Translate this page into:
Reactogenicity and persistence of IgG antibodies against SARS-CoV-2 among recipients of ChAdOx1 nCoV-19 vaccine: A single center experience from Sri Lanka
Address for correspondence: Dr. Dumitha Govindapala, Department of Clinical Sciences, Faculty of Medicine, General Sir John Kotelawala Defence University, Rathmalana, Sri Lanka. E-mail: dumithagovindapala@kdu.ac.lk
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Qassim Uninversity and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objectives:
Actual world data on vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are imperative for future immunization decisions. We studied the reactogenicity and IgG response in a cohort dually vaccinated with the ChAdOx1 nCoV-19 vaccine.
Methods:
This prospective study recruited 494 ChAdOx1 nCoV-19 vaccine recipients at the University Hospital KDU between January 30 and February 5, 2021, and followed up for 9 months. The two doses of the vaccine were administered 3-month apart, followed by a booster dose with the BNT162b2 (Pfizer-BioNTech) vaccine 6 months later. One-week post-vaccination surveillance ascertained the reactogenicity of the vaccine. Seroprevalence of IgG antibodies before each vaccination dose was determined using a commercially available quantitative ELISA kit (WANTAI SARS-CoV-2 IgG Quantitative ELISA Beijing China). Reactogenicity profiles after vaccination doses were compared. Association of pre-vaccination seropositivity and demographic variables with antibody levels was assessed.
Results:
Reactogenicity was reported by 78.5% (329/419) and 25.4% (104/410) participants after the first and second doses, respectively, with a significantly high mean total score of vaccine-related symptoms following the first dose (P = 0.015). Post-first dose seroconversion rate was 97.1%, and the immune response was more robust among pre-vaccination seropositive participants and females. Following the second dose, 100% seroconversion was observed. Subgroup analysis of 196 participants revealed persistent antibodies at nine months with a rise in the previously measured levels among 78.1% compared to 21.9% with declining titers. Antibody waning was significantly associated with pre-vaccination seropositivity (P = 0.015) and female gender (P = 0.022).
Conclusions:
High seroconversion rates and longevity of antibody response in the absence of serious concerns regarding reactogenicity suggest that the vaccine is immunogenic and safe. Significant antibody waning among females and pre-vaccination seropositive participants warrant further research.
Keywords
ChAdOx1 nCoV-19 vaccine
coronavirus disease 2019
IgG seroprevalence
reactogenicity
SARS-CoV-2
Introduction
Vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was considered the most plausible approach to end the coronavirus disease 2019 (COVID-19) pandemic. Thus, many vaccines were developed within a short duration and rapidly deployed globally. Mitigation of the COVID-19 pandemic was observed after commencing the mass vaccination programs, especially in regions with high vaccination coverage.[1] The ChAdOx1 nCoV-19 was the first approved vaccine in Sri Lanka and the vaccination program commenced on January 29, 2021, with the immunization of healthcare workers in the country.
The acceptability of a vaccine is mainly dependent on its safety profile. The safety of the ChAdOx1 nCoV-19 vaccine was demonstrated in Phase 1–3 clinical trials.[2] Nonetheless, there were safety concerns after commencing the vaccine rollout programs in many countries.[3] Although booster dosing against COVID-19 is recommended, vaccine hesitancy and refusal are observed among people due to the fear of adverse reactions.[4,5] Therefore, data on reactogenicity to COVID vaccines among different populations are essential in alleviating vaccine hesitancy.
The post-vaccination immune response is dynamic and has yet to be elucidated fully. Although humoral immunity constitutes only a part of the immune response to the vaccine, it is far easier to detect due to its widespread use and standardization.[6] Antibodies prevent systemic dissemination of SARS-CoV-2 and protect against mortality.[7] Hence, accurate measurement of the antibody response following vaccination plays an important role in determining the efficacy of the vaccine. Many factors affect the antibody response depending on the individual and the type of vaccine.[8]
A clear understanding of real-world data on immunogenicity is crucial to plan future vaccine strategies in the most cost-effective way, especially in resource-limited settings. Dual comparative investigations of reactogenicity and immunogenicity are essential to ascertain the safety of the vaccines against SARS-CoV-2, the potential need for booster doses in different populations and to decide the interval between periodical boosters. In the present work, we assessed the reactogenicity, humoral immune response, persistence of IgG response and factors associated with antibody waning among a cohort of ChAdOx1 nCoV-19 recipients from Sri Lanka.
Methods
Study setting and population
This prospective cohort study invited all staff members of the University Hospital KDU, who received the prime dose of the ChAdOx1 nCoV-19 vaccine from January 30 to February 5, 2021, at the hospital vaccination center. All consented recipients were recruited consecutively using the vaccination register as the sampling frame and followed up until the booster dose. The second dose of the ChAdOx1 nCoV-19 vaccine was administered after 3 months (April–May 2022), followed by a booster dose with BNT162b2 (Pfizer-BioNTech) vaccine at nine months (October–November 2022) of the prime dose. Recipients of other types of vaccines and the staff members who received the ChAdOx1 nCoV-19 vaccine at other centers or at different time intervals were excluded from the study.
Data collection
The data regarding the demographic characteristics, comorbidities, the previous diagnosis of COVID-19 or exposure,and post-vaccination reactogenicity were gathered using a pre-tested structured questionnaire. The recipients were observed for immediate reactions during the first 30 min at the vaccination center. Then active surveillance of the post-vaccination symptoms was done for up to 1 week, where recipients were enquired telephonically 72-h and 1 week after each dose of vaccination. A scoring system was devised to quantify the reactogenicity, which allocated one mark for each post-vaccination symptom with a maximum score of five.
Blood sampling
Blood samples (3 mL) were drawn at three-time points. Sampling was done before the first dose of the vaccine (baseline) to assess the pre-vaccination antibody status of the participants. The other two samples were collected just before the second dose and the booster dose (3 months and nine months after the first dose of the vaccine, respectively) [Figure 1]. Blood sampling was done under strict aseptic conditions by two qualified phlebotomists, where the skin was disinfected with a 70% alcohol swab for 30 s and allowed to dry completely before the collection of samples. Serum was separated according to the standard operating procedures, aliquoted, and stored at −70°C at the research laboratory of KDU until the antibody test was performed.

- Flowchart of participant selection and serology sample collection
SARS CoV-2 antibody measurement
IgG antibodies against SARS-CoV-2 were estimated using a commercially available quantitative ELISA kit (WANTAI SARS-CoV-2 IgG Quantitative ELISA Beijing China; WS-1396) as per the instructions of the manufacturer. Pre-vaccination serology samples were analyzed without dilution, while post-vaccination samples were diluted 4 times with specimen diluent before processing. According to the manufacturer, the detection range spanned from 1.0U/mL~32.0U/mL. Values higher than 1.0 U/mL were considered positive. The specificity of the ELISA kit was 99.4% and the sensitivity was around 81.16%.[9]
Statistical analysis
Descriptive statistics were performed and data for categorical variables were presented as frequencies and proportions. Median and interquartile range were used to describe continuous data with a skewed distribution. The Shapiro-Wilk test was used to assess the distribution normality of data. Comparisons between groups were done using the Chi-squared test, t-test and ANOVA. IBM SPSS statistics version 25 was used to analyze all data presented in this study. Statistical significance was defined as P < 0.05.
Ethical considerations
Ethics approval for this study was obtained from Ethics Review Committee, General Sir John Kotelawala Defence University (Reference: RP/2021/08). Informed written consent was obtained from all study participants at the beginning of the survey and before collection of blood samples.
Results
Demographic characteristics
A total of 494 staff members consented to participate in this study and for the successive blood sampling at 3 months after the first dose and 6 months after the second dose of vaccination, 382 and 221 responded, respectively. Hundred and ninety-six (39.7%) study participants provided blood samples at all 3-time points [Figure 1].
Majority of the study population were below 40 years (339/494, 68.6%) with a median age of 33 years (IQR = 28–43). More than half the participants were males (265/494, 53.6%). Comorbidities were infrequent in this study group (94/494, 19%), with hypertension (33/494, 6.7%) and diabetes mellitus (32/494, 6.5%) being the most reported pre-existing medical conditions. Less than 1% of the study participants were diagnosed with COVID-19, while 57 (11.5%) were quarantined due to high-risk exposures before the vaccination. The sociodemographic characteristics of the participants who provided blood samples at all 3-time points (subgroup) were comparable to the whole group [Table 1].
Reactogenicity profile
Of the 494 participants, post-vaccination surveillance was completed by 419 (84.8%) and 410 (83.0%) after the first and second doses of the vaccine, respectively. Following the first dose, 329 (78.5%) participants reported experiencing at least one vaccine-related symptom. In contrast, post-vaccination symptoms were less prevalent (104/410, 25.4%) after the second dose. Systemic reactions were more frequent among the first dose recipients (317/419, 75.9%), whereas local symptoms were predominant (74/410, 18.0%) after the second dose. Fever was the commonest post-vaccination symptom among first dose recipients (241/419, 58.7%), followed by pain/tenderness at the injection site (233/419, 55.6%) and body/muscle aches (208/419, 49.6%). Following the second dose, pain/tenderness at the injection site was the most frequent symptom (72/410, 17.6%) [Figure 2].
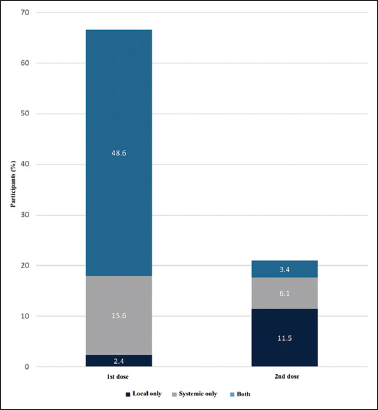
- Proportions of reported local and systemic reactions after 1st dose versus 2nd dose of ChAdOx1 nCoV-19 vaccine
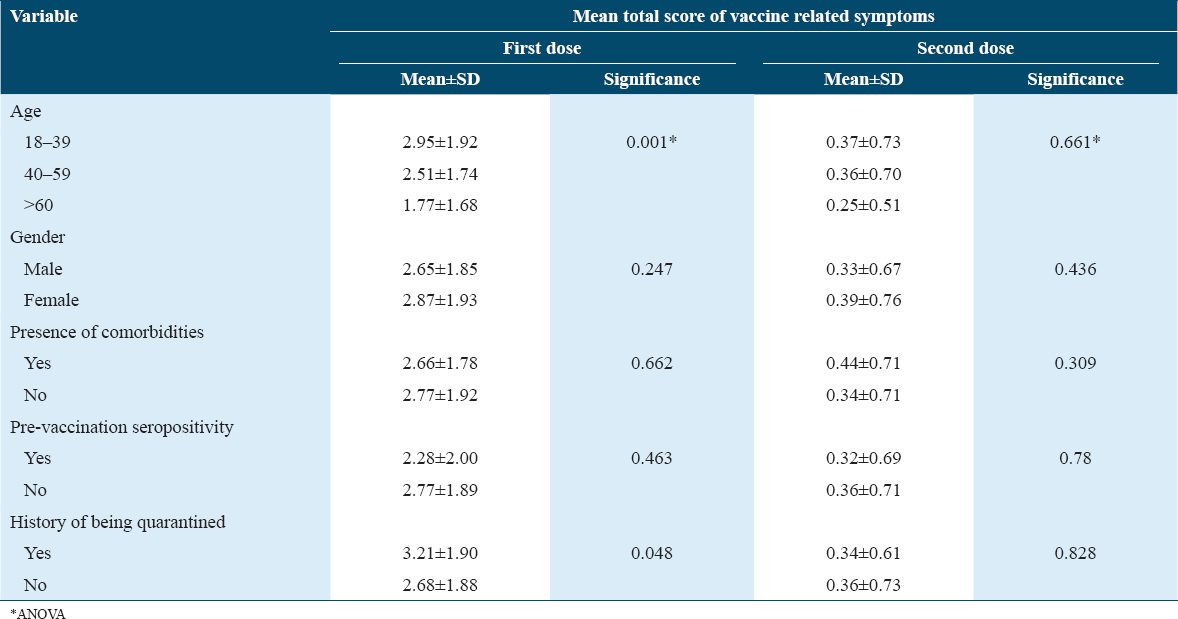
The total score of vaccine-related symptoms was calculated and the mean total score was significantly higher following the first dose compared to the second dose (2.75 ± 1.89 vs. 0.36 ± 0.71, respectively, P < 0.001). Post-vaccination symptoms were more frequent among younger participants (<40 years) than in recipients above 40 years. The mean total score of symptoms observed across age groups was statistically significant after the first dose (P < 0.01) but not after the second dose. Females had a high mean total score than men following both doses of vaccination. Nonetheless, the observed difference was statistically not significant [Table 2].
Humoral immune response
Pre-vaccination antibodies were detected in 30 participants (30/494, 6.1%), indicating previous SARS-CoV-2 infection. The antibody levels were low, with a mean antibody titer of 0.5 U/mL ± 2.6. Two of the three participants, who had a previous diagnosis of COVID-19, tested negative for pre-vaccination antibodies. Noteworthy, most of the pre-vaccination seropositive participants (25/30, 83.3%) were not being quarantined or diagnosed with COVID-19 previously.
Excluding the participants who were lost to follow-up, a positive humoral immune response was observed among 371/382 (97.1%) 3 months after receiving the first dose of the vaccine with a mean antibody titer of 22.5 U/mL ± 14.9. The post-first dose antibody response was more profound among seropositive participants before vaccination than those who were seronegative (32.7 U/mL vs. 21.9 U/mL, P = 0.002). The mean antibody titers were significantly higher among females than in males after the first dose (24.2 U/mL vs. 21.0 U/mL, P = 0.041). At 3 months, post-first dose mean antibody levels were comparable between participants <40 years and more than 40 years. Similarly, no significant difference in the mean antibody titers was observed between participants with comorbidities and without comorbidities.
Six months after the second dose of vaccination (nine months after the first dose), only 221 (44.7%) participants responded, and IgG antibodies were detected in all of them with a higher mean antibody titer (39.3 U/mL ± 14.2) [Figure 3].
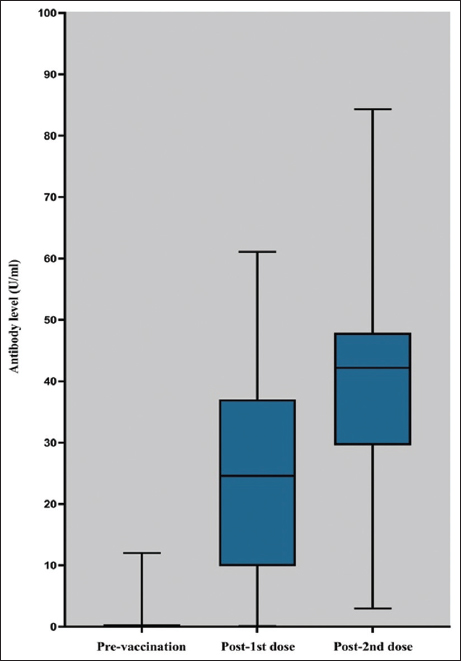
- Seroprevalence of IgG antibodies before and after vaccination among recipients of ChAdOx1 nCoV-19 vaccine
Subgroup analysis of the participants who have completed serological sampling at all 3-time points
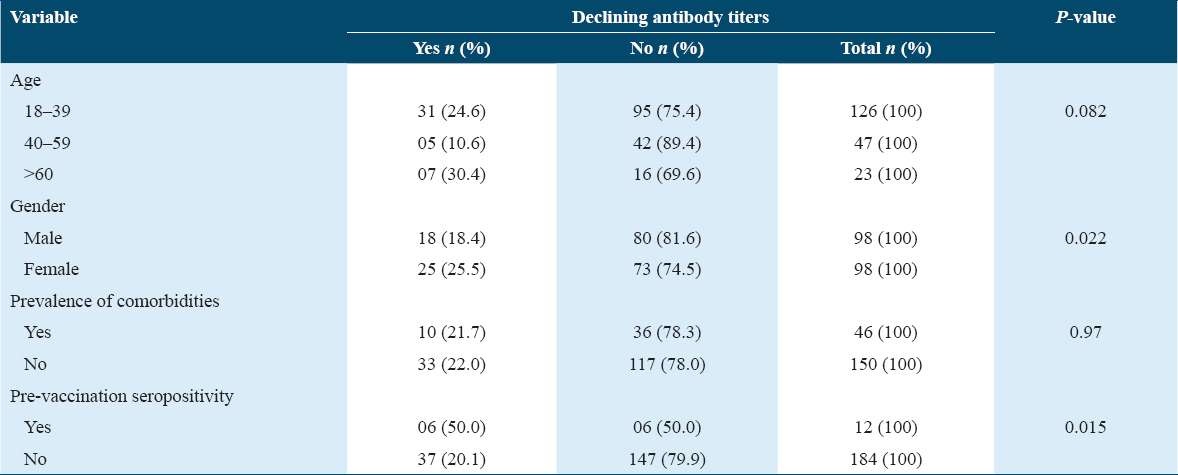
Blood samples were drawn from 196 participants at all three-time points and baseline IgG antibodies were positive in 12 (6.1%) of them. Three months after receiving the first dose 97.0% seroconversion rate was observed, where participants with pre-vaccination seropositivity elicited a higher antibody response than individuals who were seronegative at baseline (mean Ab titer 35.3 vs. 23.7 P = 0.015). Six months after the second dose, all 196 participants were seropositive and a rise in the previously measured antibody levels was found in 153/196 (78.1%) of them while declining antibody titers were observed in 43/196 (21.9%). One participant had SARS-CoV-2 infection after the first dose of vaccination. None of the participants reported breakthrough infection following the second dose until the time of the third blood sample collection.
Antibody waning at 6 months showed a significant association with pre-vaccination seropositivity (P = 0.015) and female gender (P = 0.022). Antibody decline was frequent among participants older than 60 years (n = 7/23, 30.4%), while the age category 40–59 years included the least proportion of participants with declining titers (n = 5/47, 10.6%). However, the observed difference in antibody waning across age groups was statistically not significant. Moreover, comorbid status of the recipient had no significant association with declining IgG levels [Table 3].
Discussion
This study sought the reactogenicity and the longevity of the humoral immune response in a cohort dually vaccinated with the ChAdOx1 nCoV-19 vaccine. We found high seroconversion rates with the persistence of IgG antibodies for up to 9 months in the studied population. Pre-vaccination seropositivity and female gender were associated with higher post-first dose IgG levels, as was a faster decay of IgG levels. A significant reduction in reactogenicity was observed following the second dose of vaccination.
In the present study, post-vaccination reactions were reported to be much lower than anticipated in clinical trials.[10] Fever was the predominant systemic reaction to the first dose of vaccination in our population compared to fatigue, muscle aches, and headache reported in numerous studies.[11-13] Notably, two regional studies also reported fever as the commonest systemic symptom following the first dose of vaccination.[14,15] In accordance with previous studies on the ChAdOx1 nCoV-19 vaccine, the reactogenicity to the second dose was significantly less among our population compared to the first dose.[10,14,15] Interestingly, this lower reactogenicity with each additional dose was not common for all vaccines, where the recipients of mRNA vaccines showed more frequent and severe adverse reactions following the second dose.[16] Female gender, young age and prior SARS-CoV-2 infection were associated with high vaccine reactogenicity in the previous studies.[11,12,17] We observed similar high reactogenicity among young adults (<40 years) following the first dose but not after the second dose. No association between participants’ degree of reactogenicity and gender or baseline seropositivity could be identified. Neither thrombotic events linked to the vaccine nor life-threatening adverse reactions were observed in our population.
Despite our study population being potentially more at risk of being exposed to COVID-19 patients, the pre-vaccination seropositivity was lower (6.1%) than that of the general population (24.46%).[18] Participants with pre-vaccination seropositivity demonstrated a more robust post-first dose immune response in terms of the magnitude of antibody levels than the seronegative group. The pre-vaccination seropositivity indicates the previous SARS-CoV-2 infections despite almost all being asymptomatic and undiagnosed in our population. The natural infection stimulates B and T cells, whereas B cells get stimulated following vaccination. Therefore, the observed profound antibody response following the first dose among pre-vaccination seropositive participants is attributed to the vaccine’s booster effect and has reported in the literature.[19,20]
When comparing the seroconversion rates and antibody tires following the first and second doses, our findings were in accordance with the original phase 2/3 trials. The clinical trials observed the development of IgG antibodies within 2 weeks after vaccination and a further rise in titers with a seroconversion rate of 99% at 14 days following the second dose.[21] However, the post-vaccination seropositivity rates in the real-world data were inconsistent across the various geographical areas. A study from Saudi Arabia reported a lower seroconversion rate (79.6%) after the first dose of the ChAdOx1 nCoV-19 vaccine but 98.3% seropositivity following the second dose.[22] The post-vaccination seropositivity at 28 days after the first dose was 81.9% among healthcare workers in Eastern India.[23] In the same subcontinent, the seroconversion rate was 69.7% among healthcare workers in a cardiac center following the second dose of vaccination.[24] Our study observed 97.1% and 100% seropositivity following the first and second doses. Compared to the 4–6 weeks dosing interval in the Indian studies, a 12-week gap was maintained between the two doses in Sri Lanka to ensure maximum deployment of the first doses to many people in the country. The robust seroconversion rates in our study may reflect the effectiveness of the adapted vaccination strategy. Better immunogenicity and higher vaccine efficacy had been reported in the previous studies with a longer interval between the two doses.[25]
On evaluating the durability and magnitude of IgG response among 196 participants who provided blood samples at all-time points, a rise in the previously measured antibody titers were observed in the majority 6 months after the second dose (nine months after the first dose). None of them reported SARS-CoV-2 infection between the second and third blood sample collection. Nonetheless, the possibility of asymptomatic infection cannot be ruled out and may have influenced the antibody titers at 6 months. Although the kinetics of antibody response to vaccines against SARS-CoV-2 infection is not fully understood, waning has been observed after immunization.[26] The durability of the post-vaccination humoral immunity and the degree of antibody decline reported in the previous studies were incongruous. In addition to the variations in vaccine regimes, geographical and racial differences and exposure to SARS-CoV-2 may influence recipients’ post-vaccination humoral immune response. A 6-month follow-up study of Indian healthcare workers revealed a two-fold decrease in antibody titers among ChAdOx1 nCoV-19 recipients following the second dose of the vaccination, where the decline in IgG levels was observed from the 3rd month onwards.[23] A study among 30 healthcare workers in India showed a consistent rise in the antibody titers up to 3 weeks after the first dose and a steady fall from the 1st week after the second dose. The reported trend was common for both previously SARS-CoV-2 infected and non-infected individuals.[19] Thus, persistent higher antibody titers observed among more than two-thirds of our study population is exceptional.
The protection imparted by the IgG antibodies against SARS-CoV-2 infection and the required protection threshold has not been established yet.[27] Nonetheless, the significant correlation between neutralizing and anti-S IgG antibodies shown in the previous studies suggests that these antibodies are effective in neutralizing the virus.[28,29] IgG antibodies can be detected easily in most laboratories. Hence, a selective booster vaccination strategy than a mass vaccination approach based on IgG levels is presumably more cost-effective, especially for developing countries. Such a targeted immunization strategy to maximize the limited resources imparts the need for further studies on the usefulness of IgG antibody levels in booster dose decisions.
Despite profound immune response to the first vaccination dose, the significant antibody waning among females and pre-vaccination seropositive participants following the second dose is striking. In contrast to our finding, immunization after SARS-CoV-2 infection had shown a more durable humoral immune response as compared to two-dose vaccination in a SARS-CoV-2-naïve Individuals.[30] Nonetheless, confirming the findings of many studies, the antibody decay showed no statistically significant difference across age or according to comorbid status.[23] As old age and comorbidity are predictors of severe COVID-19, the observed sustained humoral immunity following vaccination in these groups suggest prolonged protection against severe disease.[31,32]
To the best of our knowledge, the present study is the first to compare the reactogenicity and immunogenicity of the ChAdOx1 nCoV-19 vaccine in Sri Lanka. However, given our limited resources, blood samples were drawn only at 3-time points for the evaluation of humoral immune response and neutralizing titers were not estimated. This is the main limitation of our study, where serial blood samples would have given a better understanding of the antibody response. We only studied the reactogenicity up to 1 week following each dose of the vaccine and the lack of data on long-term adverse effects of the vaccine is another limitation. The study included only a cohort of hospital staff, which limits the generalizability of the results. Nevertheless, this study provides insight into adapting comprehensive and effective vaccination strategies, especially in a resource-limited setting.
Conclusions
Although post-vaccination symptoms were common after the first dose, a significant reduction in the reactogenicity was observed following the second dose. None of the participants had vaccine-related thrombotic events or life-threatening adverse reactions. Post-first dose seroconversion rates were high, and the participants remained seropositive even nine months after the first dose. Therefore, the ChAdOx1 nCoV-19 vaccine is safe and immunogenic in the studied population. The observed sustained humoral response following vaccination with waning among a small proportion of participants suggests the possibility of selective booster immunization based on IgG levels than mass vaccination of populations against SARS-CoV-2. However, the significant waning of antibodies following the second dose among pre-vaccination seropositive participants and females needs broader population studies for a better understanding of the dynamics of immune response to the ChAdOx1 nCoV-19 vaccine.
Author’s Declaration Statements
Availability of data and material
The supporting data and material of this study are available from the corresponding author on request. Participant’s de-identification will be maintained in sharing the data.
Conflict of interest statement
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Ethics approval for this study was obtained from of the General Sir John Kotelawala Defence University (Reference: RP/2021/08). Informed written consent was obtained from all participants of this study. Participants consented for publication of analyzed data without revealing their identity.
Funding
This work was supported by the General Sir John Kotelawala Defence University (Grant number: KDU/RG/2021/FOM/005). The funding source had no involvement in conduction of the research or preparation of the manuscript.
Author Contributions
Conceptualization: DG, Design of research study: DG, DN, WW, NSF, USS, Data acquisition: DG, USS, TDW, DN, TNS, PK UK, Data analysis: DG, DD, WW, USS, NSF, PK, TNS, UK, Interpretation of results: DG, DD, DL, NSF, PK, TNS, UK, Writing the manuscript: DG, DD, DL, WW, ADS, NSF.
Funding acquisition: DG, All authors contributed and approved the final draft of the manuscript.
Acknowledgment
The authors acknowledged Kotelawala Defence University for financial assistance.
References
- BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412-23.
- [Google Scholar]
- Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2:An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99-111.
- [Google Scholar]
- 2022. Coronavirus Vaccine-summary of Yellow Card Reporting. Available from: https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting#yellow-card-reports
- Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England. Nat Med. 2022;28:831-7.
- [Google Scholar]
- COVID-19 vaccine acceptance, hesitancy and refusal among Iraqi Kurdish population. Int J Health Sci (Qassim). 2022;16:10-6.
- [Google Scholar]
- SARS-CoV-2 serologic assay needs for the next phase of the US COVID-19 pandemic response. Open Forum Infect Dis. 2021;8:ofaa555.
- [Google Scholar]
- Low anti-SARS-CoV-2 S antibody levels predict increased mortality and dissemination of viral components in the blood of critical COVID-19 patients. J Intern Med. 2022;291:232-40.
- [Google Scholar]
- Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019;32:e00084.
- [Google Scholar]
- 2021. Wantai SARS CoV2 IgG Quantitative Elisa for IgG Antibody to COVID-19. Available from: https://www.bioscience.co.uk/userfiles/pdf/WANTAI_SARS_CoV_2_IgG_ELISA_Quantitative_CE_IFU.pdf
- Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002):A single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396:1979-93.
- [Google Scholar]
- Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID symptom study app in the UK:A prospective observational study. Lancet Infect Dis. 2021;21:939-49.
- [Google Scholar]
- Safety of ChAdOx1 nCoV-19 vaccine:Independent evidence from two EU States. Vaccines (Basel). 2021;9:673.
- [Google Scholar]
- Prevalence of severe adverse events among health professionals after receiving the first dose of the ChAdOx1 nCoV-19 coronavirus vaccine (Covishield) in Togo, March 2021. Arch Public Health. 2021;79:207.
- [Google Scholar]
- Adverse events following the first dose of ChAdOx1 nCoV-19 (COVISHIELD) vaccine in the first phase of vaccine roll out in Nepal. J Patan Acad Health Sci. 2021;8:9-17.
- [Google Scholar]
- Adverse events following COVISHIELD vaccination among adult population in Bangladesh. SN Compr Clin Med. 2021;3:2207-13.
- [Google Scholar]
- Reactogenicity after the first and second doses of BNT162b2 mRNA coronavirus disease vaccine:A single-center study. Clin Exp Vaccine Res. 2021;10:282-9.
- [Google Scholar]
- Survey of adverse events after the first dose of the ChAdOx1 nCoV-19 vaccine:A single-center experience in Korea. Infect Chemother. 2021;53:557-61.
- [Google Scholar]
- Seroprevalence of SARS-CoV-2 infection in the Colombo municipality region, Sri Lanka. Front Public Health. 2021;9:724398.
- [Google Scholar]
- Antibody response against SARS-CoV-2 following ChAdOx1(Astezeneca AZD 1222) vaccine in health care workers in a tertiary care hospital in India. Mod Med. 2021;28:307-13.
- [Google Scholar]
- Prior SARS-CoV-2 infection rescues B and T cell responses to variants after first vaccine dose. Science. 2021;372:1418-23.
- [Google Scholar]
- Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2:A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467-78.
- [Google Scholar]
- Reactogenicity and immunogenicity of the Pfizer and AstraZeneca COVID-19 vaccines. Front Immunol. 2021;12:794642.
- [Google Scholar]
- Persistence of antibodies against spike glycoprotein of SARS-CoV-2 in healthcare workers post double dose of BBV-152 and AZD1222 vaccines. Front Med (Lausanne). 2021;8:2469.
- [Google Scholar]
- Study of immunogenicity, safety and efficacy of covishield vaccine among health care workers in a tertiary cardiac care centre. Indian J Med Microbiol. 2022;40:200-3.
- [Google Scholar]
- Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine:A pooled analysis of four randomised trials. Lancet. 2021;397:881-91.
- [Google Scholar]
- Immunological memory to SARS-CoV-2 assessed for up to eight months after infection. Science. 2021;371:eabf4063.
- [Google Scholar]
- Persistence of antibody and cellular immune responses in coronavirus disease 2019 patients over nine months after infection. J Infect Dis. 2021;224:586-94.
- [Google Scholar]
- BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics:A prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir Med. 2021;9:999-1009.
- [Google Scholar]
- Antibody response to the BNT162b2 mRNA COVID-19 vaccine in subjects with prior SARS-CoV-2 infection. Viruses. 2021;13:422.
- [Google Scholar]
- Humoral immunity in dually vaccinated SARS-CoV-2-naïve individuals and in booster-vaccinated COVID-19-convalescent subjects. Infection. 2022;50:1475-81.
- [Google Scholar]
- Clinical and epidemiological characteristics and outcomes of Coronavirus disease-19 patients in a large longitudinal study. Int J Health Sci (Qassim). 2021;15:29-41.
- [Google Scholar]
- Association of comorbidities with the COVID-19 severity and hospitalization:A study among the recovered individuals in Bangladesh. Int J Health Sci (Qassim). 2022;16:30-45.
- [Google Scholar]







