Translate this page into:
Periodontal health status in patients with lung cancer: Case–control study
Address for correspondence: Dr. Umesh Pratap Verma, Department of Periodontology, Faculty of Dental Sciences, King George’s Medical University, Uttar Pradesh, Lucknow, India. Mobile: 9450195422. E-mail: umeshpratapverma@kgmcindia.edu
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Qassim Uninversity and was migrated to Scientific Scholar after the change of Publisher.
Abstract
ABSTRACT
Objective:
The objective of this study was to assess the periodontal health status of individuals with lung cancer in the North Indian population. In addition, the study aimed to determine the levels of human beta-defensin2 (Hbd-2) in the gingival crevicular fluid (GCF) and serum samples collected from the participants.
Methods:
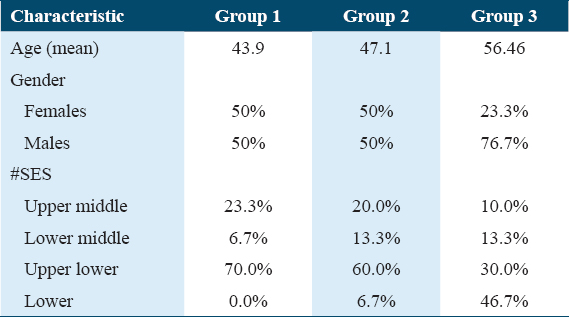
The study consisted of a total of 90 participants, who were categorized into three groups: Group 1 included 30 healthy individuals, Group 2 comprised 30 patients with chronic periodontitis, and Group 3 involved 30 patients diagnosed with both lung cancer and chronic periodontitis. Various periodontal parameters, including plaque index, gingival index, probing pocket depth, and clinical attachment level (CAL), were assessed in addition to the analysis of human beta defensin2 levels in both the GCF and serum samples of all participants.
Results:
The study results revealed that all clinical parameters assessed were higher in Group 3 compared to both Group 2 and Group 1. Specifically, the levels of hBD-2 in the GCF were measured as 52.29 ± 46.41 pg/mL in Group 1, 27.15 ± 28.76 pg/mL in Group 2, and 86.01 ± 68.82 pg/mL in Group 3. When comparing the hBD-2 levels in serum, the values were found to be 813.72 ± 269.43 pg/mL in Group 1, 591.50 ± 263.91 pg/mL in Group 2, and 1093.04 ± 674.55 pg/mL in Group 3. These intergroup comparisons indicate variations in hBD-2 levels among the different groups.
Conclusions:
The study findings demonstrated significantly higher clinical and biochemical markers in patients with both lung cancer and chronic periodontitis, in comparison to individuals with chronic periodontitis alone and healthy participants. These results suggest that Hbd-2 could potentially serve as a valuable diagnostic biomarker for identifying and distinguishing individuals with both lung cancer and chronic periodontitis.
Keywords
Chronic periodontitis
human beta defensin-2
lung cancer
Introduction
Lung cancer is the most frequently diagnosed cancer and ranked second among cancer diagnoses. Worldwide in 2020 lung cancer was the main causative factor due to cancer death, describing around one in five (18.0%) deaths and one in ten (11.4%) cancer diagnoses. Males are twice as susceptible to incidence and mortality rates as compared females.[1] In India, among males, the top five leading sites of cancer are lung (10.6%), mouth (8.4%), prostate (6.1%), tongue (5.9%), and stomach (4.8%). Among females, the estimated top five leading sites of cancer include breast (28.8%), cervix (10.6%), ovary (6.2%), corpus uteri (3.7%), and lung (3.7%).[2] Lung cancer is influenced by various risk factors, including tobacco use, environmental pollution, occupational exposure, residential radon exposure, and pre-existing chronic pulmonary conditions. These factors contribute to the development and progression of lung cancer.[3,4] Apart from other risk factors Socioeconomic status (SES) plays a significant role as it is highly related to certain lung carcinoma risk factors, such as tobacco smoking habits.[5] Periodontal disease is a chronic inflammatory condition of the periodontium. It damages the tissues that support teeth and eventually results in tooth loss.[6] Periodontal diseases are a significant health concern worldwide, impacting both developed and developing countries. These conditions affect a considerable portion of the global population, with estimates ranging from 20% to 50% of individuals being affected.[7] Overall, the prevalence of periodontal disease in India is 51.0%. The pooled prevalence of gingivitis is 46.6% and periodontitis is 51%.[8] When periodontal infections are present, the body responds by producing more tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, C-reactive protein, and matrix metalloproteinases.[9-12] Exposure to the chronic inflammation brought on by poor dental health may initiate and promote the development of lung cancer. Nitrosamine, a powerful carcinogen, can be produced by poor dental health.[13] The anatomical relationship between the oral cavity and the lungs, in addition to smoking as a risk factor, provides numerous opportunities for oral flora to influence lung flora in both health and sickness.[14] Even genetic variants have the potential to facilitate cancer development and can play a crucial role in early diagnosis, as well as aiding in the design of targeted treatment and prevention strategies.[15]
In the oral cavity, gingival epithelial cells are the first barrier against invading microorganisms from dental plaque. In addition, it acts as a physical barrier against pathogenic microbes and it also actively participates in defense by secreting antimicrobial peptides.[16] When bacteria or inflammation stimulate epithelial and mucosal surfaces, natural antibiotics called antimicrobial peptides are produced. One of the antimicrobial peptides defensins not only has a high level of microbicidal activity against a wide variety of bacteria, but it also enhances the acquired immune response.[17] Hence, the goal of our study is to describe the association between Chronic Periodontitis and Lung Cancer through Clinical and Biochemical evaluation of human beta-defensin-2 in serum and GCF of recruited participants.
Methods
A total of 90 people were enlisted and split into three groups. Group 1 (30 healthy participants), Group 2 (30 Patients having chronic periodontitis), and Group 3 (30 Patients having lung cancer with chronic periodontitis) from either patient attending the Department of Periodontology and Respiratory MedicineKing George’s Medical University, U.P, Lucknow between October 2021 to September 2022. The Institutional Ethics Committee at King George’s Medical University in Lucknow, Uttar Pradesh, granted its approval (Ref. code: III PGTSC-IIA/P37). After receiving signed informed consent, participants in the study were told of its goals and scope.
Inclusion criteria
The ages of the participants ranged from 20 to 75 years. Healthy previously untreated male or female patients of moderate-to-severe (clinical attachment level [CAL] 3–4 mm), generalized (>30% of the sites involved) chronic periodontitis, fulfilling the criteria by Armitage[18] were included, with at least one site in either quadrant (maxillary/mandibular) having a mean probing pocket depth (PPD) of ≥5 mm, CAL ≥4 mm and bleeding on probing and histopathologically confirmed lung cancer patients. At six sites on each tooth, the following clinical parameters were evaluated: Gingival index (GI) (Löe and Silness, 1963), plaque index (PI), CAL, and PPD.
Collection and storage of samples
The region was meticulously cleansed before gingival crevicular fluid (GCF) collection, and cotton rolls were used to isolate the tooth. A mild resistance was felt after 3–4 paper points (Sure-Endo, size #20, 6% taper) were gently inserted into the pocket. They were kept still for 30 s. Blood or saliva-covered paper points were discarded. Paper points were put into an Eppendorf tube that had been sterilized and contained 50 mL of phosphate buffer solution at 4°C after GCF collection. Each individual had two milliliters of peripheral venous blood drawn from them using a standard venipuncture technique. After that, the samples were put into tubes filled with 8% liquid EDTA. A centrifuge machine was used to centrifuge GCF samples for 10 min at 1000 g and blood samples for 10 min at 3000 g.[19] For the investigation, the supernatants from GCF and serum samples were separated.
Biochemical analysis
Determination of human beta-defensin 2 (HBD-2) level in serum and GCF
Serum and GCF hBD-2 concentrations were studied by the commercially available Hbd-2 ELISA Kit (CatalogNo: E-EL-H0996, Elabscience, USA). The ELISA kit used the Sandwich-ELISA principle.
Statistical analysis
To analyze the data, SPSS version 26 was used. The p-value was set at 0.05 to be significant and P < 0.001 was considered highly significant. The study’s power was put at 80% (ensures the study’s ability to detect an effect), while the confidence level was set at 95%.
Results
Clinical findings
In Group1 (mean age 43.9 years) and Group 2 (mean age 47.1 years), while in Group 3 (mean age 56.46 years) [Figure 1].
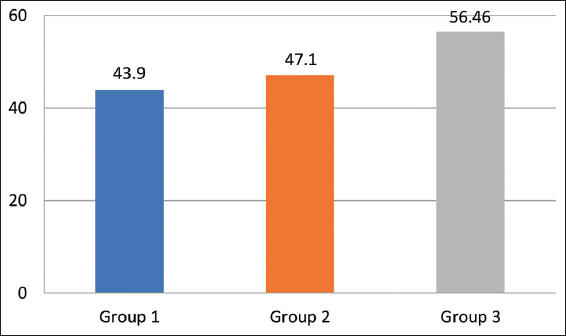
- Mean values of the age of the participants in the three groups
There were an equal number of males 15 (50%), and females 15 (50%) in Group 1 and 2 while in Group 3, there were 23 (76.7%) males and females 7(23.3%) [Figure 2].
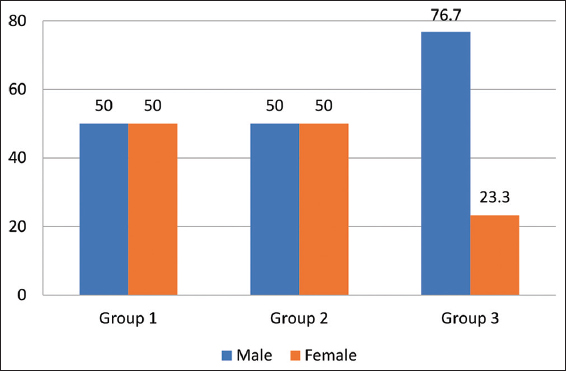
- Percentage distribution of participants as per their gender
According to Kuppuswamy’s socioeconomic scale 2020, in Group 1, majority of the cases were upper lower class (70%) while in Group 2, mostly belonged to the upper lower class (60%), and in Group 3, lower class was 46.7% [Figure 3]. Table 1 shows the overall demographic characteristics of the samples.
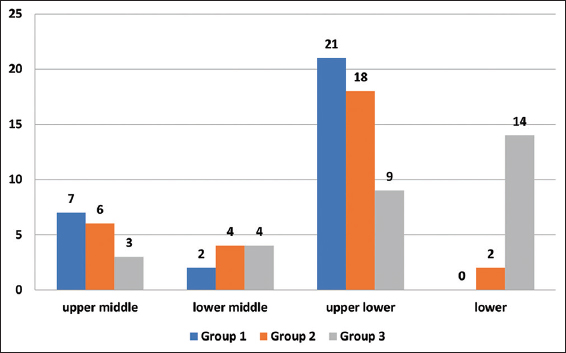
- Frequency distribution of participants as per SES in the three groups
Clinical variables
All recorded clinical parameters (GI, PI, PD, and CAL,) were significantly higher in Group 3 as compared to Groups 2 and 1 (P < 0.001) [Table 2].
Laboratory findings
On intergroup comparison of hBD-2 in GCF of Group 1, Group 2, and Group 3 values were found to be 52.29 ± 46.41 pg/mL, 27.15±28.76 pg/mL, and 86.01 ± 68.82 pg/mL, respectively (P < 0.01) [Figure 4].
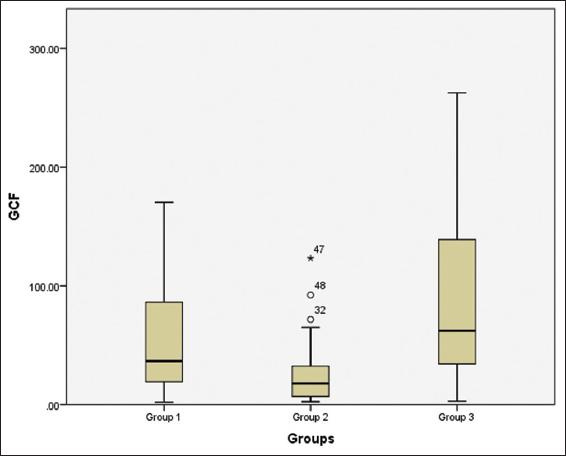
- Box plot showing the value of HBD-2 in GCF of the study participants in the three groups
The above box plot shows us the values for the minimum, the maximum, and the median value for the GCF of the patients of the three groups along with the 25th and the 75th percentile. We can also appreciate the presence of two outliers, no 32, 48, and one extreme outlier no 47 above the maximum value in Group 2. There are no outliers seen in Group 1 and 3. These have been classed as outliers by the SPSS version 20, Outliers are cases with values between 1.5 and 3 times the interquartile range, that is, beyond the whiskers.
Intergroup comparison of hBD-2 in serum of Group 1, Group 2, and Group 3 was found to be 813.72 ± 269.43 pg/mL, 591.50 ± 263.91 pg/mL, and 1093.04 ± 674.55 pg/mL respectively (P < 0.01) [Figure 5].
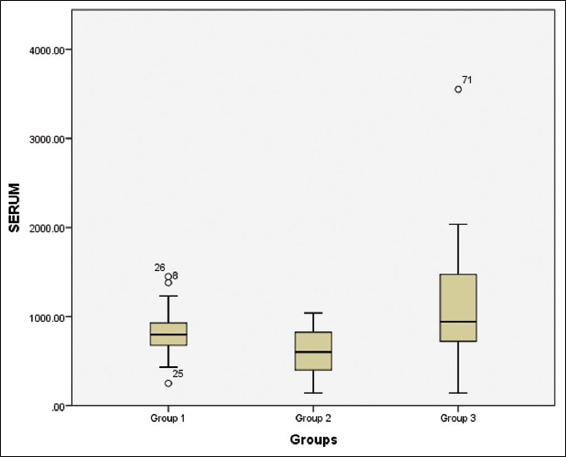
- Box plot displaying the value of HBD-2 in the Serum of the study participants in the three groups
The above box plot shows us the values for the minimum, the maximum, and the median value for the GCF of the patients of the three groups along with the 25th and the 75th percentile. We can also appreciate the presence of 3 outliers, no 8, 25, and 26 above and below the maximum and minimum value in Group 1. There are no outliers seen in Group 2 and 3. These have been classed as outliers by the SPSS version 20; Outliers are cases with values between 1.5 and 3 times the interquartile range, that is, beyond the whiskers.
Discussion
Periodontal disease, which often coexists with systemic or metabolic disorders, is highly prevalent within the population.[20] The correlation between periodontal disease and lung cancer has gathered significant attention due to their shared risk factors, including smoking, alcohol consumption, and diabetes.[21-23] The influence of oral pathogens on cancer signaling pathways is among the potential explanations for the association between periodontitis and the heightened risk of developing cancer, specifically lung cancer.[24] Furthermore, health education intervention for chronic disease patients in the target population resulted in significant but small positive changes in their lifestyles.[25]
In the current case–control study, a significantly high proportion of males were found to be diagnosed with lung cancer (76.7%). This is in accordance with results that showed that Men are more than twice as likely as women to be diagnosed with lung cancer and die of old age from it globally. As men are more prone to smoke tobacco, which is the main cause of the gender gap.[26] Michaud et al.[10] in their research showed a stronger link between severe periodontitis and an elevated risk of developing lung carcinoma in males than in females. On the contrary, based on the research conducted by Pesch et al. it was found that the incidence of lung cancer among women in industrialized nations is on the rise, while it is decreasing among men. This trend can be attributed to historical patterns of tobacco use among women, wherein they initially adopted smoking habits and later discontinued them.[27] According to Alshamsan (2020) a higher number of cancer cases were reported in female patients compared to males. The male-to-female ratio was 1:1.19, indicating that for every one male diagnosed with cancer, around 1.19 females were diagnosed.[28]
The lung cancer group had a higher mean age and there was a significant difference found between the groups’ mean ages (P < 0.001). This implies that the biological aging process also likely plays a role because as we age, our telomeres shorten, our levels of the metabolite NAD+ drop, and our cells become less resilient to DNA damage and less able to detect abnormal cells Siegel et al. (2020),[4] Campisi (2013),[29] Shay (2016).[30] Similarly to this, older people have a higher chance of developing all types of cancer including lung cancer Thun et al. (2006),[31] Danaei et al.,[32] as well as periodontal disease Newmann et al. (2006).[33] The socioeconomic status between the groups varied in our study and findings resonated with a study conducted by Yoon et al. (2018)[34] which stated that lung cancer patients were more likely to be less educated, earn less, had low incomes and most of them were illiterate groups. A similar study was conducted on periodontitis patients where the people surveyed of lower SES found symptoms of periodontal disease in more advanced stage Kim et al. (2018).[35]
Group 3 patients had a higher mean value of PI as compared to Group 2 and 1. Additional proof in favor of a causal relationship between cancer risk and periodontal disease comes from the biological plausibility of the relationships discovered between Porphyromonas gingivalis in dental plaque. As P. gingivalis is usually found in deep periodontal pockets, it has also been detected as microcolonies close to the pocket epithelium in the superficial layers of subgingival plaque Tan et al. (2014).[36] According to research, P. gingivalis predominantly encourages carcinogenesis by altering the cell cycle and immunological regulation Perera et al. (2016),[37] Arjunan et al. (2018).[38] P. gingivalis has been shown to suppress apoptosis in gingival epithelial cells by upregulating the JAK1/STAT pathway and downregulating the Bcl2 family of proteins, which typically regulates cell death through the mitochondria. P. gingivalis enhances the expression of several matrix metalloproteinase enzymes so that they can infiltrate epithelial cells, which facilitates the breakdown of collagen and intensifies the inflammatory, promalignant environment.[37]
While comparing the GI values of various group pairs at baseline, a highly significant difference was observed between groups (P < 0.001). According to Mohanty et al. (2019),[39] the red complex species Tannerella forsythia, Treponema denticola, and P. gingivalis showed a very strong correlation between pocket depth and bleeding on probing. They were also strongly linked to the clinical development of chronic periodontitis.[40,41] In a study, it was discovered that P. gingivalis (red complex pathogen usually associated with bleeding of gums) and Fusobacterium nucleatum can accelerate the development of tumor by triggering the toll-like receptors (TLR) on oral epithelial cells and IL6/STAT3 pathway Gallimidi et al. (2015).[42] TLR activation has been associated with immune system evasion, invasion, cellular proliferation, and inflammation Basith et al. (2012),[43] Park et al. (2011).[44]
On intergroup comparison of PPD at baseline, a highly significant difference was found between various groups (P < 0.001). A similar result was shown by a study where PPD was associated with an elevated risk of lung cancer Chrysanthakopoulos (2016).[45]
A highly significant difference between the groups was discovered during the intergroup comparison of CAL (P < 0.001). This outcome is consistent with a study that found that severe periodontitis (>30% of sites with attachment loss >3 mm) was associated with a higher risk of all cancers (lung cancer) than no or moderate periodontitis (3 mm), even after controlling for smoking and other variables. Patients with lung cancer and chronic periodontitis showed strong correlations, according to Michaud et al. (2018).[10] Similar findings were found in studies where the periodontal disease was associated with an increased risk of lung cancer Michaud et al. (2016),[46] Chung et al. (2019),[47] Chen et al. (2020),[48] and Chung and Chan (2020).[49] As PI, GI, PPD, and CAL are related to chronic periodontitis which is a chronic inflammatory condition of the periodontium. Free radicals and active intermediates produced by chronic inflammatory processes can lead to oxidative/nitrosative stress, which can lead to DNA mutations or impair DNA repair mechanisms Federico et al. (2007).[50] In addition, oral bacteria may encourage the production of known carcinogens including acetaldehyde and nitrosamines Ziebarth et al. (1997),[51] Meurman (2010).[52] Thus, a chronic inflammatory milieu can be created by dysbiotic microbiota and chronic inflammation.
Human beta defensin-2 is small cationic antimicrobial peptides which usually expressed in the presence of infection or inflammation. The hBD-2 has an anti-inflammatory impact by directly neutralizing lipopolysaccharide and preventing the release of pro-inflammatory cytokines like TNF-α.[53,54] By activating nearby dendritic cells, it also aids in the recruitment of neutrophils and connects innate and adaptive immune responses.[55] Hence, the GCF and serum were analyzed in this study for hBD-2 after the collection of sample from all groups. The level of Hbd-2 was lowest in group two patients in our study, results suggest that the expression and secretion of hBDs may be suppressed during periodontitis Gursoy and Könönen (2012)[55] and Brancatisano et al. (2011).[56] The result of our study resonated with the study conducted by Bissell et al., (2004)[57] and Hamanaka et al., (2001).[58] The study described that periodontally healthy tissues had higher levels of hBD-2 mRNA expression than periodontally diseased tissues. However, a study conducted by Pereira et al. (2020),[59] Öztürk et al. (2020)[19] explained that individuals with periodontitis presented higher levels of hBD-2 in the GCF. The intergroup comparison level of hBD-2in the serum of Group 3 was more. This result is in agreement with Arimura et al. (2004)[60] who had recorded significantly elevated concentrations of hBD-2 in the blood serum of lung cancer patient and other studies have documented an up-regulation of hBD-2 mRNAs in lung cancer compared to normal lung tissue samples Shestakova et al. (2008).[61] In contrast serum, hBD-2 levels in CP (group 2) were lower compared with those in healthy controls and lung cancer patients, and similar observations were detected in a study conducted by Öztürk et al. (2020).[19]
Conclusion
In our study, we found that all the clinical and biochemical factors that we measured were related to each other. In all groups, statistically significant differences were present in terms of PI, GI, PPD, and CAL. Interestingly, we observed that lung cancer patients with chronic periodontitis were more likely to be men. Moreover, we observed that the lung cancer and chronic periodontitis patients were generally older than the healthy participants. The Kuppuswamy socioeconomic scale (2020) was used to evaluate SES among groups. There was a significant difference found amongst groups. Lower SES patients were found to be more in lung cancer and chronic periodontitis patients as compared to healthy participants.
HBD-2 levels in GCF and serum were higher in lung cancer patients compared to healthy participants, while they were lowest in the chronic periodontitis group. Reduced production of HBD-2 was hypothesized in chronic periodontitis patients. HBD-2 shows potential as a diagnostic marker for lung cancer and chronic periodontitis, but further research is needed for validation.
Limitations
Indeed, to establish a more conclusive relationship between chronic periodontitis and lung cancer patients, further studies with larger sample sizes and interventions would be necessary. Increasing the number of participants in the study would provide a more comprehensive understanding of the potential connection between these two conditions. In addition, implementing specific interventions or treatments targeted at managing chronic periodontitis and examining their impact on lung cancer outcomes could shed more light on the relationship between these two health issues.
Acknowledgements
We are appreciative of the Department of Periodontology, microbiology, and Respiratory Medicine faculty and residents for their invaluable assistance with the study effort.
Authors’ Declaration Statements
Ethical approval and patients consent
The Institutional Ethics Committee at King George’s Medical University in Lucknow, Uttar Pradesh, granted its approval (Ref. code: III PGTSC-IIA/P37) and written informed consents were also taken from the participated patients.
Conflicts of interest
Nil.
Funding statement
Nil.
Authors’ contribution
All authors contributed equally.
References
- Global Cancer Statistics 2020:GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-49.
- [Google Scholar]
- Cancer incidence estimates for 2022 &projection for 2025:Result from National Cancer Registry Programme, India. Indian J Med Res. 2022;156:598-607.
- [Google Scholar]
- Munden RF. Lung cancer epidemiology, risk factors, and prevention. Radiol Clin North Am. 2012;50:863-76.
- [Google Scholar]
- An epidemiological study of risk factors for lung cancer in Guangzhou, China. Lung Cancer. 1996;14(Suppl 1):S9-37.
- [Google Scholar]
- Effect of non-surgical periodontal therapy on the serum and crevicular fluid interleukin-10 levels in chronic periodontitis - A systematic review and meta-analysis. Int J Health Sci (Qassim). 2021;15:34-46.
- [Google Scholar]
- Prevalence of periodontal disease among adults in India:A systematic review and meta-analysis. J Oral Biol Craniofac Res. 2020;10:800-6.
- [Google Scholar]
- Southern community cohort study:Establishing a cohort to investigate health disparities. J Natl Med Assoc. 2005;97:972-9.
- [Google Scholar]
- Periodontal disease assessed using clinical dental measurements and cancer risk in the ARIC Study. J Natl Cancer Inst. 2018;110:843-54.
- [Google Scholar]
- Cancer risk among gingivitis and periodontitis patients:A nationwide cohort study. QJM. 2014;107:283-90.
- [Google Scholar]
- The common risk factor approach:A rational basis for promoting oral health. Community Dent Oral Epidemiol. 2000;28:399-406.
- [Google Scholar]
- Tooth loss is associated with increased risk of gastric non-cardia adenocarcinoma in a cohort of Finnish smokers. Scand J Gastroenterol. 2005;40:681-7.
- [Google Scholar]
- Oral-lung microbiome interactions in lung diseases. Periodontol 2000. 2020;83:234-41.
- [Google Scholar]
- Meta-analysis reveals no correlation of caveolin-1 G14713A (G>A) gene polymorphism with increased cancer risk in Taiwanese population. Int J Health Sci (Qassim). 2018;12:3-9.
- [Google Scholar]
- Antimicrobial peptides in the oral environment:Expression and function in health and disease. Curr Issues Mol Biol. 2005;7:119-33.
- [Google Scholar]
- Defensins:Antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3:710-20.
- [Google Scholar]
- Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1-6.
- [Google Scholar]
- Comparison of gingival crevicular fluid and serum human beta-defensin-2 levels between periodontal health and disease. Oral Dis. 2021;27:993-1000.
- [Google Scholar]
- Evaluation of cardiac biomarkers in smokers and non-smokers with chronic periodontitis. Int J Health Sci (Qassim). 2020;14:26-32.
- [Google Scholar]
- Alcohol consumption and risk of lung cancer:A pooled analysis of cohort studies. Am J Clin Nutr. 2005;82:657-67.
- [Google Scholar]
- Periodontal pathogens as risk factors of cardiovascular diseases, diabetes, rheumatoid arthritis, cancer, and chronic obstructive pulmonary disease-is there cause for consideration? Microorganisms. 2019;7:424.
- [Google Scholar]
- Impact of health education on compliance among patients of chronic diseases in Al Qassim, Saudi Arabia. Int J Health Sci (Qassim). 2010;4:139-48.
- [Google Scholar]
- Cigarette smoking and lung cancer-relative risk estimates for the major histological types from a pooled analysis of case-control studies. Int J Cancer. 2012;131:1210-9.
- [Google Scholar]
- Trends of cancer incidence in Qassim Region, a descriptive analysis of data from the Saudi Cancer registry 2002-2016. Int J Health Sci (Qassim). 2022;16:21-31.
- [Google Scholar]
- Role of telomeres and telomerase in aging and cancer. Cancer Discov. 2016;6:584-93.
- [Google Scholar]
- Comparative Risk Assessment collaborating group (Cancers). Causes of cancer in the world:Comparative risk assessment of nine behavioural and environmental risk factors. Lancet. 2005;366:1784-93.
- [Google Scholar]
- Epidemiology of gingival and periodontal diseases. In: Newmann MG, Takei HH, eds. Text Book of Carranza's Clinical Periodontology. St Louis, Missouri: Saunders; 2006. p. :86-91.
- [Google Scholar]
- Association of oral health with lung cancer risk in a low-income population of African Americans and European Americans in the Southeastern United States. Lung Cancer. 2019;127:90-5.
- [Google Scholar]
- Socioeconomic status and self-reported periodontal symptoms in community-dwelling individuals:Data from the Korea Community Health Surveys of 2011 and 2013. Int Dent J. 2018;68:411-9.
- [Google Scholar]
- Porphyromonas gingivalis and treponema denticola exhibit metabolic symbioses. PLoS Pathog. 2014;10:e1003955.
- [Google Scholar]
- Emerging role of bacteria in oral carcinogenesis:A review with special reference to perio-pathogenic bacteria. J Oral Microbiol. 2016;8:32762.
- [Google Scholar]
- Oral pathobiont activates anti-apoptotic pathway, promoting both immune suppression and oncogenic cell proliferation. Sci Rep. 2018;8:16607.
- [Google Scholar]
- Red complex:Polymicrobial conglomerate in oral flora:A review. J Family Med Prim Care. 2019;8:3480-6.
- [Google Scholar]
- Life below the gum line:Pathogenic mechanisms of Porphyromonas gingivalis. Microbiol Mol Biol Rev. 1998;62:1244-63.
- [Google Scholar]
- Periodontal pathogens Porphyromonas gingivalis and Fusobacterium nucleatum promote tumor progression in an oral-specific chemical carcinogenesis model. Oncotarget. 2015;6:22613-23.
- [Google Scholar]
- Roles of toll-like receptors in cancer:A double-edged sword for defense and offense. Arch Pharm Res. 2012;35:1297-316.
- [Google Scholar]
- Toll-like receptor 5 activation promotes migration and invasion of salivary gland adenocarcinoma. J Oral Pathol Med. 2011;40:187-93.
- [Google Scholar]
- Correlation between periodontal disease indices and lung cancer in Greek adults:A case-control study. Exp Oncol. 2016;38:49-53.
- [Google Scholar]
- Periodontal disease and risk of all cancers among male never smokers:An updated analysis of the Health Professionals Follow-up Study. Ann Oncol. 2016;27:941-7.
- [Google Scholar]
- Periodontal disease and tooth loss are associated with lung cancer risk. Biomed Res Int. 2020;2020:5107696.
- [Google Scholar]
- Association between periodontitis and all-cause and cancer mortality:Retrospective elderly community cohort study. BMC Oral Health. 2020;20:168.
- [Google Scholar]
- Chronic inflammation and oxidative stress in human carcinogenesis. Int J Cancer. 2007;121:2381-6.
- [Google Scholar]
- N-nitrosation of medicinal drugs catalysed by bacteria from human saliva and gastro-intestinal tract, including Helicobacter pylori . Carcinogenesis. 1997;18:383-9.
- [Google Scholar]
- Antibacterial and lipopolysaccharide (LPS)-neutralising activity of human cationic antimicrobial peptides against periodontopathogens. Int J Antimicrob Agents. 2010;35:138-45.
- [Google Scholar]
- Susceptibilities of periodontopathogenic and cariogenic bacteria to antibacterial peptides, {beta}-defensins and LL37, produced by human epithelial cells. J Antimicrob Chemother. 2005;55:888-96.
- [Google Scholar]
- Understanding the roles of gingival beta-defensins. J Oral Microbiol. 2012;4:15127.
- [Google Scholar]
- Reduced human beta defensin 3 in individuals with periodontal disease. J Dent Res. 2011;90:241-5.
- [Google Scholar]
- Expression of beta-defensins in gingival health and in periodontal disease. J Oral Pathol Med. 2004;33:278-85.
- [Google Scholar]
- Expression of human beta-defensin 2 (hBD-2) in Helicobacter pylori induced gastritis:Antibacterial effect of hBD-2 against Helicobacter pylori. Gut. 2001;49:481-7.
- [Google Scholar]
- Gingival crevicular fluid levels of human beta-defensin 2 and 3 in healthy and diseased sites of individuals with and without periodontitis. J Int Acad Periodontol. 2020;22:90-9.
- [Google Scholar]
- Elevated serum beta-defensins concentrations in patients with lung cancer. Anticancer Res. 2004;24:4051-7.
- [Google Scholar]
- Expression of human beta-defensins-1, 2 and 4 mRNA in human lung tumor tissue:A pilot study. Exp Oncol. 2008;30:153-6.
- [Google Scholar]







