Translate this page into:
Metabolic consequences of flavonoid and saponin extracts from Gongronema Latifolium leaves in offspring of rats that consumed sucrose during lactation
Address for correspondence: David Chibuike Ikwuka, Department of Medical Physiology, College of Medicine and Pharmacy, University of Rwanda Huye Campus, Huye, Southern Province, Rwanda. Phone: +250791374933. E-mail: d.c.ikwuka@ur.ac.rw
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Qassim Uninversity and was migrated to Scientific Scholar after the change of Publisher.
Abstract
ABSTRACT
Objectives:
This study examines the metabolic consequences of saponin and flavonoid extracts of Gongronema latifolium leaves in rat offspring whose mothers consumed sucrose during breastfeeding.
Methods:
Thirty-two female albino Wistar rats were randomly assigned to control group, given water only: sucrose group, given sucrose solution only; flavonoid groups, given sucrose solution and 100 mg/kg b.w. and 200 mg/kg b.w. of flavonoid and saponin groups, given sucrose solution and 100 mg/kg b.w. and 200 mg/kg b.w. of saponin extracts, for 3 weeks during lactation. Then the body, hepatic and pancreatic weights, food intake, glucose tolerance, lipid profile, insulin, and leptin levels of their offspring were measured.
Results:
There was a significant decrease in the body weight (BW), food intake, and glucose level among the flavonoid and saponin groups compared to the control group. However, when compared to the sucrose group, there was a significant decrease in food intake, blood glucose level, triglyceride, and very low-density lipoprotein cholesterol levels and a significant increase in the BW. There was no significant difference in insulin and leptin levels, hepatic, and pancreatic weights among groups.
Conclusion:
This study shows that G. lactifolium consumption among lactating rats maintains metabolic homeostatic as it protects against elevated blood glucose level and dyslipidemia in offspring post-weaning. It also suggests that the hypoglycemic and hypolipidemic properties of G. latifolium maybe as a result of saponin and flavonoids inherent in the plant.
Keywords
Cardiovascular disease
flavonoid
glucose level
Gongronema latifolium
hypoglycemic
hypolipidemic
lactation
metabolic syndrome
saponin
Introduction
The incidence of metabolic syndrome (MetS) has become an issue of global concern, as it is not a respecter of socioeconomic, race, country, or ethnicity; it is fueled by rapid urbanization, nutrition transition, and increasingly sedentary lifestyle.[1] Central obesity, insulin resistance, hypertension, fasting hyperglycemia, and dyslipidemia compute the spike in morbidity and mortality.[2,3]
Nutrition plays a pivotal role in shaping an individual’s growth, organ development, body composition, and physiological function in the early developmental stage. Several experimental animal studies and clinical and epidemiological studies have shown that the nutrient a child is in contact with during the prenatal period and/or in early childhood is a crucial risk factor for obesity and MetS over their lifetime.[4] Studies have demonstrated the relationship between the consumption of sucrose by a mother during pregnancy and breastfeeding and the occurrence of insulin resistance, hypertension, obesity as well as other metabolic dysfunctions in the progenyin later life.[5,6] Furthermore, Katchy et al.,[7] reported the metabolic homeostatic role of Gongronema latifolium on rat off spring whose mothers consumed sucrose during breastfeeding as the extract prevented increased blood glucose level and dyslipidemia in offspring later in life.
In recent years, plant-based drugs are gaining momentum due to their potency in ameliorating certain health challenges and low toxicity.[8] The use of medicinal plants and nutrients in managing MetS is also gaining increasing attention and studies have reported the metabolic homeostasis, hypoglycemic, and hypolipidemic activities of certain plants including G. latifolium.[7,9-12] However, despite several reports on the hypoglycemic, hypolipidemic, and metabolic homeostasis of crude extracts of G. latifolium, little has been reported on the effects of its bioactive phytochemicals. Therefore, this study examined the metabolic consequences of flavonoid and saponin extracts of G. latifolium on the rat offspring whose mothers consumed sucrose during breastfeeding.
Materials and Methods
Plant material
G. latifolium leaves were sourced locally, identified, and authenticated by the chief taxonomist of the Plant Science Unit of the Department of Biotechnology, University of Nigeria, Nsukka.
Preparation of flavonoid-rich and saponin-rich extracts
2,000 g of G. latifolium leaf was rinsed in water and dried under shade. It was then pulverized to a coarse powder using a Thomas-Wiley laboratory mill (model 4). Flavonoid-rich extract was prepared following the methods according to Arora and Itankar[13] with slight adjustments. Petroleum ether was used to defat 1 kg of the crude powder and 50% acetone for extraction using the cold maceration method. The extraction period was 3 days with fresh solvent replaced every 24 h. The resulting solution was again filtered into a volumetric flask. A rotary evaporator was used to dry the concentrate. The extract rich in saponin was prepared following the methods described by Harborne[14] with slight modifications. The dry leaves of G. latifolium were cut into little pieces. For 72 h, the dry herb thrice was thoroughly extracted with 80% MeOH, each extraction lasting 24 h. The filtered constituents were mixed and using rotavapor at 40°C its volume was reduced to 500 mL, hexane (4 × 250 mL) was used to defat the methanol extract, and afterward, the moisture was evaporated (rotavapor, 40°C).
Experimental animals and design
In this study, 32 female matured non-pregnant albino Wistar rats weighing 180–210 g were randomly assigned into ventilated metallic cages, with 12 h light and dark cycle at a temperature of 22°C and were provided standard commercial pelleted feed from vital feed, Nigeria Ltd. and water ad libitum. They were allowed 7 days to acclimatize before the experiment. At pro-estrous, the fertility of the male Wistar rats was confirmed before they were paired with the female rats in the ratio of 1:2 for proper mating. The 1st day that sperm was found in the vaginal smear of the female rats was considered day 1 of pregnancy.[15] The Wistar rats were given chow and water ad libitum throughout gestation. After delivery, the litters were culled eight pups per dam. The dams together with their pups, were randomly assigned to seven groups. Group 1 (control), dams were given water only; group 2 (sucrose group), dams were given sucrose solution (30% weight/volume) only; group 3, dams were given sucrose solution (30% weight/volume) and 100 mg/kg of flavonoid extract; group 4, dams were given sucrose solution (30% weight/volume) and 200 mg/kg of flavonoid extract; group 5, dams were given sucrose solution (30% weight/volume) and 100 mg/kg of saponin extract; group 6, dams were given sucrose solution (30% weight/volume) and 200 mg/kg of saponin extract; and group 7 (standard control), dams were given sucrose solution (30% weight/volume) and 5 mg/kg metformin. After the 3 weeks period of lactation, the pups were weaned on post-natal day (PND) 21. The off spring were then separated into different cages according to their groups and were giving unlimited access to food (chow) and water.
Measurement of food intake
Food intake was measured using a method by Katchy et al.,[7] and D’Alessandro et al.[5] The quantity of food administered to each animal (offspring) was weighed using a digital weighing scale and recorded daily from PND 22 to PND 42. Before the administration of the next meal, the leftovers were gathered and the quantity was recorded, then the value was subtracted from the original quantity that was administered the day before.
Chow consumed (g) = Quantity given (g) – Quantity leftover (g)
Body, hepatic, and pancreatic weight measurement
The body weights (BWs) of the pups were recorded weekly from PND 1 to PND 42 while the liver and pancreatic weights were measured on PND 42 using a digital weighing balance.
Oral glucose tolerance determination
The pups underwent an overnight (12 h) fast and 2 g/kg BW glucose solution was given orally on PND 42. Then blood was collected at 0, 30, 60, and 90 min intervals from the tail vein and one touch basic glucometer was used to determine the blood glucose levels in mg/dL.
Lipid profile and insulin level determination
The cardiac puncture method was used to get blood from the pups at PND 42 into specimen blood vessels; they were allowed time for clotting and centrifuged. The serum separation was used for insulin level and lipid profile determination. The determination of triglyceride and cholesterol levels was by glycerol phosphate oxidase method using Enzymatic Colorimetric Diagnostic Kits from Randox Laboratories, United Kingdom.[16] High-density lipoprotein cholesterol (HDL-C) was determined using the method according to Burstein et al.[17] Calculation of low-density lipoprotein cholesterol (LDL-C) is:
LDL-C (mg/dL) = TC - {HDL-C + TG/5},
VLDL-C (mg/dL) = TG/5
Where LDL-C is low-density lipoprotein cholesterol, TC is Total cholesterol, HDL-C is high-density lipoprotein cholesterol, and VLDL-C is very low-density lipoprotein cholesterol, TG: Tryglycerides.
An insulin enzyme-linked immunoassay (ELISA) kit was used to determine serum insulin level (uIU/mL).
Serum leptin measurement
After an overnight fast which lasted for 12 h, blood samples of the pups were collected into EDTA bottles from the tail vein on PND 42. They were allowed time for clotting and centrifuged. Serum leptin measurement (ng/mL) was done using a specialized leptin ELISA kit.
Data analysis
A one-way analysis of variance (ANOVA) was use to evaluate the difference of mean followed Student’s Newman-Keuls post hoc test using IBM Statistical Package for the Social Sciences software version 20. The value of P < 5% (0.05) was considered statistically significant. The data are represented as mean ± standard error of the mean (SEM).
Ethical approval
Ethical approval for the study was obtained from the Research and Ethics Committee of the University of Nigeria Enugu Campus, Enugu, Nigeria with protocol number 085/09/2022.
Results
As shown in Table 1, maternal consumption of flavonoid and sapon in extracts of G. latifolium significantly decreased offspring BW when compared to control. However, the extracts significantly increased offspring BW when it was compared to the sucrose group and this competes favorably with the result of metformin on offspring BW.
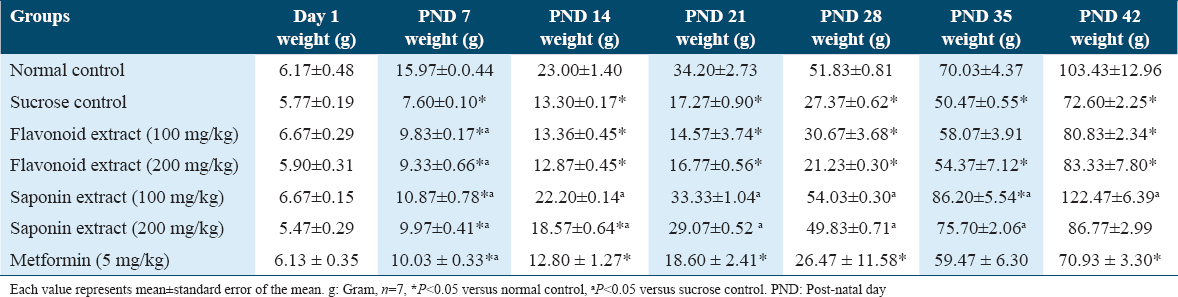
As shown in Table 2, flavonoid and saponin extracts of G. latifolium showed no significant difference in the offspring’s hepatic and pancreatic weights when it was compared to control and sucrose groups.
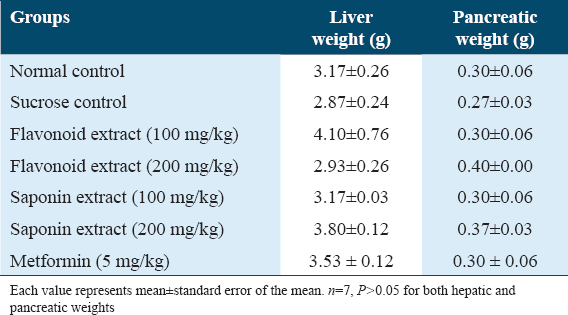
Table 3 shows the effect of maternal consumption of flavonoid and saponin extracts of G. latifolium during lactation on offspring’s food intake post-weaning when it was compared with the control and sucrose groups from PND 22 to PND 42. The result revealed a significant reduction (P < 0.05) in offspring food intake when Flavonoid 100 mg/kg, saponin 100 mg/kg, and saponin 200 mg/kg were compared to normal control in the 4th, 5th, and 6th weeks. While Flavonoid 200 mg/kg significantly decreased BW in the 4th week but showed no significant difference in the 5th and 6th weeks. Furthermore, offspring food intake in flavonoid 100 mg/kg, saponin 100 mg/kg, and saponin 200 mg/kg groups revealed a significant reduction (P < 0.05) when it was compared to the sucrose group on the 6th week. However, animals treated with saponin 100 mg/kg showed the highest level of decrease in food intake.
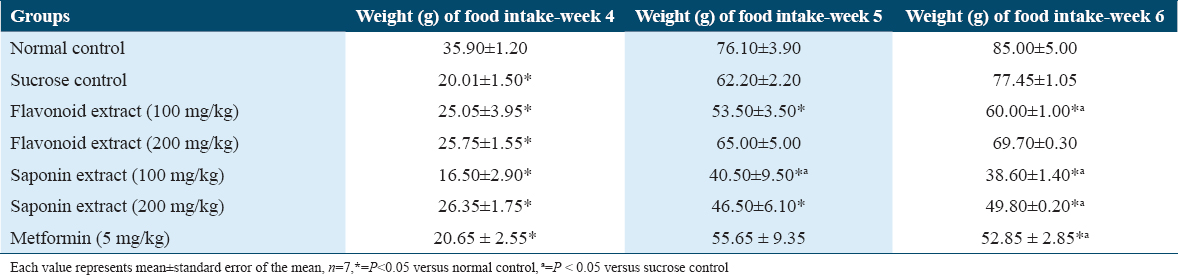
As shown in Table 4, maternal consumption of flavonoid (100 mg/kg) and saponin (100 mg/kg and 200 mg/kg) extracts of G. latifolium significantly decreased blood glucose levels when it was compared to control and sucrose groups. However, the blood glucose of offspring treated with saponin 200 mg/kg showed the highest level of decrease.
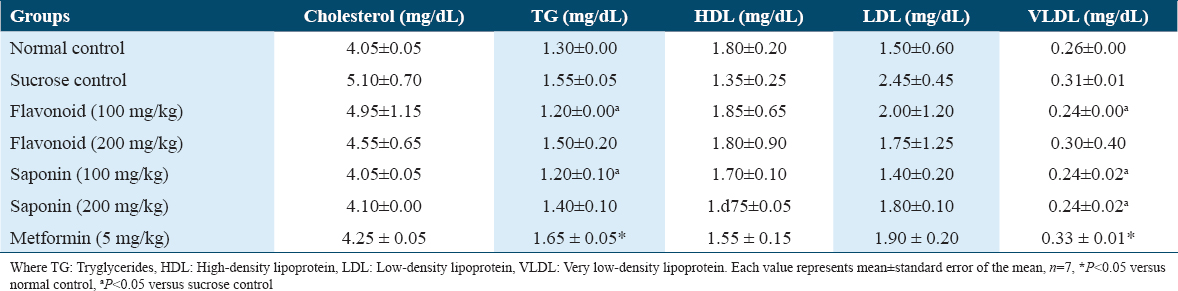
As shown in Table 5, flavonoid and saponin extracts of G. latifolium had no significant effect on cholesterol, tryglycerides, HDL, LDL, and VLDL levels when they were compared to the control group. However, there was a significant decrease in triglyceride and VLDL levels when they were compared to the sucrose group.
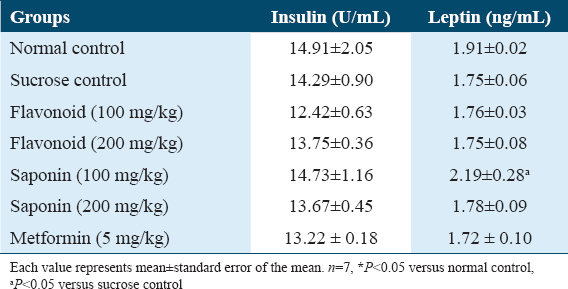
As shown in Table 6, maternal consumption of flavonoid and saponin extracts of G. latifolium showed no significant difference in insulin levels when they were compared to control and sucrose groups. It also had no significant effect on leptin levels when they were compared to control. However, leptin levels of animals treated with saponin extract 100 mg/kg significantly increased when they were compared to the sucrose group.
Discussion
MetS is a collection of metabolic dysregulations that includes insulin resistance, obesity, hypertension, and atherogenic dyslipidemia that occur together and it is linked with a heightened risk of developing diabetes and cardiovascular disease.[3] This study investigated the metabolic consequences of flavonoid and saponin extracts of G. latifolium leaves in the rat offspring whose mothers were fed sucrose during breastfeeding.
This study shows a decrease in BW of pups whose dams consumed sucrose during lactation compared to a control whose dams did not consume sucrose. Moreover, it is in agreement with the study of Katchy et al.,[18] and in disagreement with the studies of Toop et al.,[19] and Kendig et al.,[6] who showed that sucrose consumption during lactation had no effect on BW of the pups. The study also showed that flavonoid and saponin of G. latifolium L decreased offspring BW when compared to the control group and this agrees with reports of Katchy et al.,[7] and Amadi et al.[12] as well as confirms the reports of Nwaka et al.,[20] who noted that the reduction in BW of rats given G. Latifolium extracts could be a result of saponin inherent in the plant, as animals treated with saponin extract showed the highest decrease in BW. The decrease is assumed to be a result of adenosine monophosphate activation and protein kinase activation by G. latifolium saponins and polyphenols that are absorbable in the hepatic system, adipose tissues, and skeletal muscle which prompts a reduction in lipogenesis and gluconeogenesis, inhibits anabolism, mitochondrial biogenesis (catabolism), and increases lipolysis,[21,22] therefore leading to a reduction in BW. It also showed that flavonoid and saponin extracts of G. latifolium increased offspring BW when compared to the sucrose group and this corroborates previous reports that G. latifolium leave extract reverses rapid and significant weight loss which characterizes diabetes mellitus.[23,24]
Administration of flavonoid and saponin extracts of G. latifolium to lactating rats caused a decrease in food intake of the pups post-weaning and this indicates that components of the extract infiltrate the breast milk in the course of lactation to program decreased pups’ food consumption.[12]
Flavonoid and saponin extracts of G. latifolium reduced blood glucose level when compared to control and sucrose groups and this suggests that the hypoglycemic and antidiabetic properties of crude extract of G. latifolium is due to the presence of flavonoid and mostly saponin inherent in the plant. Mechanisms through which flavonoid and saponin extract of G. latifolium lower hyperglycemia remains incompletely understood. The enzyme Glucokinase or Hexokinase D in the beta cells of the pancreas acts as the glucose sensor that determines insulin secretion threshold and in the hepatic parenchyma cells, it assists phosphorylation of glucose through hyperglycemia and as hexokinase D acts as a glucose sensor in hypothalamic neurons, with a vital role in the sympathomimetic reaction to increased blood sugar level. It shows that flavonoid and saponin act as catalysts and stimulators of the hexokinase D, to detect elevated level of sugar and concurrently phosphorylate the sugar to glucose-6-phosphate, which is an active step in blood glucose clearance and glycolysis.[25]
This study also showed that flavonoid and saponin extracts of G. latifolium decreased triglyceride, LDL, VLDL, and total cholesterol concentrations and increased HDL concentration when they were compared to sucrose group and the hypolipidemic activity of flavonoid and saponin of G. latifolium extracts may be due to the modification of the hydrophilic-hydrophobic interface betwixt the aqueous fraction of plasma and cholesterol by the extract in support of hypocholesterolemia and hypolipidemia.[25] However, the lipid profile was not affected by flavonoid and saponin extracts when they were compared to normal control.
The few potential limitations of this study are: first of all, even though Wistar rats are used in research due to practical and ethical reasons, metabolic responses in Wistar rats may not perfectly represent those in humans, thereby restricting the immediate applicability of the findings to human population; secondly, the consumption and metabolism of saponin and flavonoid may differ between rat pups, thereby affecting the observed metabolic consequences; thirdly, the study focused on specific metabolic indices, possibly neglecting important metabolic effects and markers such as hormone-sensitive lipase, skeletal muscle glycogen synthase, and lipoprotein lipase which may increase the risk of MetS; finally, despite the researchers’ effectiveness, there may be unseen variables that influenced the outcome, leading to biased conclusions. To increase the accuracy and generalizability of the results, future research could address these limitations.
Conclusion
This study established that the metabolic homeostatic effect of flavonoid and saponin extracts of G. latifolium as the maternal consumption of the extracts during lactation protected against elevated blood glucose levels and dyslipidemia in offspring post-weaning and also suggested that the hypoglycemic and hypolipidemic effect of G. latifolium may be as a result of saponin and flavonoid inherent in the plant. Further research should explore more underlying mechanisms by which flavonoid and saponin extracts of G. latifolium elicited sugar-lowering effects and reverse dyslipidemia is warranted to fully harness the therapeutic potentials of G. latifolium in the management of obesity, diabetes, and MetS.
Authors’ Declaration Statements
Ethical approval
Ethical approval for the study was obtained from the Research and Ethics Committee of the University of Nigeria Enugu Campus, Enugu, Nigeria with protocol number 085/09/2022.
Consent for publication
None
Availability of data and material
To be provided by the corresponding author on request.
Competing interests
In accordance with the standardized disclosure form recommended by the International Committee of Medical Journal Editors (IJMJE), each author hereby affirms the following: Funding and Support: The authors affirm that they did not receive any financial assistance or services from any organization for the research presented in this submission. Financial Associations: All authors assert that they do not currently have any financial affiliations or have had any financial interactions within the last 3 years with organizations that may have a vested interest in the research presented. Other Associations: The authors confirm that there are no other personal or professional relationships or activities that might be perceived as having an influence on the research submitted.
Funding statement
None
Authors’ contributions
All authors contributed equally. The author CNO did the conceptualization and designed the study. Authors CNO, DCI, and OMO performed the methodology. Authors CNO, BUA, and AK did the formal analysis. Authors CNO, DCI, and OMO wrote the original draft. BUA supervised the study. All the authors contributed to drafting the work, critically revised the manuscript, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
References
- Metabolic syndrome:A closer look at the growing epidemic and its associated pathologies. Obes Rev. 2015;16:1-12.
- [Google Scholar]
- Harmonizing the metabolic syndrome:A joint interim statement of the international diabetes federation task force on epidemiology and prevention;National heart, lung, and blood institute;American heart association;World heart federation;International atherosclerosis society;And international association for the study of obesity. Circulation. 2009;120:1640-5.
- [Google Scholar]
- Metabolic syndrome:Updates on pathophysiology and management in 2021. Int J Mol Sci. 2022;23:786.
- [Google Scholar]
- Developmental origins of the metabolic syndrome:Prediction, plasticity, and programming. Physiol Rev. 2005;85:571-633.
- [Google Scholar]
- Maternal sucrose-rich diet and fetal programming:Changes in hepatic lipogenic and oxidative enzymes and glucose homeostasis in adult offspring. Food Funct. 2014;5:446-53.
- [Google Scholar]
- Metabolic effects of access to sucrose drink in female rats and transmission of some effects to their offspring. PLoS One. 2015;10:e0131107.
- [Google Scholar]
- Consumption of Gongronema latifolium aqueous leaf extract during lactation may improve metabolic homeostasis in young adult offspring. Pak J Biol Sci. 2020;23:1201-9.
- [Google Scholar]
- Hematological and histological effect of fractionated neem leaf extract in healthy Wistar rats. Physiol Pharmacol. 2020;24:314-21.
- [Google Scholar]
- Antihyperglycemic effect of aqueous and ethanolic extracts of Gongronema latifolium leaves on glucose and glycogen metabolism in livers of normal and streptozotocin-induced diabetic rats. Life Sci. 2003;73:1925-38.
- [Google Scholar]
- Comparative chemical composition of leaves of some antidiabetic medicinal plants:Azadirachta indica, Vernonia amygdalina and Gongronema latifolium. Afr J Biotechnol. 2009;8:4685-9.
- [Google Scholar]
- Effect of Gongronema latifolium crude leaf extract on some cardiac enzymes of alloxan-induced diabetic rats. Afr J Biochem Res. 2009;3:366-9.
- [Google Scholar]
- Postweaning administration of aqueous leaf extract of Gongronema latifolium may improve obesity indices in young adult offspring. Nigeria J Exp Clin Biosci. 2021;9:186-91.
- [Google Scholar]
- Extraction, isolation and identification of flavonoid from Chenopodium album aerial parts. J Tradit Complement Med. 2018;8:476-82.
- [Google Scholar]
- Phytochemical Methods:A Guide to Modern Techniques of Plant Analysis. London: Springer Dordrecht; 1998.
- Reproductive characteristics of the female laboratory rat. Afr J Biotechnol. 2013;12:2510-4.
- [Google Scholar]
- Determination of blood glucose using an oxidase-peroxidase system with a non-carcinogenic chromogen. J Clin Pathol. 1969;22:158-61.
- [Google Scholar]
- Rapid method for the isolation of lipoproteins from human serum by precipitation with polyanions. J Lipid Res. 1970;11:583-95.
- [Google Scholar]
- Maternal consumption of sucrose during lactation may program metabolic dysfunction in young offspring. Niger J Exp Clin Biosci. 2019;7:17-22.
- [Google Scholar]
- Consumption of sucrose, but not high fructose corn syrup, leads to increased adiposity and dyslipidaemia in the pregnant and lactating rat. J Dev Orig Health Dis. 2015;6:38-46.
- [Google Scholar]
- Comparative effect of ethanolic extract of Gongronema latifolium (Utazi) and Vitex doniana (Uchakiri) leaves on the body weight and lipid profile of Wistar albino rats. Glob J Biotechnol Biochem. 2015;10:126-30.
- [Google Scholar]
- AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest. 2006;116:1176-83.
- [Google Scholar]
- AMPK:A nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251-62.
- [Google Scholar]
- Effect of magnesium pre-treatment on alloxan induced hyperglycemia in rats. Afr Health Sci. 2011;11:79-84.
- [Google Scholar]
- Protective effect of ethanolic extract of Gongronema latifolium leaves in alloxan-induced diabetic rats. IOSR J Pharm Biol Sci. 2013;7:63-8.
- [Google Scholar]
- Saponin fraction of Gongronema latifolium reverses dyslipidemia and catalyzes glucokinase in lowering blood glucose sugar in streptozocin-induced diabetic rats. J Adv Med Pharm Sci. 2017;14:1-12.
- [Google Scholar]







