Translate this page into:
MicroRNA-183-5p negatively regulates interleukin-8 expression in cervical cancer cells
Address for correspondence: Dr. Zafar Rasheed, Department of Pathology, College of Medicine, Qassim University, Buraidah - 51452, Saudi Arabia. E-mail: zafarrasheed@qu.edu.sa
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Qassim Uninversity and was migrated to Scientific Scholar after the change of Publisher.
Abstract
ABSTRACT
Objectives:
Interleukin-8 (IL-8) and microRNA-183-5p (hsa-miR-183-5p) have been implicated in the development of cervical cancer, yet their relationship has not been explored. This study aims to determine whether phorbol 12-myristate 13-acetate (PMA)-induced IL-8 expression is regulated by hsa-miR-183-5p in cervical cancer cells.
Methods:
Bioinformatics algorithms were employed to predict the potential binding of hsa-miR-183-5p to the 3’UTR of IL-8 mRNA. CaSKi cervical cancer cells were used as a model to investigate this regulation. The expression levels of hsa-miR-183-5p and IL-8 were measured using Taqman assays through real-time polymerase chain reaction, while IL-8 protein levels were quantified in culture media through IL-8 specific Sandwich enzyme-linked immunosorbent assays. Luciferase reporter assays and transfections with pre- or anti-miR-183-5p were conducted to validate the binding of hsa-miR-183-5p to IL-8 mRNA’s 3’UTR.
Results:
The bioinformatics tool TargetScan identified a seed-matched sequence for hsa-miR-183-5p in the 3’UTR of IL-8 mRNA. PMA-induced IL-8 expression was inversely correlated with hsa-miR-183-5p down regulation in cervical cancer cells. hsa-miR-183-5p significantly reduced luciferase activity in the 3’UTR-IL-8 reporter assay. Transfection with pre-miR-183-5p led to a notable decrease in IL-8 mRNA and protein secretion, while anti-miR-183-5p transfection caused a significant increase in IL-8 mRNA and protein levels in PMA-treated cells.
Conclusion:
This study is the first to demonstrate that hsa-miR-183-5p directly regulates IL-8 expression in cervical cancer cells. Both IL-8 and hsa-miR-183-5p could serve as potential therapeutic targets in the treatment of cervical cancer.
Keywords
CaSKi cell line
cervical cancer
hsa-miR-183-5p
interleukin-8
microRNA
Introduction
Cervical cancer is still a commonly diagnosed malignancy in women globally. While early detection and treatment through surgery, chemotherapy, and radiation improve clinical outcomes, the prognosis for advanced stages remains poor.[1] Cancer progression and metastasis are the leading causes of death in cervical cancer patients.[1,2] This study, therefore, explored the molecular mechanisms involved in cervical cancer cells activation and their role in inflammation. MicroRNAs (miRNAs) are small, single-stranded RNA molecules consisting of 18–22 nucleotides. Studies have shown that miRNAs are involved in regulating malignant cell growth, differentiation, and apoptosis.[3] miRNAs play pivotal roles in cancer development and progression, functioning as either oncogenes or tumor suppressors.[3,4] However, the role of miR-183 in cancer biology is particularly complex.[5-12] In certain cancers, such as osteosarcoma, nasopharyngeal carcinoma, and cervical carcinoma, miR-183 has been reported to act as a tumor suppressor.[8,12,13] Conversely, in other cancer types, including prostate, bladder, liver, and breast cancers, miR-183 is identified as a cancer promoter.[5-7,9-11] However, its involvement in cancer biology has been well-established. Specifically, miR-183 has been linked to the metastasis of several cancers, such as osteosarcoma, pancreatic neuroendocrine tumors, prostate cancer, bladder cancer, and liver cancer.[5-11] In colorectal cancer, elevated miR-183 levels in primary tumor tissues compared to adjacent normal tissues suggest that the Wnt/CTNNB1/miR-183 signaling pathway could serve as a biomarker for cancer recurrence and prognosis.[10] Moreover, miR-183 promotes cancer cell growth by inhibiting Fox1 in non-small cell lung carcinoma.[11] Importantly, studies also reported the role of miR-183 in cervical cancer cells as it suppressed cervical cancer cells aggression through integrin subunit beta-1 (ITGB1) targeting,[12] indicating that miR-183 serves as a hidden anti-oncogene by targeting the metastasis-promoter gene. Despite of these supportive evidences of miR-183’s role in various cancers including cervical cancer, but the mechanisms involved in regulation of inflammatory genes such as interleukin-8 (IL-8) remain uninvestigated.
IL-8 is a chemokine that plays several pro-tumorigenic roles within the tumor microenvironment, such as promoting tumor cell proliferation and inducing a migratory or mesenchymal phenotype.[14-16] It also enhances tumor angiogenesis and attracts immunosuppressive cells to the tumor site. Higher baseline levels of IL-8 have been associated with poorer clinical outcomes in various cancers.[14,15] Studies have further indicated that IL-8 contributes to resistance against chemotherapy and molecularly targeted treatments.[14,15] Recent clinical studies involving immune checkpoint inhibitor (ICI) therapy have revealed that IL-8-driven myeloid infiltration into tumors leads to resistance to ICIs, with peripheral IL-8 levels serving as potential predictors of ICI therapy outcomes.[16,17] Preclinical studies suggest that targeting IL-8 or its receptors can enhance immune cell-mediated tumor destruction, and combining IL-8/IL-8R blockade with ICIs improves the overall anti-tumor response.[15-17] Thus, it is now clear that elevated expression of IL-8 promotes cancer development. While several strategies for reducing IL-8 levels have been explored, selective inhibition of IL-8 has garnered the most attention as a potential therapeutic approach. Since miRNAs are emerging as selective regulators of gene expression and may play significant roles in cancer development and progression, this study was the first to investigate whether hsa-miR-183-5p regulates IL-8 expression in cervical cancer cells. These findings could contribute to the development of new therapeutic approaches for treating cervical cancer and other malignancies.
Materials and Methods
Bioinformatics approach
TargetScan computer-based approach was used (https://www.targetscan.org/accessed on March 05, 2024) for the bioinformatic-based scanning of 3’UTR of IL-8 for the prediction of seed-matched sequence of miRNAs. The method used to predict miRNA sequences in the 3’UTR of pathogenic genes followed the same protocol outlined in prior studies.[18,19]
Cervical cancer cells and their culture
Cervical cancer cell line CaSki was brought from the American type culture collection (ATCC) and the cells were cultured as described by ATCC manufactured instructions. Briefly, CaSKi cancer cells were cultured in RPMI-1640 supplemented with 10% fetal bovine serum at 37°C with 5% CO2 as described previously.[20]
Treatment of cervical cancer cells with phorbol 12-myristate 13-acetate (PMA) and identification of miRNAs
CaSKi cervical cancer cells (3 × 106 cells/mL) were seeded in 6-well culture plates using complete RPMI-1640 medium and subjected to serum starvation for 12 h or overnight. After starvation, the cells were treated with 75 nM PMA for 24 h as described previously.[21,22] Total RNA, including miRNA fractions, was extracted using the mirVana miRNA Isolation Kit (catalog # AM1560, Ambion, CA, USA).
Luciferase reporter assay
CaSKi cervical cancer cells were co-transfected with a reporter construct containing the complete IL-8 3’UTR and a Renilla luciferase reporter, either alone or alongside pre-miR-183-5p or a pre-miR-negative control (pre-miR-NC) (Ambion, Austin, Texas, USA), using HiPerfect Transfection Reagent (Qiagen, Valencia, USA) as described previously.[22] After 24 h, the cells were lysed, and luciferase activity was assessed using the dual-luciferase reporter assay system (Promega, Madison, WI, USA) following the manufacturer’s instructions. Firefly luciferase activity was normalized to that of Renilla luciferase for comparison.
Transfection of cervical cancer cells with pre- and anti-miRNAs
CaSKi cervical cancer cells were transfected with pre-miRNAs or anti-miRNAs (100 nM; Ambion/Qiagen) at a final concentration of 100 nM using HiPerfect Transfection Reagent (Qiagen), following the protocol outlined in previous studies.[22] After transfection, the cells were treated with 75 nM PMA for 24 h to evaluate IL-8 expression.
Reverse transcription and real-time polymerase chain reaction (RT-PCR)
A total of 1.0 μg of extracted RNA was reverse-transcribed into cDNA using the SuperScript First Strand cDNA synthesis kit (catalog #75780, Affymetrix Inc., OH, USA) in accordance with the manufacturer’s protocol. IL-8 mRNA and hsa-miR-183-5p expression were quantified using TaqMan Gene Expression Assays (Applied Biosystems, Foster City, CA), with GAPDH and RNU6B serving as endogenous controls. PCR amplification and data collection were conducted on the step one RT-PCR system (Applied Biosystems) and relative expression levels were calculated using the ΔΔcomputed tomography method.[23]
Enzyme-linked immunosorbent assays (ELISA)
The amount of IL-8 secreted into the culture medium was measured using IL-8-specific sandwich ELISAs (R and D systems). The absorbance was recorded at 450 nm using a multimode microplate reader (Anthos 3100, Salzburg, Austria), following established procedures.[24]
Results
Bioinformatic prediction of hsa-miR-183-5p targeting the 3’UTR of IL-8 mRNA (ENST00000307407.3)
Using the TargetScan algorithm, the 3’UTR of human IL-8 mRNA (ENST00000307407.3) was analyzed for potential binding sites of hsa-miR-183-5p. The analysis revealed that the 3′UTR of IL-8 is 1,252 nucleotides long and contains conserved sequences for several miRNAs, including hsa-miR-183-5p [Figure 1]. To explore the evolutionary conservation of miR-183-5p binding, TargetScan also predicted binding sequences in other species such as chimpanzees, rhesus monkeys, and squirrels [Figure 2a]. The predicted interaction between hsa-miR-183-5p and the IL-8 mRNA occurs at nucleotides 410–416 of the 3’UTR, as illustrated in Figure 2b.
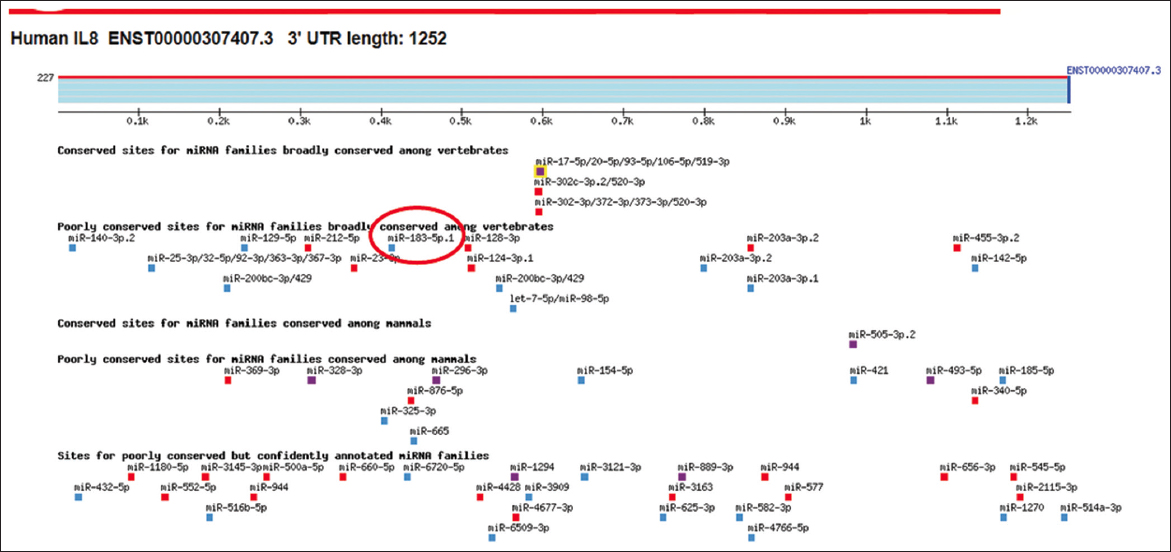
- Bioinformatic based scanning of 3’UTR of human interleukin-8 (IL-8) mRNA sequence (ENST00000307407.3). MicroRNA hsa-miR-183-5p showed in red circle in the 3`UTR of IL-8 mRNA sequence
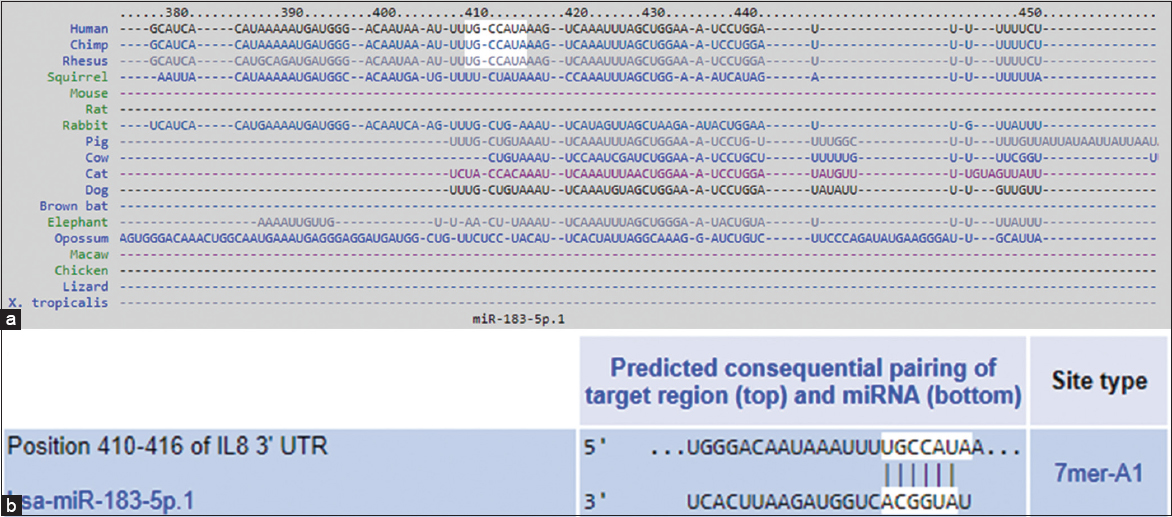
- (a) TargetScan predicted duplex of hsa-miR-183-5p with the seed sequence in the 3’UTR of interleukin-8 (IL-8) mRNA in human, chimp, rhesus and in other species. (b) TargetScan predicted duplex of hsa-miR-183-5p with the seed sequence in the 3’UTR of human IL-8 mRNA at position 410–416 bases
Correlation between hsa-miR-183-5p and IL-8 expression in unstimulated and PMA-stimulated cervical cancer cells
We investigated the relationship between hsa-miR-183-5p and IL-8 expression in both unstimulated and PMA-stimulated CaSKi cervical cancer cells. An inverse correlation was observed between hsa-miR-183-5p and IL-8 expression in these cells (P < 0.05). Specifically, hsa-miR-183-5p levels were significantly lower in PMA-stimulated cells compared to unstimulated cells [Figure 3a; P < 0.05], whereas IL-8 mRNA expression was significantly elevated in PMA-stimulated cells [Figure 3b; P < 0.0001]. In addition, IL-8 protein secretion in the culture medium was markedly higher in PMA-stimulated cells than in unstimulated cells [Figure 3c; P < 0.0001].

- Phorbol 12-myristate 13-acetate (PMA) down-regulates hsa-miR-183-5p and up-regulates the interleukin-8 (IL-8) expression in cervical cancer cells (a) expression of hsa-miR-183-5p in PMA-stimulated human cervical cancer cells CaSKi. *P<0.05 versus untreated cells. (b) IL-8 mRNA expression. #P<0.0001 versus untreated cancer cells. (c) IL-8 protein secretion in the culture medium of PMA-treated CASKi cancer cells. @P<0.0001 versus untreated cancer cells. Untreated cancer cells were used as control and expression of RNU6B/GAPDH was used as an endogenous control. IL-8 protein was determined by IL-8-specific sandwich enzyme-linked immunosorbent assays. The results are expressed as the mean±standard deviation from three independent experiments
Verification of hsa-miR-183-5p binding to the 3’UTR of IL-8 mRNA
To confirm the bioinformatic prediction that hsa-miR-183-5p targets the 3’UTR of IL-8 mRNA, a reporter construct containing the full 3’UTR of IL-8 was used. Co-transfection of CaSKi cells with pre-miR-183-5p resulted in a significant reduction in luciferase activity [Figure 4a; P < 0.01], validating the direct interaction between hsa-miR-183-5p and the seed-matched sequence in the IL-8 3’UTR.
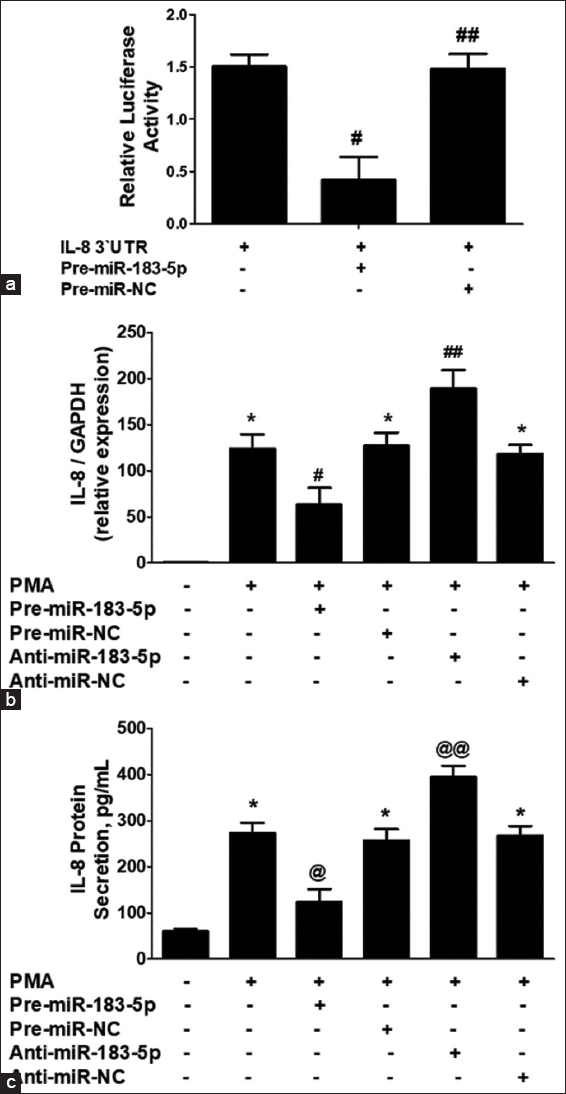
- (a) Luciferase activity in CaSKi cancer cells transfected with the reporter vector and pre-miR-183-5p. Transfection CaSKi cancer cells with pre-miR negative control (pre-miR-NC) was used as a negative control. #P<0.01 versus interleukin-8 (IL-8) 3’UTR alone. ##P>0.05 versus IL-8 3’UTR alone. (b) IL-8 mRNA expression in pre-miR-183-5p and anti-miR-183-5p transfected CasKi cervical cancer cells after 24 h of phorbol 12-myristate 13-acetate (PMA) stimulation. *P<0.001 versus untreated cancer cells; #P<0.05 versus*; ##P<0.05 versus*; ##P<0.05 versus#. (c) IL-8 protein expression in pre-miR-183-5p and anti-miR-183-5p transfected CasKi cervical cancer cells after 24 h of PMA stimulation. *P<0.001 versus untreated cancer cells; *P<0.05 versus @; @@P<0.05 versus*; @@P<0.05 versus@. Gene expression in unstimulated cancer cells was used as a control and expression of RNU6B/GAPDH was used as an endogenous control. IL-8 protein was determined by IL-8-specific sandwich enzyme-linked immunosorbent assays. The results are expressed as the mean±standard deviation from three independent experiments
Regulation of IL-8 mRNA and protein by miR-183-5p in cervical cancer cells
In this set of experiments, CaSKi cells were transfected with either pre-miR-183-5p or anti-miR-183-5p and then stimulated with PMA for 24 h. The IL-8 mRNA and protein levels were measured [Figure 4]. Transfection with pre-miR-183-5p significantly reduced IL-8 mRNA levels [Figure 4b, bar 2; P < 0.0001], while anti-miR-183-5p transfection significantly increased IL-8 mRNA in PMA-stimulated cells [Figure 4b, bar 5; P < 0.01]. Controls using pre-miR-NC and anti-miR-NC with PMA stimulation showed no significant changes in IL-8 mRNA compared to non-transfected cells [Figure 4b, bars 4 and 6; P > 0.05].
To assess whether the changes in IL-8 mRNA were reflected at the protein level, IL-8 secretion was measured in the culture medium. Transfection with pre-miR-183-5p resulted in a significant reduction in IL-8 protein levels [Figure 4c, bar 2; P < 0.001], while anti-miR-183-5p transfection significantly increased IL-8 protein in PMA-stimulated cells [Figure 4c, bar 5; P < 0.01]. Controls transfected with pre-miR-NC or anti-miR-NC showed no significant change in IL-8 secretion compared to non-transfected cells [Figure 4c, bars 4 and 6; P > 0.05]. These findings confirm that hsa-miR-183-5p negatively regulates both IL-8 mRNA and protein levels by targeting the seed sequence in the IL-8 3’UTR in cervical cancer cells.
Discussion
This study is the first to demonstrate that miRNAs hsa-miR-183-5p negatively regulates the expression of IL-8 in human cervical cancer cells simulated with PMA, suggesting its potential as a tumor-suppressive factor. miRNAs are widely recognized for their critical roles in cancer development and progression, acting as either oncogenes or tumor suppressors.[25] For example, in osteosarcoma, miR-183 functions as a tumor suppressor by inhibiting the LRP6-Wnt/β-catenin signaling pathway.[8] Similarly, miR-183 is downregulated in nasopharyngeal carcinoma spheroids, where it acts as a tumor suppressor.[13] However, several studies reported pro-oncogenic or cancer promoting activity of miR-183 in different cancer types such as prostate cancer, bladder cancer, liver cancer, breast cancer.[5-7,26,27] The present study was designed to solve these controversial findings, in cervical cancer, miR-183 was reported to inhibit the cancer promoting activity as it suppresses the aggressiveness of the cervical cancer cells through targeting ITGB-1.[12] Not only have these, the level of miR-183 was reported to be lower in clinical tissues of cervical cancer patients as compared with the levels of this miRNAs in the adjacent non-cancerous tissues,[12] indicating the anti-oncogenic role of miR-183 in cervical cancer tissues. In order to investigate more on miR-183 in cervical cancer, the present study supported the anti-oncogenic role of miR-183 in cervical cancer.
IL-8 (also known as CXCL8), a pro-inflammatory chemokine, has been studied extensively as a prognostic biomarker in several cancers, including cervical cancer.[28,29] However, its regulatory role against miR-183 has never been explored. In light of these literatures of IL-8 and miR-183 in cervical cancer, this study investigated the regulation of IL-8 by miR-183-5p in cervical cancer cells. We identified a direct regulatory relationship between miR-183-5p and IL-8 in CaSki cells. Specifically, the seed sequence of hsa-miR-183-5p (7 mer-A1 nucleotides) was complementary to nucleotides 410–416 of the IL-8 3’UTR. This seed sequence is conserved across multiple species, including humans, chimpanzees, and rhesus monkeys. Most animal miRNAs bind their targets with mismatches or bulges, but the key to target recognition lies in the complementarity of nucleotides 2–8.[30] This type of interaction typically promotes the repression of mRNA translation, rather than mRNA degradation, which is the dominant mechanism of miRNA regulation in animals.[30] After identifying the seed-matched sequence in the IL-8 3’UTR, we analyzed miR-183-5p expression in cervical cancer cells. The data analysis showed an inverse correlation between miR-183-5p and IL-8 expression. Specifically, PMA-stimulated cells showed a decrease in miR-183-5p expression and a corresponding increase in IL-8 mRNA and protein levels, indicating a direct link between the two molecules.
To confirm that the 3’UTR of IL-8 is a direct target of hsa-miR-183-5p, we used a luciferase reporter assay. Overexpression of hsa-miR-183-5p significantly reduced luciferase activity in CaSki cells, confirming direct binding between the miRNA and the IL-8 3’UTR. Furthermore, transfection of pre-miR-183-5p resulted in the silencing of IL-8 mRNA and protein secretion, while anti-miR-183-5p led to upregulation of both mRNA and protein levels in PMA-stimulated cells. These findings confirm that hsa-miR-183-5p directly regulates IL-8 expression by binding to its 3’UTR in cervical cancer cells. In short, these findings suggest that miR-183-5p plays a significant role in modulating IL-8 expression in cervical cancer cells, providing new insights into its potential as a therapeutic target for cancer treatment.
Conclusion
This study is the first to demonstrate that hsa-miR-183-5p directly targets IL-8 mRNA in human cervical cancer cells by binding to a conserved site within its 3’UTR. The data observed an inverse correlation between hsa-miR-183-5p expression and the mRNA and protein levels of IL-8 in PMA-stimulated cervical cancer cells. These findings highlight hsa-miR-183-5p as a potential regulator of IL-8 expression, offering a promising avenue for developing therapeutic strategies aimed at managing inflammation in cervical cancer.
Ethics Approval and Consent to Participate
Not application.
Competing Interest
The author declares no competing interests.
Availability of Data and Material
All data and materials used in this study are available with the author and will be provided upon reasonable request.
Acknowledgment
The author expresses sincere gratitude to Mr. Casimero A. Victoria for his valuable assistance in data collection.
References
- A review of cervical cancer:Incidence and disparities. J Natl Med Assoc. 2020;112:229-32.
- [Google Scholar]
- The potential role of miRNAs in the pathogenesis of gallbladder cancer-a focus on signaling pathways interplay. Pathol Res Pract. 2023;248:154682.
- [Google Scholar]
- MicroRNA-183-5p:A new potential marker for prostate cancer. Indian J Clin Biochem. 2019;34:207-12.
- [Google Scholar]
- Urinary exosomal microRNA-96-5p and microRNA-183-5p expression as potential biomarkers of bladder cancer. Mol Biol Rep. 2021;48:4361-71.
- [Google Scholar]
- MicroRNA-183-5p contributes to malignant progression through targeting PDCD4 in human hepatocellular carcinoma. Biosci Rep. 2020;40:BSR20201761.
- [Google Scholar]
- MiR-183 inhibits osteosarcoma cell growth and invasion by regulating LRP6-Wnt/b-catenin signaling pathway. Biochem Biophys Res Commun. 2018;496:1197-203.
- [Google Scholar]
- MEG3 suppresses human pancreatic neuroendocrine tumor cells growth and metastasis by down-regulation of Mir-183. Cell Physiol Biochem. 2017;44:345-56.
- [Google Scholar]
- Wnt/catenin b1/microRNA 183 predicts recurrence and prognosis of patients with colorectal cancer. Oncol Lett. 2018;15:4451-56.
- [Google Scholar]
- MiR-183 promotes growth of non-small cell lung cancer cells through FoxO1 inhibition. Tumour Biol. 2015;36:8121-6.
- [Google Scholar]
- MicroRNA-183-5p inhibits aggressiveness of cervical cancer cells by targeting integrin subunit beta 1 (ITGB1) Med Sci Monit. 2018;24:7137-45.
- [Google Scholar]
- MiR-183 overexpression inhibits tumorigenesis and enhances DDP-induced cytotoxicity by targeting MTA1 in nasopharyngeal carcinoma. Tumour Biol. 2017;39:1010428317703825.
- [Google Scholar]
- Interleukin-8:A chemokine at the intersection of cancer plasticity, angiogenesis, and immune suppression. Pharmacol Ther. 2021;219:107692.
- [Google Scholar]
- Interleukin-8 in cancer pathogenesis, treatment and follow-up. Cancer Treat Rev. 2017;60:24-31.
- [Google Scholar]
- Prognostic role of IL-8 in cancer patients treated with immune checkpoint inhibitors:A system review and meta-analysis. Front Oncol. 2023;13:1176574.
- [Google Scholar]
- MicroRNA-125b-5p regulates IL-1b induced inflammatory genes via targeting TRAF6-mediated MAPKs and NF-kB signaling in human osteoarthritic chondrocytes. Sci Rep. 2019;9:6882. Erratum in: Sci Rep 2019;9:14729
- [Google Scholar]
- Bioinformatics approach:A powerful tool for microRNA research. Int J Health Sci (Qassim). 2017;11:1-3.
- [Google Scholar]
- Evaluation of the antitumour activity of Rinvanil and Phenylacetylrinvanil on the cervical cancer tumour cell lines HeLa, CaSKi and ViBo. Eur J Pharmacol. 2015;758:129-36.
- [Google Scholar]
- Polyphenol-rich pomegranate fruit extract (POMx) suppresses PMACI-induced expression of pro-inflammatory cytokines by inhibiting the activation of MAP Kinases and NF-kappaB in human KU812 cells. J Inflamm (Lond). 2009;6:1.
- [Google Scholar]
- MicroRNA-26a-5p regulates the expression of inducible nitric oxide synthase via activation of NF-kB pathway in human osteoarthritis chondrocytes. Arch Biochem Biophys. 2016;594:61-7.
- [Google Scholar]
- A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45.
- [Google Scholar]
- Butrin, isobutrin, and butein from medicinal plant Butea monosperma selectively inhibit nuclear factor-kappaB in activated human mast cells:Suppression of tumor necrosis factor-alpha, interleukin (IL)-6, and IL-8. J Pharmacol Exp Ther. 2010;333:354-63.
- [Google Scholar]
- MicroRNA-183 cluster:A promising biomarker and therapeutic target in gastrointestinal malignancies. Am J Cancer Res. 2023;13:6147-75.
- [Google Scholar]
- IL-8 is upregulated in cervical cancer tissues and is associated with the proliferation and migration of HeLa cervical cancer cells. Oncol Lett. 2018;15:1350-56.
- [Google Scholar]
- CXCL8 in tumor biology and its implications for clinical translation. Front Mol Biosci. 2022;9:723846.
- [Google Scholar]







