for completing the Jenga games:
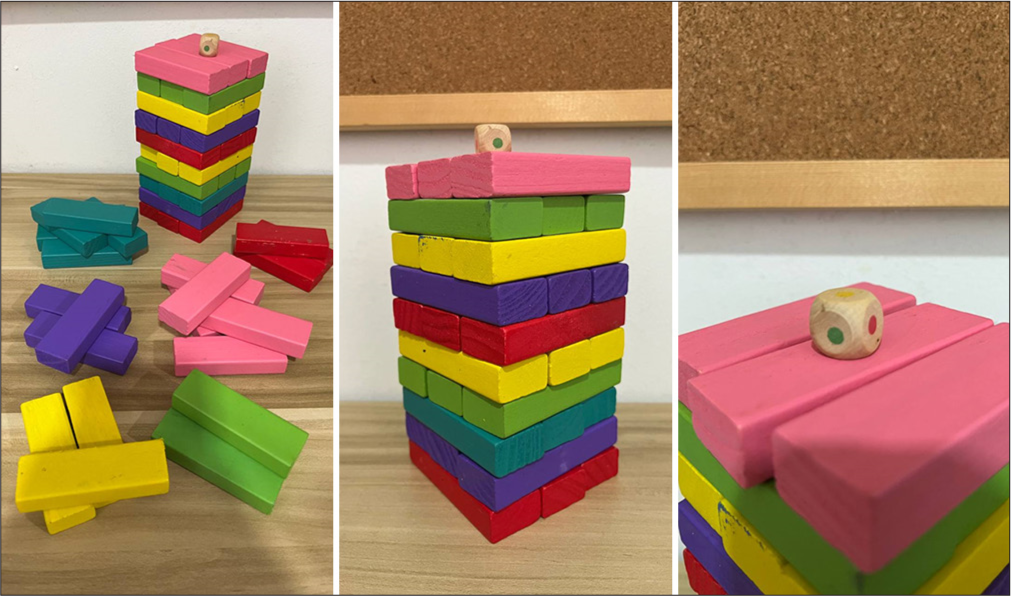
- The Jenga.
The participants were briefly explained about the procedure of the Jenga games and demonstrated the steps to play. The participants needed to build a tower of 10 levels, each consisting of three blocks of the same color. A colored dice determines the color for each level of the Jenga Tower. The dice consisted of six colors, with each side having the same color as that of the wooden blocks. The time began when the participants started to grip the first block and stopped when they completed three rows of Jenga blocks to form one level of the Jenga tower. The sum of the time completed in seconds and the accuracy of each level of the challenge were recorded [Table 1].
To begin the challenge, the wooden blocks were placed on the table and grouped according to the same color in front of the participants.
The participants then threw dice to determine the color to build each level of the Jenga tower. The participants were required to stack in three rows of the same-colored blocks to form one level of the Jenga tower.
To form the next level, the participants needed to arrange the block transversely, and this procedure was repeated until a Jenga tower with ten levels was built.
The time the participants took to form the Jenga tower was recorded in seconds, and its accuracy was calculated when the participants chose the color of the blocks the same as the dice to form one level of the Jenga tower.
| Jenga level | 1st | 2nd | 3rd | 4th | 5th | 6th | 7th | 8th | 9th | 10th | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Time (seconds) | 10 | 8 | 6 | 7 | 8 | 9 | 7 | 8 | 11 | 8 | 82 |
| Accuracy | ✔ | ✔ | ✘ | ✔ | ✔ | ✔ | ✔ | ✔ | ✘ | ✔ | 8/10 |
✔: Accurate, ✘: Not accurate
Statistical analysis
Data were analyzed using SPSS version 30.0, with all statistical tests set at P < 0.05 as a significant level. The Pearson Chi-square tests were used to compare differences in time and accuracy of completing Jenga games among the variables of interest. The 50th percentile of mean scores was used to categorize completion time and accuracy of the Jenga games, as well as the anxiety and depression levels into two categories. The binary logistic regression analyses for dichotomous data (shorter vs. longer time; higher vs. lower accuracy) were used to determine the influence of the variables of interest on the time and accuracy of Jenga games.
RESULTS
Participants’ characteristic
Table 2 presents the characteristics of the study participants (n = 101, mean age = 72.30 ± 6.83 years). The mean time to complete the Jenga tower was 79.42 ± 24.06 s, and the majority presented with a longer time (54.5%). In terms of accuracy, most participants scored perfect accuracy (55.4%).
| Variables | Mean±SD (min-max) | Frequency (%) |
|---|---|---|
| Age (years) | 72.3±6.83 | |
| <75 | (60–89) | 61 (60.4) |
| ≥75 | 40 (39.6) | |
| Gender | ||
| Male | 45 (44.6) | |
| Female | 56 (55.4) | |
| Education levels | ||
| Primary | 5 (5.0) | |
| Secondary | 47 (46.5) | |
| Higher | 49 (48.5) | |
| Marital status | ||
| Married | 75 (74.3) | |
| Single | 14 (13.9) | |
| Divorced/Widowed | 12 (11.9) | |
| Cognitive function (MoCA) | 25.61±2.32 | |
| Normal (≥26) | (20–30) | 44 (43.6) |
| MCI (<26) | 57 (56.4) | |
| Anxiety levels | 1.28±1.73 | |
| Very low (0–2) | (0–6) | 75 (74.3) |
| Low (3–6) | 26 (25.7) | |
| Depression levels | 5.89±3.77 | |
| Low (0–9) | (2–16) | 79 (78.2) |
| Mild depression (10–18) | 22 (21.8) | |
| Time to complete (sec) | 79.42±24.06 | |
| Faster (<68) | (46–154) | 46 (45.5) |
| Slower (≥68) | 55 (54.5) | |
| Accuracy | 9.04±1.19 | |
| Low (<10) | (6–10) | 45 (44.6) |
| High (10) | 56 (55.4) |
SD: Standard deviation, MoCA: Montreal cognitive assessment, MCI: Mild cognitive impairment
Comparison of time and accuracy in completing Jenga games among older adults with different characteristics
As shown in Table 3, the analysis demonstrated that age has a significant relationship with both time and accuracy, in which participants aged 75 years or older were more likely to have slower completion times (P = 0.010) and lower accuracy levels (P < 0.001). Education level also showed a significant relationship with cognitive performance, with participants who had higher education achieving faster completion time (P = 0.004) and higher accuracy (P < 0.001). Similarly, participants with normal cognitive function (MoCA ≥26) were faster in completing the game (P = 0.009) and displayed higher accuracy (P < 0.001). In contrast, the analyses did not find significant relationships by gender, marital status, anxiety, or depression levels with time and accuracy in Jenga performance (All P > 0.05).
| Characteristics | Time | Accuracy | ||||
|---|---|---|---|---|---|---|
| Faster | Slower | P-value | Low | High | P-value | |
| Age (years) | ||||||
| <75 | 21 | 39 | 0.010** | 16 | 44 | <0.001** |
| ≥75 | 5 | 36 | 29 | 12 | ||
| Gender | ||||||
| Male | 13 | 32 | 0.517 | 18 | 27 | 0.409 |
| Female | 13 | 43 | 27 | 29 | ||
| Education levels | ||||||
| Higher | 19 | 30 | 0.004** | 13 | 36 | <0.001** |
| Secondary/Primary | 7 | 45 | 32 | 20 | ||
| Marital status | ||||||
| Married | 20 | 55 | 0.718 | 31 | 44 | 0.269 |
| Single/Divorced/Widowed | 6 | 20 | 14 | 12 | ||
| Cognitive function (MoCA) | ||||||
| Normal (≥26) | 17 | 27 | 0.009** | 10 | 34 | <0.001** |
| MCI (<26) | 9 | 48 | 35 | 22 | ||
| Anxiety levels | ||||||
| Very low (0–2) | 21 | 54 | 0.378 | 32 | 43 | 0.517 |
| Low (3–6) | 5 | 21 | 13 | 13 | ||
| Depression levels | ||||||
| Low (0–9) | 23 | 56 | 0.142 | 33 | 46 | 0.286 |
| Mild depression (10–18) | 3 | 19 | 12 | 10 | ||
Pearson Chi-square test significant at *P<0.05, **P<0.01. MoCA: Montreal cognitive assessment, MCI: Mild cognitive impairment
Binary logistic regression analysis of factors associated with time and accuracy in Jenga games
Table 4 shows the results of the binary logistic regression with only significant variables from the Pearson Chi-square tests included in the analysis. Age has a significant association with both time and accuracy, with older individuals (≥75) showing higher odds of taking longer to complete the task and differing performance in accuracy, while a higher education level is associated with significantly higher odds of faster completion time and better accuracy. Normal cognitive function (MoCA ≥26) is associated with both faster completion time and better accuracy in the Jenga game.
| Predictors | Time | Accuracy | ||||||
|---|---|---|---|---|---|---|---|---|
| B | P-value | OR | 95% CI | B | P-value | OR | 95% CI | |
| Age (years) | ||||||||
| <75 | 0.62 | 0.06 | 1.86 | 1.32, 11.36 | −1.89 | <0.001** | 0.15 | 0.06, 0.36 |
| ≥75 | 1.35 | 0.04* | 3.88 | 1.01 | <0.001** | 2.75 | ||
| Education | ||||||||
| Higher | 1.40 | 0.05* | 4.07 | 1.52, 10.87 | −1.49 | <0.001** | 0.23 | 0.10, 0.53 |
| Lower | 0.46 | 0.12 | 1.58 | 1.02 | 0.002** | 2.77 | ||
| Cognitive function | ||||||||
| Normal (≥26) | 1.21 | 0.011* | 3.36 | 1.32, 8.55 | −1.69 | <0.001** | 0.18 | 0.08, 0.04 |
| MCI (<26) | 0.46 | 0.135 | 1.59 | 1.22 | 3.40 | |||
Regression model is significant at *P<0.05 and **P<0.01. MCI: Mild cognitive impairment, OR: Odd ratio, CI: Confidence interval
DISCUSSION
The current study examined how different sociodemographic factors, cognitive function, and mental health differed in Jenga performance (time and accuracy) and whether these factors can predict Jenga performance. The findings of this study align with previous research, highlighting the importance of cognitive function, mental health, and sociodemographic factors in predicting task performance among older adults. Consistent with the CRT, participants with higher cognitive function scores performed better in terms of task completion time and accuracy, supporting the significance of cognitive reserve with enhanced executive function and problem-solving skills.[15] In addition, the negative impact of anxiety and depression on performance supports prior research, indicating that emotional distress hinders cognitive processing and decision-making.[22] Moreover, the observed influence of age and education level corresponds with studies emphasizing the role of lifelong learning and cognitive engagement in maintaining cognitive reserve.[21] Older participants showed reduced performance, consistent with age-related declines in cognitive flexibility and working memory documented in a prior study.[24] Similarly, participants with higher educational attainment performed better, supporting evidence that education promotes resilience against cognitive decline through enhanced cognitive reserve.
Specifically, this current study found that older adults aged ≥75 years took more time to complete and were less accurate in the Jenga performance. It has been suggested that as individuals age, their fluid cognitive functions, such as problem-solving capabilities, processing speed, and reasoning skills, tend to decline.[29] The current finding is also consistent with an earlier study that younger participants exhibited higher accuracy and speed in completing the task, leading to more correct responses.[30] Playing brain games such as Jenga requires cognitive skills, such as strategic thinking and precision planning, because players must carefully decide which block to remove or place, understand the spatial relationships between blocks, and maintain stability while anticipating each move’s consequences, which requires planning several steps. Older adults may find brain games challenging as they need to control their focus and emotions to play calmly and carefully to prevent errors and make accurate or precise movements to prevent the Jenga Tower from falling.[31] These results suggest that older adults may be more adept at solving difficulties quickly by applying their critical thinking skills.
The present study also discovered a connection between age, time taken, and accuracy when building the Jenga tower, suggesting that age could impact reaction time and precision outcomes. This finding aligns with a previous report that revealed that older age is linked to diminished performance, including movements, prolonged processing responses, and reduced accuracy.[30]
Age was also a factor in predicting how well someone performs the Jenga games, supporting the belief that as people age, their cognitive abilities decrease, affecting how quickly and accurately they can complete cognitive tasks.[2] A previous study also reported that performance in all cognitive domains decreases with age; in particular, tests related to working memory, flexibility, and visuoconstructive abilities were influenced by age.[32] Accordingly, the association of age with Jenga performance may be attenuated by the effects of education, mainly in the visuoconstructive domain.[32] This finding enhances the understanding of age-related declines in task performance, highlighting the protective role of cognitive reserve acquired through lifelong education and engagement.[17] The current finding supports the importance of considering age differences when researching older adult populations. The time required to complete the task and the accuracy of the task performance are critical measures in older adults due to the decline in reaction time and precision, which are parts of the executive function.
The current study found that gender was not significantly different in brain games, though the majority of males performed better in completing the given task. A previous study found that males had significantly better scores than females in cognitive performance, including executive function, but not in memory and psychomotor speed.[33] Females have a higher risk of cognitive decline because, on average, females have lower education levels and a reduction in reproductive hormones such as estrogen, which serves as a protective factor of cognitive function.[33]
Regarding education, those with a higher level completed tasks faster and had higher accuracy in completing Jenga games, indicating that education is fundamental to mental function. This finding supports a previous argument that years of education have a significant positive association with cognitive function.[33,34] Furthermore, education level is vital in cognitive performance and task completion because it involves thinking and problem-solving. The interaction between education and cognitive status in influencing task performance could be due to the complexity of cognitive aging and suggests that interventions aimed at enhancing cognitive function in older adults should consider individual differences in educational background and cognitive baseline.[20] Moreover, there is a significant interaction between leisure activity and educational level, such that the beneficial effect of leisure activities on cognitive function was more significant in educated individuals than in their uneducated counterparts, and only those educated benefited from cognitive activities.[12]
In this study, married participants played the Jenga games in a shorter time and had higher accuracy than those who were single, divorced, or widowed, though the difference was not statistically significant. Marital status was screened because, according to one study, older adults who were single, widowed, or divorced had worse cognitive function than those who were married.[35] Marriage may enhance opportunities for greater cognitive stimulation as married individuals typically engage in more social interactions and committed partnerships, which offer fundamental protection against cognitive decline.[36]
Older adults who scored 26 or higher on the MoCA performed better in the Jenga games. These findings are consistent with a previous study that suggested individuals with moderate and moderate-to-severe cognitive impairment presented with slower reaction times and were less accurate in task completion than those with normal cognitive function.[20]
The participants with lower anxiety and depression scores took a shorter time and had higher accuracy in completing the Jenga games, but they were not significantly different. The non-significant findings could be attributed to the lack of symptoms of anxiety and depression among the participants as the majority scored in the lower ranges. Accordingly, older adults who experience anxiety symptoms often experience severe depression, but anxiety may not increase cognitive dysfunction and recognition tasks.[37] However, late-life depression is often accompanied by cognitive impairment,[38] which could be due to the interaction between neurobiological changes, neurotransmitter imbalances, and elevated levels of inflammatory markers. Late-life depression can also cause chronic stress and dysregulation of the hypothalamic-pituitary-adrenal axis, leading to increased levels of cortisol, which can damage brain regions, particularly the hippocampus, which is involved in cognitive functioning.[39] In terms of relationship, there was no significant correlation between the level of anxiety and time and accuracy in completing the Jenga games. The current finding is also consistent with a previous study that did not show a significant correlation in assessing the level of anxiety using Jenga games, which could be due to differences in the background of the study population.[19] Morimoto et al. found a correlation between depressive episodes and cognitive impairment but did not progress to dementia.[38] Mental and cognitive function must be evaluated in terms of time and accuracy, as older adults with mental disorders may have challenges in completing tasks efficiently, as the individual focuses more on the threat rather than completing the task.
It is important to note the limitations of this study, in which the participants were recruited from the institutions for older adults who may have psychosocial circumstances. Ultimately, these results cannot be applied to communities or broader populations.
CONCLUSION
The findings of this study reinforce the theoretical background of the study, suggesting that both cognitive and emotional factors, alongside demographic characteristics, play crucial roles in predicting performance on cognitively demanding tasks such as Jenga. In addition, this study has also contributed to a deeper understanding of the cognitive reserve and executive functions in older people in relation to brain games. Methodologically, Jenga may be a feasible tool for cognitive assessment for institutionalized older adults. This study reveals several factors that influence cognitive tasks and can implicate clinical practice to mitigate cognitive decline, creating personalized approaches that focus on an individual’s baseline education and cognitive ability.
It is important to note the limitations of this study, in which the participants were recruited from the institutions for older adults who may have psychosocial circumstances. Ultimately, these results cannot be applied to communities or broader populations. In addition, this study used only Jenga games which may limit generalization to other specific cognitive tasks. Future studies are recommended to adopt a longitudinal approach and consider conducting follow-up assessments to maintain regular contact with participants to track better outcomes, as well as the use of various brain games.
Acknowledgments
We would like to thank all the managers of the institutions where the data collection took place for the permission given and for facilitating the process.
Author’s contributions
LS:Data collection, wrote the original manuscript draft, and data management; LNA: Data collection, obtaining study resources, and data management; AS: Data analysis, reviewed and edited the manuscript; DS: Data analysis, reviewed and edited the manuscript; MJ: Principal investigator, data analysis, reviewed and edited the manuscript.
Ethical approval
The study design was approved by the UiTM Research Ethics Committee (FERC/FSK/MR/2021/0186).
Declaration of patient consent
Patient’s consent is not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
The data collection process was supported by the GERAN DANA UITM SELANGOR (DUCS Faculty: 600 UiTMSEL (PI) 5/4 (151/2022)).
Conflicts of interest
There are no conflicts of interest.
Availability of data and material
The datasets supporting this study’s findings will be available upon request from the corresponding author.
References
- Global cognitive impairment prevalence and incidence in community-dwelling older adults-a systematic review. Geriatrics. 2020;5:84.
- [CrossRef] [PubMed] [Google Scholar]
- Affective problems and decline in cognitive state in older adults: a systematic review and meta-analysis. Psychol Med. 2019;49:353-65.
- [CrossRef] [PubMed] [Google Scholar]
- Neurotransmitters-key factors in neurological and neurodegenerative disorders of the central nervous system. Int J Mol Sci. 2022;23:5954.
- [CrossRef] [PubMed] [Google Scholar]
- Neurotransmitter and behaviour, examining biological foundations of human behavior United States: IGI Global; 2020. p. :80-93. Available from: https://www.igi-global.com/chapter/neurotransmitter-and-behaviour/249989 [Last accessed on 2025 Mar 21]
- [CrossRef] [PubMed] [Google Scholar]
- Anxiety, depression and other factors associated with voice handicaps in active older people during the COVID-19 pandemic. J Voice 2023:S0892-1997(23)00167-4.
- [Google Scholar]
- Sleep quality, depression, and cognitive function in non-demented older adults. J Alzheimers Dis. 2020;76:1637-50.
- [CrossRef] [PubMed] [Google Scholar]
- Depression, anxiety, and apathy in mild cognitive impairment: current perspectives. Front Aging Neurosci. 2020;12:9.
- [CrossRef] [PubMed] [Google Scholar]
- Schizophrenia, bipolar and major depressive disorders: overview of clinical features, neurotransmitter alterations, pharmacological interventions, and impact of oxidative stress in the disease process. ACS Chem Neurosci. 2022;13:2784-802.
- [CrossRef] [PubMed] [Google Scholar]
- Consequences of physical inactivity in older adults: a systematic review of reviews and meta-analyses. Scand J Med Sci Sports. 2020;30:816-27.
- [CrossRef] [PubMed] [Google Scholar]
- Fatty acids, antioxidants and physical activity in brain ageing. Nutrients. 2017;9:1263.
- [CrossRef] [PubMed] [Google Scholar]
- Oxidative stress: role of physical exercise and antioxidant nutraceuticals in adulthood and aging. Oncotarget. 2018;9:17181.
- [CrossRef] [PubMed] [Google Scholar]
- Leisure activities, education, and cognitive impairment in chinese older adults: a population-based longitudinal study. Int Psychogeriatr. 2017;29:727-39.
- [CrossRef] [PubMed] [Google Scholar]
- Exploring the impact of puzzle games for the elderly from experiential learning. Think Skills Creat. 2022;45:101107.
- [CrossRef] [Google Scholar]
- Promoting cognitive brain health and sustained attention in adults and older adults through e-games. J Soc Stud Educ Res. 2024;15:1-22.
- [Google Scholar]
- Game-based brain training for improving cognitive function in community-dwelling older adults: a systematic review and meta-regression. Arch Gerontol Geriatr. 2021;92:104260.
- [CrossRef] [PubMed] [Google Scholar]
- Cooperative learning strategy in critical reading english text through team game tournament (tgt) and jenga. J English Pedagogy Linguist Lit Teach. 2021;9:44-61.
- [CrossRef] [Google Scholar]
- Effects of physical, virtual reality-based, and brain exercise on physical, cognition, and preference in older persons: a randomised controlled trial. Eur Rev Aging Phys Act. 2018;15:10.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluating effect of anxiety on eye-hand coordination using jenga game in female nursing professionals: an observational study. Int J Innov Sci Res Technol. 2020;5:550-5.
- [CrossRef] [Google Scholar]
- Evaluation of the reaction time and accuracy rate in normal subjects, mci, and dementia using serious games. Appl Sci. 2021;11:628.
- [CrossRef] [Google Scholar]
- Cognitive reserve hypothesis in frontotemporal dementia: A FDG-PET Study. Neuroimage Clin. 2021;29:102535.
- [CrossRef] [PubMed] [Google Scholar]
- How can cognitive reserve promote cognitive and neurobehavioral health? Arch Clin Neuropsychol. 2021;36:1291-5.
- [CrossRef] [PubMed] [Google Scholar]
- Evidence that ageing yields improvements as well as declines across attention and executive functions. Nat Hum Behav. 2022;6:97-110.
- [CrossRef] [PubMed] [Google Scholar]
- Which intellectual activities are related to cognitive reserve? introduction and testing a three-dimensional model. Psychol Res. 2024;88:1081-91.
- [CrossRef] [PubMed] [Google Scholar]
- The montreal cognitive assessment (MoCA) Occup Med. 2015;65:764-5.
- [CrossRef] [PubMed] [Google Scholar]
- Validation and cut-off scores of montreal cognitive assessment for elderly visually impaired. J Health Transl Med. 2022;25:140-4.
- [Google Scholar]
- Validity and reliability of the beck anxiety inventory (BAI) for family caregivers of children with cancer. Int J Environ Res Public Health. 2020;17:7765.
- [CrossRef] [PubMed] [Google Scholar]
- Theories of cognitive aging and work In: work across the lifespan. United States: Elsevier Academic Press; 2019. p. :17-45.
- [CrossRef] [PubMed] [Google Scholar]
- Age-related increases in reaction time result from slower preparation, not delayed initiation. J Neurophysiol. 2022;128:582-92.
- [CrossRef] [PubMed] [Google Scholar]
- Development of accounting jenga as a learning media to improve students motivation. J Pendidikan Akuntansi Indones. 2017;15:14815.
- [CrossRef] [Google Scholar]
- Cognitive vulnerability in aging may be modulated by education and reserve in healthy people. Front Aging Neurosci. 2017;9:340.
- [CrossRef] [PubMed] [Google Scholar]
- Gender Differences in cognitive function and its associated factors among older adults with type 2 diabetes. Geriatr Nurs. 2023;52:165-71.
- [CrossRef] [PubMed] [Google Scholar]
- Cognitive Impairment among Older adults living in the community and in nursing home in Indonesia: A Pilot Study. Dement Neuropsychol. 2022;16:347-53.
- [CrossRef] [PubMed] [Google Scholar]
- Associations between cognitive function and marital status in the United States, South Africa, Mexico, and China. SSM Popul Health. 2022;20:101288.
- [CrossRef] [PubMed] [Google Scholar]
- Marriage and risk of dementia: systematic review and meta-analysis of observational studies. J Neurol Neurosurg Psychiatry. 2018;89:231-8.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of anxiety on cognition in older adult inpatients with depression: results from a multicenter observational study. Heliyon. 2019;5:e02235.
- [CrossRef] [PubMed] [Google Scholar]
- Cognitive impairment in depressed older adults: implications for prognosis and treatment. Psychiatric Ann. 2014;44:138-42.
- [CrossRef] [PubMed] [Google Scholar]
- High cortisol and the risk of dementia and alzheimer's disease: a review of the literature. Front Aging Neurosci. 2019;11:43.
- [CrossRef] [PubMed] [Google Scholar]








