Translate this page into:
Restoration of renal hemodynamics and functions by Nigella sativa administration in dinitrophenol-induced hypoxia in rat’s animal model
Address for correspondence: Ola M. Omran, Department of Pathology, College of Medicine, Qassim University, Buraidah, Qassim Region, Saudi Arabia/Department of Pathology, Faculty of Medicine Assiut University, Assiut, Egypt. E-mail: ola67oh@yahoo.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Qassim Uninversity and was migrated to Scientific Scholar after the change of Publisher.
Abstract
ABSTRACT
Objective:
Hypoxia is one of the principal causes of renal diseases. This study aimed to evaluate the effects of Nigella sativa on dinitrophenol (DNP)-induced hypoxia renal damage in rats.
Methods:
Forty adult male rats were incorporated in this study. The rats were divided into four groups: control group, N. sativa group, DNP hypoxic group, and DNP + N. sativa group receiving N. sativa (400 mg/kg body weight). Serum and renal tissue erythropoietin (EPO) hormone and hypoxia-inducible factor-2α (HIF-2α) levels were measured. Renal oxidative stress biomarkers, inflammatory biomarkers, renal hemodynamics, and histopathological examination were evaluated.
Results:
Administration of N. sativa highly significantly normalized serum EPO level, HIF-2α (P < 0.001 for each) in DNP + N. sativa treated rats as compared to DNP hypoxic rats. Furthermore, it highly significantly improved renal oxidative stress evident by decreased renal tissues malondialdehyde and increased superoxide dismutase, total thiol, and catalase activity (P < 0.001 for each). Furthermore, a highly significant decline of renal intercellular adhesion molecule-1, myeloperoxidase, and interleukin-6 was observed in DNP + N. sativa rats (P < 0.001 for each). Improvements in renal hemodynamics and kidney functions were also found after N. sativa administration (with P < 0.001 for all parameters). In addition, N. sativa treatment reduced renal histopathological changes of the DNP + N. sativa group. Our results were statistically analyzed using the Prism software package (GraphPad version 8.0).
Conclusion:
N. sativa has an alleviating effect on DNP-induced hypoxia renal damage and can restore kidney functions in rats’ animal models. These effects were through antioxidant, anti-inflammatory, and hemodynamic mechanisms.
Keywords
Dinitrophenol
erythropoietin
hypoxia-inducible factor-2α
hypoxia
inflammatory biomarkers
Nigella sativa
oxidative stress
renal damage
Introduction
Hypoxia is a situation of diminished oxygen (O2) availability and is involved in the pathophysiology of various human diseases.[1] The kidneys take about 1/5 of the cardiac output at rest and have a low oxygen extraction ratio, large regional differences in renal blood flow (RBF) and oxygenation are to blame toward this.[2] Low O2 tensions are caused by the peculiar arrangement of the kidney’s vascular system, where the rate of regional blood input to the inner and outer medulla is less than that to the renal cortex. This results in a physiological condition of ongoing hypoxia.
Under various illness situations, this could have an impact on renal physiology and result in the development of renal pathology. In addition, kidney tubules are distinguished by a restricted capacity to produce energy in anaerobic environments. As a result, the metabolic activities necessary for salt reabsorption use oxygen very quickly. Due to these features, the kidney is more susceptible to physiological and environmental stresses that cause ischemia and may increase the risk of harm from hypoxia.[3]
The dominant mechanism by which hypoxia produces renal diseases is increased reactive oxygen species (ROS) that leads to cellular toxicity and renal damage as well as induction of continuous inflammatory infiltrates. It was assumed that this process stemmed mainly from the response of the leukocytes to cytokines derived from ischemic renal endothelium.[3] Hypoxia causes macrophage accumulation in the renal tissue, which produces profibrotic cytokines by activating renal fibroblasts, which are also activated directly by hypoxia to increase the deposition of extracellular matrix. Fibroblast activation, together with inflammatory cell recruitment and tubular epithelial cell damage, leads to tubule-interstitial fibrosis.[4] The latter aggravates the hypoxic state, which eventually leads to chronic kidney disease (CKD). Moreover, tubule-interstitial fibrosis is aggravated by the augmentation of endothelin expression and then vasoconstriction.[5,6]
Erythropoietin (EPO) hormone is expressed and synthesized by peritubular interstitial fibroblasts in the inner cortex of the kidney under hypoxic conditions as a result of a sustained reduction in corticomedullary O2 tension.[7] Hypoxia-inducible factors (HIFs) provide the functional connection between variations in tissue O2 tension and altered EPO transcription. The most significant is HIF-2, which is present in some sporadic endothelial, glomerular, and interstitial cells of the renal cortex.[8] According to Liu et al.,[9] HIF (including HIF-1, 2, and 3) is significantly entangled with the protective response to hypoxia and has been demonstrated to be activated in renal illness.
The protective mechanisms, including EPO production in response to hypoxia, might not be maintained with chronic hypoxia and aging that consecutively result in progressive microvascular decline and decreased expression of cytoprotective molecules.[10] As a result, strategies might be proposed and tested for renoprotection under hypoxic conditions.
The seeds of Nigella sativa which is commonly known as black cumin is a plant from the Ranunculaceae (buttercup) family are used in folk (herbal) medicine all over the world. It is used for the treatment and prevention of a number of diseases and conditions, including asthma, diarrhea, and dyslipidemia. N. sativa has been extensively studied for its biological activities and therapeutic potential and has been shown to possess a wide spectrum of activities such as antidiabetic,[11] reduced ischemia-reperfusion injury,[12] antiepileptic,[13] antibacterial,[14] antinociceptive,[15] and smooth muscle relaxant effects.[16] In a recent study, Shakiba and Fatemeh reported the protective effects of N. sativa in ischemia stroke through different mechanisms including antioxidative stress, anti-inflammation, anti-apoptosis, neuroprotective, and vascular protective effects. These properties make N. sativa promising candidates for developing potential agents for the prevention and treatment of ischemic and hypoxic states.[17]
2,4-dinitrophenol (DNP), a chemical mitochondrial uncoupler, uncouples oxidative phosphorylation in biological systems. DNP increases systemic O2 consumption while at the same time reducing the synthesis of high-energy phosphate bonds in mitochondria. Tissue hypoxia may result from these effects.[18]
In living cells, DNP acts as a protonophore, an agent that can shuttle protons (hydrogen cations) across biological membranes. It dissipates the proton gradient across the mitochondrial membrane, collapsing the proton motive force that the cell uses to produce most of its adenosine triphosphate (ATP) chemical energy. Instead of producing ATP, the energy of the proton gradient is lost as heat.[19]
Up till now, no available research has addressed the renoprotective effects of N. sativa against hypoxic states. Therefore, the current work examined the hypothesis that administration of N. sativa can improve renal hemodynamics and attenuate the DNP-induced hypoxia renal damage and investigated the possible underlying protective mechanisms.
Materials and Methods
Preparation of hydroalcoholic extract of N. sativa
N. sativa seeds were received from the Medicinal and Aromatic Research Department, Horticulture Research Station, Agriculture Research Center, Alexandria, Egypt. For the preparation of the hydroalcoholic extract, N. sativa seeds underwent washing, drying, and grinding into a fine powder using electric micronization before being reduced to powder. Prepare a mixture of water and alcohol (e.g., ethanol) in a desired ratio (e.g., 1:1 or 3:1). In a Soxhlet extractor, 100 g of powdered seeds were extracted with 70% ethanol (80% v/v) for 48 h and subsequently, the mixture was filtered to remove solid particles and concentrated using evaporation under reduced pressure at 40°C (yield: 7.5% w/w). Until use, the resultant extract was stored at −20°C.[20]
Animals
Forty mature male Sprague Dawley rats, 4 months old, weighing between 250 and 300 g, were involved in the investigation. The Taif University College of Medicine’s Ethical Committee gave its approval to the project (approval No: 8208). The Institute of Laboratory Animal Resources Commission’s recommendations and standards for the care and usage of laboratory animals were followed during the animal experiment and Helsinki regulations.
Experimental procedures
Rats were randomly categorized into four groups (n = 10/group).
Control group
Treated with vehicles.
N. sativa group
Received ethanol extract of N. sativa seed daily by oral gavages 400 mg/kg body weight for 30 days.[21]
DNP hypoxic group
Received DNP, 30 mg/kg body weight/day, 1 mL dissolved in 1.5% methylcellulose by oral gavage for 30 days.[18]
DNP + N. sativa group
Received DNP + ethanol extract of N. sativa 400 mg/kg body weight orally for 30 days.
Topical anesthetics and lidocaine cream were applied to the tail vein area to numb the skin and reduce pain during the blood collection. Blood sampling from the tail vein was obtained 48 h after the experiment for measuring serum levels of EPO and HIF-2α. Then, at the end of the experiment (day 30), blood samples were gathered for the estimation of biochemical parameters. Blood samples collected in sodium citrate and plain tubes were immediately centrifuged at 2500 rpm for 15 min, and the resulting blood sera were stored at −80°C for further analysis. The animals were sacrificed by decapitation, and both kidneys were excised and weighed; half of each kidney was formalin-fixed and then processed for histopathological examination, and the other half was snap-frozen into liquid nitrogen and stored at −80°C for renal tissue homogenization and subsequent biochemical analysis. The protein concentration of the supernatant of tissue homogenate was estimated based on the method of Lowry et al.[22]
Biochemical analysis
Assessment of hypoxia biomarkers
Serum and tissue levels of EPO hormone were estimated by enzyme-linked immunosorbent assay (ELISA) method using Rat EPO, ELISA kit Cusabio, Biotech. Co. (Hubei, China). Serum and tissue HIF-2α levels were detected by a HIF-2α ELISA kit; SunRed Biotechn. Co. (Shangai, China). The procedure for biomarker estimation was followed according to the manufacturer’s protocol for each kit.
Assessment of oxidative stress biomarkers
Malondialdehyde (MDA), superoxide dismutase (SOD), total thiol, and catalase (CAT) activity in renal homogenate: MDA, the product of lipid peroxidation was quantitated by colorimetric method, employing the thiobarbituric acid reactive substance assay according to Satoh.[23] The procedure for MDA estimation involves that MDA in the sample reacts with a chromogenic reagent, typically thiobarbituric acid (TBA), under acidic conditions. Heating the reaction mixture promotes the MDA-TBA reaction, followed by cooling and centrifugation to separate the pink-colored complex. The absorbance of the supernatant is measured at a specific wavelength using a spectrophotometer. The MDA concentration is calculated by comparing the absorbance with a standard curve generated using known MDA concentrations.
Total thiol concentration was assessed according to the method of Ellman.[24] The procedure for measuring total thiol content involves the preparation of a sample, followed by its reaction with Ellman’s reagent. This reagent specifically reacts with thiol groups, forming a yellow-colored complex. After an incubation period, the absorbance of the complex is measured spectrophotometrically. The intensity of the yellow color is proportional to the concentration of thiol groups in the sample. The thiol concentration is calculated by comparing the absorbance with a standard curve generated using known thiol concentrations.
SOD activity was measured according to Madesh and Balasurbamanian colorimetric assay.[25] The procedure for SOD estimation involves the preparation of a sample, followed by its reaction with a compound that generates superoxide radicals. SOD converts superoxide radicals into hydrogen peroxide and molecular oxygen. The inhibition of superoxide radical formation is measured using a chromogenic or fluorescent dye. SOD activity is calculated based on the degree of inhibition and is expressed in units per milligram of protein or per gram of tissue.
CAT activity was measured by the Aebi method (1974) based on the determination rate constant of decomposing hydrogen peroxide.[26] The procedure for catalase estimation involves the preparation of a sample, followed by its reaction with hydrogen peroxide. Catalase catalyzes the breakdown of hydrogen peroxide into water and oxygen. The oxygen evolved during the reaction is measured and quantified. Catalase activity is calculated based on the rate of oxygen evolution. This procedure provides a quantitative assessment of catalase enzyme activity and is commonly used to study oxidative stress and antioxidant capacity in biological samples.
Assessment of inflammatory biomarkers
Intercellular adhesion molecule-1 (ICAM-1), interleukin-6 (IL-6), and myeloperoxidase (MPO) activity in renal homogenate: ICAM-1 was determined using a rat ELISA kit (Bosde Biotechnology Limited Company, Wuhan, China). IL-6 content was assessed with a specific rat ELISA kit (Ebioscience Co, San Diego, CA, USA). The procedures followed the manufacturer’s protocol for each kit. MPO activity was measured by the method of Mullane et al.[27] The procedure for MPO estimation involves preparing a sample and reacting it with a substrate that is oxidized by MPO. The resulting color formation is measured spectrophotometrically, and the intensity of the color is proportional to MPO activity. MPO activity is calculated using absorbance values and compared to a standard curve. The results are typically expressed in units per milligram of protein or per gram of tissue.
Assessments of kidney functions
Both serum blood urea nitrogen (BUN) and serum creatinine levels were estimated by colorimetric assay using the BUN Detection Assay kit and Creatinine Assay Colorimetric (Sigma-Aldrich) kit, respectively.[28] The urine was analyzed for total protein content using a Pierce Biotechnology, Inc. kit.[29] The procedure for biomarker estimation was followed according to the manufacturer’s protocol for each kit.
Renal hemodynamics and glomerular functions
The glomerular filtration rate (GFR), RBF, renal plasma flow (RPF), renal vascular resistance (RVR), and mean arterial pressure (MAP) were used to assess the in vivo kidney functioning at the end of the experiment (day 30). Using standard formulas,[30] renal clearances of inulin (Cin) and para-aminohippurate “PAH” (CPAH) were used to determine GFR and RPF, respectively. These formulas were as follows: Cin = Uin × V/Pin, CPAH = UPAH × V/PPAH, where Uin, Pin, UPAH, and PPAH were the concentration of inulin and PAH in urine and plasma, respectively, and V is the urine volume per min. RBF was calculated as RPF/(l - hematocrit). RVR was determined as MAP/RBF. The urine volume and flow rate were determined. By using a spectrophotometer, the concentrations of inulin and PAH in urine and plasma were measured.
Histopathological examination of renal tissues
Renal tissues obtained from experimental rats were fixed in 10% formalin. After paraffin embedding, the kidney tissue was sectioned at 5 microns and stained with hematoxylin and eosin (H&E) for histopathological assessment. Masson’s Trichrome stains were utilized to estimate the glomerular and tubular collagen and fibrous tissue. Morphological alterations were examined by Olympus light microscope and pictures were taken from 12 randomly selected regions per sample.
Statistical analysis
It was done utilizing the Prism software package (GraphPad version 8.0). A probability value of <0.05 was considered statistically significant. Means ± standard deviation were utilized to present our data. One-way analysis of variance was applied to analyze group differences, and Tukey’s post hoc multiple comparison test was employed to homogenize the results.
Results
Serum EPO hormone [Figure 1a] and HIF-2α [Figure 1b] 48 h of the experiment were highly significantly increased (P < 0.001) in DNP hypoxic and DNP + N. sativa groups. At the end of the experiment [Figure 1c-f], serum and renal tissue levels of these parameters were significantly decreased in DNP hypoxic rats (P < 0.001). Administration of N. sativa normalized EPO and HIF-2α levels nearly to control.
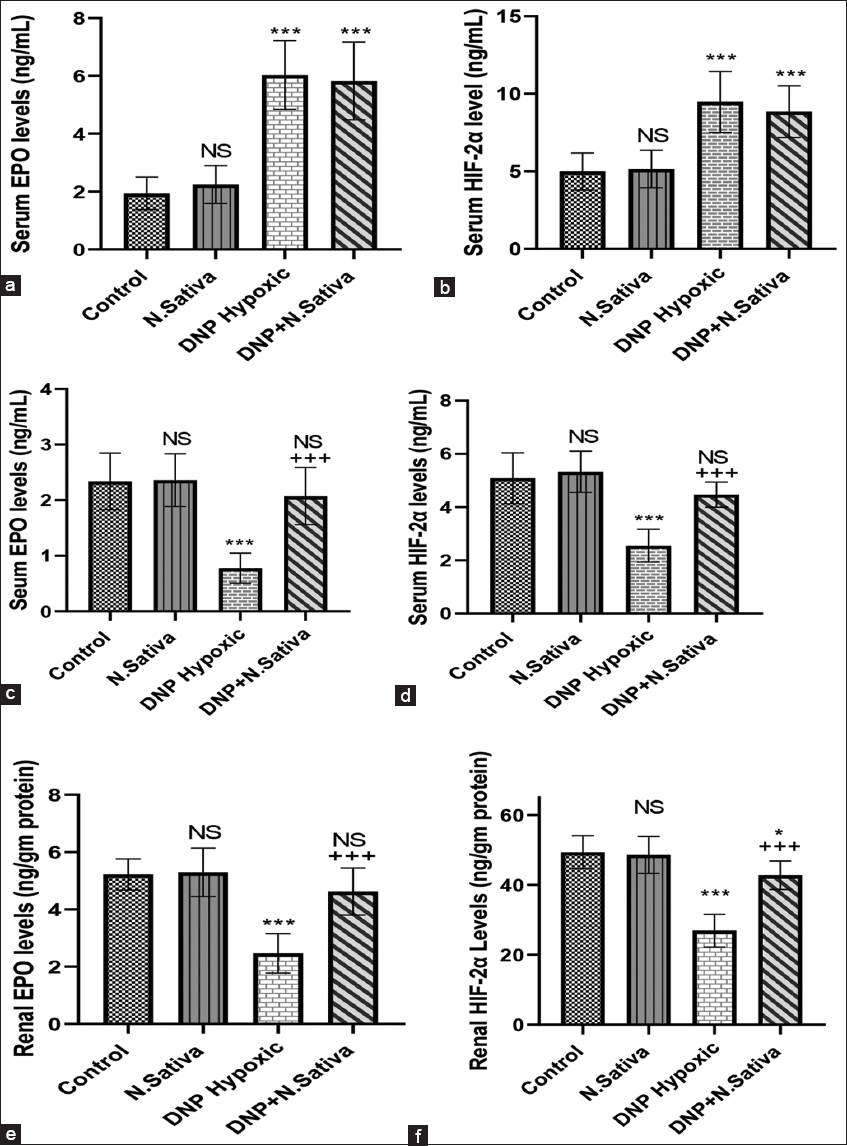
- (a-f) Serum levels of erythropoietin (EPO) hormone (a) and hypoxia-inducible factor-2α (HIF-2α) (b) 48 h of the experiment. Serum EPO hormone (c) and HIF-2α (d), renal EPO (e), and HIF-2α (f) at the end of the experiment (day 30). NS: Non-significant versus control. *: P<0.05, ***: P<0.001 versus control and Nigella sativa and + + +: P<0.001 versus dinitrophenol hypoxic group. Data are means ± standard deviation. 10 animals in each group
Renal MDA concentration was significantly increased, and total thiol concentration, CAT, and SOD activity were decreased significantly in DNP hypoxic rats (P < 0.001). Significantly decreased MDA concentration and increased total thiol, SOD, and CAT activity with N. sativa treatment (P < 0.001) as compared to DNP hypoxic rats [Figure 2a-d].
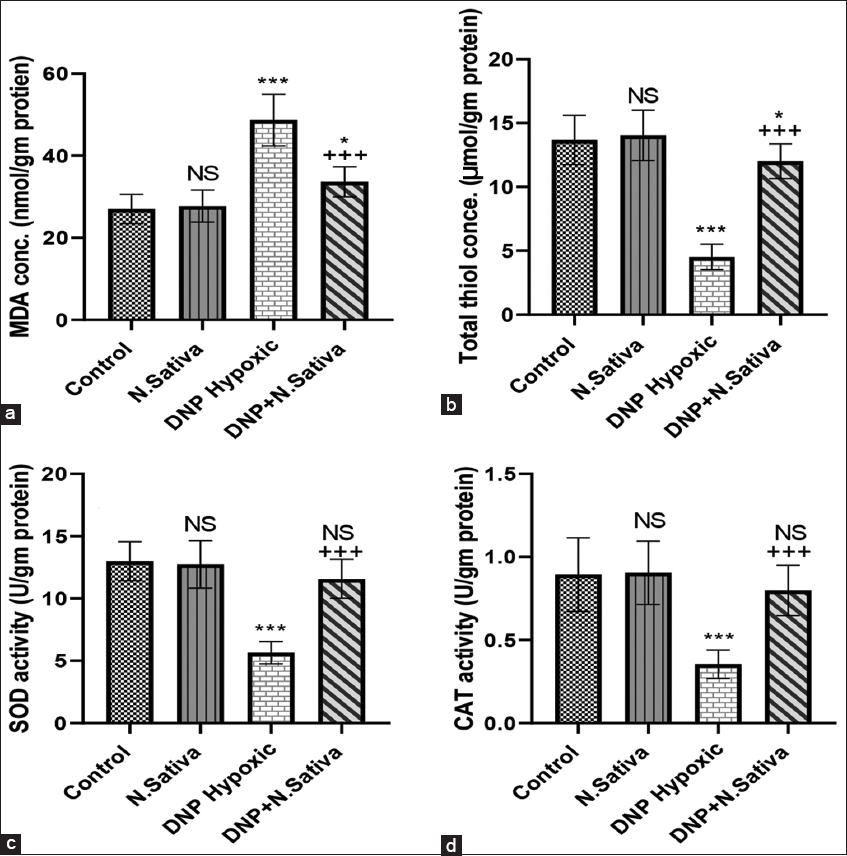
- (a-d) Renal malondialdehyde (a), total thiol (b), superoxide dismutase activity (c) and catalase activity (d) in the studied groups of animals. NS: Non-significant versus control. *: P<0.05 and ***: P<0.001 versus control and Nigella sativa. + + +: P<0.001 versus dinitrophenol hypoxic group. Data are means ± standard deviation. 10 animals in each group
In DNP hypoxic rats, levels of inflammatory biomarkers: ICAM-1, IL-6, and MPO activity were increased significantly (P < 0.001). Administration of N. sativa significantly decreased these levels in DNP + N. sativa rats (P < 0.001) in comparison to DNP hypoxic rats [Figure 3a-c].
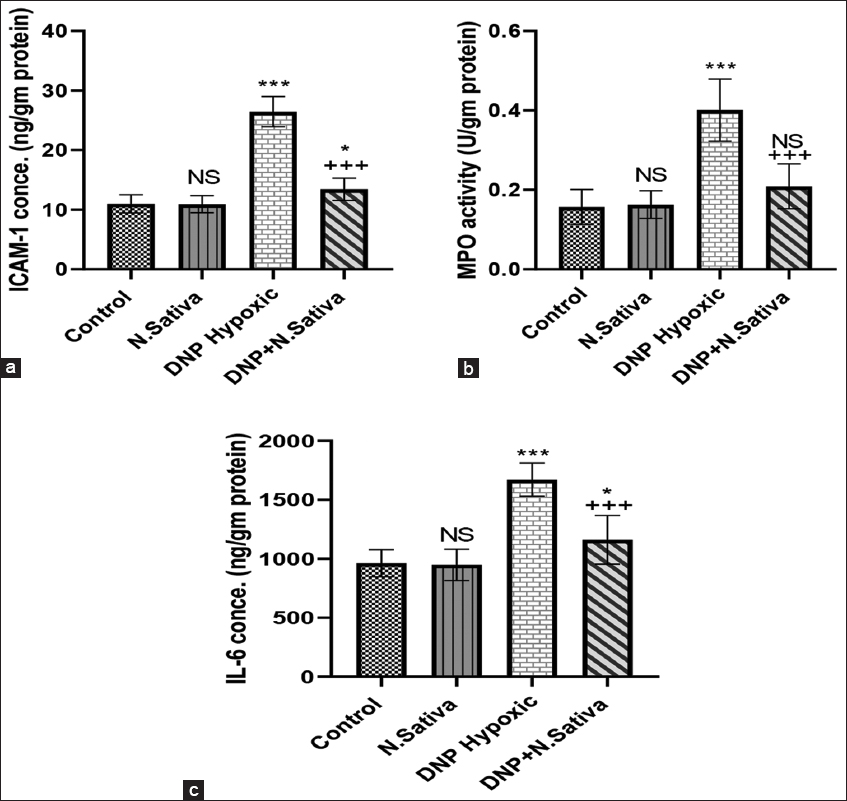
- (a-c) Renal intercellular adhesion molecule-1 (a), myeloperoxidase activity (b), and interleukin-6 (c) in the studied groups of animals. NS: Non-significant versus control. *: P<0.05, and ***: P<0.001 versus control and Nigella sativa and + + +: P<0.001 versus dinitrophenol hypoxic group. Data are means ± standard deviation. 10 animals in each group
Serum levels of BUN, creatinine, and urinary protein were significantly increased in DNP hypoxic rats (P < 0.001 for each). Treatment with N. sativa significantly decreased the levels of all these parameters (P < 0.001 for each) compared to DNP hypoxic rats. The GFR, RBF, and urine flow rate were significantly decreased in DNP hypoxic rats whereas RVR was significantly increased (P < 0.001 for each). Administration of N. sativa restored all these parameters nearly to control levels [Table 1].
Histopathological investigation [Figure 4a-h] and [Figure 5a-d] using H&E and Mason’s Trichrome-stained section, respectively, revealed that N. sativa treatment alleviated DNP-induced hypoxia renal histopathological changes.
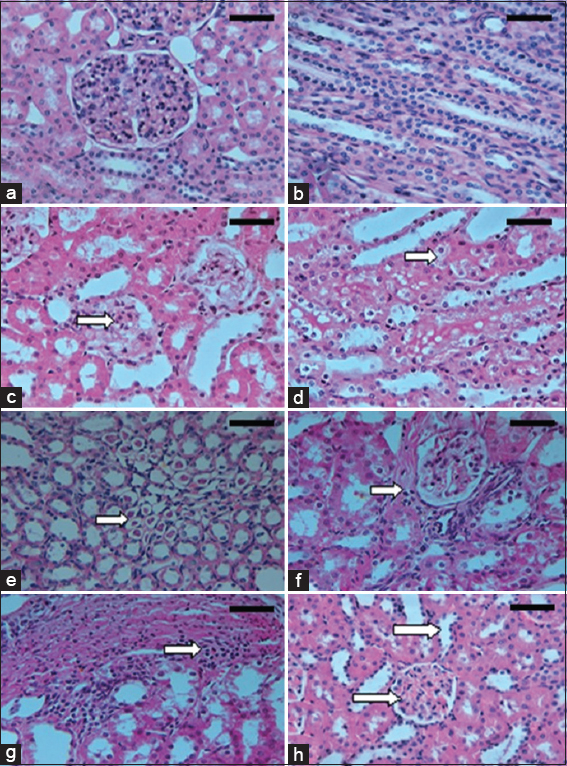
- (a-h) Microscopic appearances from kidney tissues of the experimental rats (H&E, ×400). Control group rat shows normal renal architecture with normal glomeruli and tubules of both cortex (a) and medulla (b). Dinitrophenol (DNP) hypoxic group demonstrates mild patchy expansion of mesangial matrix, with glomerular basement membrane (GBM) diffuse thickening (c), tubular epithelium shows vacuolated cytoplasm (cloudy swelling and hydropic degeneration) in renal cortex (d) and medulla with marked intratubular hyaline cast (e), interstitial fibrosis and mononuclear leukocytic infiltrates (f and g). DNP + Nigella sativa treated group shows decreased glomerular size, mesangial matrix, GBM thickness, tubular dilatation and casts formation (h). Scale bars 100 μm in (a-d) and 50 μm in (e-h)
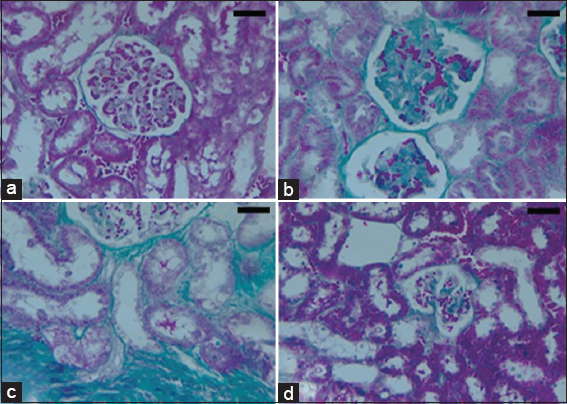
- (a-d) Microscopic appearance from kidney tissues of the experimental rats (Photomicrograph of 5 microns thickness, Mason’s Trichrome stained paraffin section, ×400). Normal kidney with normal cortical glomeruli and tubules (a). Dinitrophenol (DNP) hypoxic group shows glomerulus with diffuse glomerulosclerosis, increased mesangial matrix (b) and thickening of capillary wall with periglomerular interstitial fibrosis (c). DNP + Nigella sativa treated group demonstrates decreased glomerular size, mesangial matrix and glomerular basement membrane (d). Scale bars 100 μm in (a-d)
Discussion
Our results revealed significantly increased serum EPO hormone and HIF-2α 48 h after exposure to hypoxia in DNP hypoxic and DNP+ N. sativa-treated groups, then levels significantly decreased at the end of the experiment (day 30) in DNP hypoxic group. Luks et al. showed that EPO release starts 1–2 h after hypoxic exposure, peaking at 24–48 h, then declining through many weeks.[31] This is due to, in response to hypoxia, there is an increase in the level of gene transcription and production of both EPO and EPO receptors (EPO-R) which in turn increases the red cell mass, thereby improving tissue oxygenation. Rosenberger et al. reported that in hypoxia, renal epithelial cells respond initially through protective mechanisms that focus on stabilization of both HIF-1α and HIF- 2α.[32] With prolonged hypoxia, there is a reduction in HIF expression by destabilizing its mRNA, and this likely makes failure or downregulation of the initial protective mechanisms of the kidney.[33] Furthermore, sustained hypoxia of the renal interstitial tissue results in tubulointerstitial damage and impaired production of EPO Watts et al.[34]
In the current study, the reduction of serum levels of EPO and HIF-2α in DNP hypoxic rats than controls at the end of the experiment might be due to damage and degeneration of renal tissues induced by DNP administration for 30 days. This led to kidney failure in the production of adequate EPO amounts in response to hypoxia by peritubular interstitial fibroblasts. This was evident by the observed histopathological changes, especially peritubular interstitial fibrosis. EPO levels were significantly restored nearly to levels of control by N. sativa treatment. Similar effects were reported by Ashour who observed that thymoquinone (TQ) therapy improves serum and renal EPO production in a rat model of streptozotocin-induced diabetes.[35]
Previous studies reported that moderate levels of endogenous ROS are critical for different cell activities, but increased levels result in cellular toxicity and are associated with many diseases.[6] Oxidative stress is brought on by any disproportion between ROS production and its scavenger antioxidant system. Hypoxia, a major final step to end-stage kidney disease, can affect the kidney. Renal hypoxia induces oxidative stress, and oxidative stress, in turn, exacerbates renal hypoxia. As a result, renal damage advances more quickly as a result of the vicious loop between oxidative stress and renal hypoxia.[36,37] According to these findings, our findings demonstrated a marked elevation in oxidative stress biomarkers in DNP hypoxic rats in the form of elevated MDA levels, decreased total thiol content, SOD, and CAT activity in renal tissues, in comparison to control and N. sativa groups.
In our study, the administration of N. sativa significantly decreased MDA level and increased total thiol content, SOD, and CAT activity in renal tissues. This agrees with other studies that described the antioxidant effects of N. sativa on renal ischemia/reperfusion-induced oxidative injury in rats[38] and lipopolysaccharide (LPS)-induced renal oxidative stress in rats.[21]
The antioxidant activity of N. sativa is due to its active constituent, TQ, which exhibits potent antiradical scavenging activity, reducing ROS levels. It increases SOD, catalase, and glutathione reductase activities. Collectively, these mechanisms result in a significant decrease in MDA, an indicator of lipid peroxidation. The reduction in ROS levels is linked to the improvement of hypoxia.[39]
Our study showed significantly increased levels of inflammatory biomarkers; ICAM-1, MPO activity, and IL-6 in renal tissues of DNP hypoxic rats. Supporting our results, Dai et al. reported that hypoxia can induce mRNA expression of inflammatory cytokines, such as interleukin-1 and IL-6, activate lymphocytes, alter chemokine receptors, or induce other signaling pathways of the hypoxic inflammatory response.[40] Hypoxia also induces monocytes to express adhesion molecules and kidney cells to express vascular cell adhesion molecule 1 and ICAM-1.
Our results clarified that N. sativa treatment reduced the levels of ICAM-1, MPO activity, and IL-6 in renal tissues of the DNP + N. sativa-treated group. These results are supported by those of Beheshti et al. who reported that N. sativa suppresses the inflammatory response of LPS-induced renal tissue inflammation in rats.[21] The anti-inflammatory and antioxidant effects of N. sativa oil supplementation in diabetic hemodialysis patients were also demonstrated by Rahmani et al.[41]
Concerning the potential anti-inflammatory mechanisms of N. sativa, these may be mainly due to TQ together with its carbonyl polymer (nigellone), thymol, thymohydroquinone, alpha-hederin, limonene, and polyphenols that reduce the production of inflammatory mediators such as 5-lipoxygenase, leukotriene, and eosinophils. It has been hypothesized that TQ can inhibit nuclear factor k chain transcription in the B-cell (NF-κB) signaling pathway, and its transcription thus suppresses the expression of chemokines and pro-inflammatory cytokines such as TNF-α and IL-6.[39]
Fan et al. reported that some natural products such as ginsenoside, which is one of the main active components of ginseng, have anti-inflammatory and antioxidant properties.[42] It exhibits significant renal function protection that can reduce renal damage in renal injury, nephritis, renal fibrosis, and diabetic nephropathy models.
In addition, salidroside, a most active constituent derived from Rhodiola rosea, exerts potent antioxidative, hypoxia-resistant, and anti-inflammatory effects that have garnered significant attention as renoprotective effects, in parallel with the inhibition of oxidative stress and inflammation, salidroside holds promise as a potential therapeutic agent for kidney damage.[43]
Histopathological findings of our study [Figures 4 and 5] demonstrated significant structural changes in the DNP hypoxic group in the form of mild focal mesangial matrix expansion, diffusely thickened glomerular basement membrane with vacuolated cytoplasm of the tubular epithelial cells (cloudy swelling and hydropic degeneration) in the renal cortex and medulla with marked intratubular hyaline cast. Furthermore, interstitial mononuclear inflammatory infiltrate with periglomerular and peritubular interstitial fibrosis was detected in the DNP hypoxic group in comparison with control. These findings are supported by other studies.[44] On administration of N. sativa, the histopathological alterations were improved. This finding matches with the result of Shafiee et al., 2016.[45]
Our results showed significantly increased serum levels of creatinine and BUN, urinary protein excretion, and RVR whereas decreased GFR, RBF, and urine flow rate in DNP hypoxic rats. Supporting our results, Friederich-Persson et al. found elevated leakage of the urinary protein in the hypoxic rats.[18] It is likely that hypoxic damage affects protein reabsorption in the proximal tubule in addition to increasing protein permeability across the glomerular capillary. These two processes encourage proteinuria. Fine et al. found initial hypoxic glomerular injury reduced RBF in peritubular capillaries and induced low tissue PO2, which enhanced tubulointerstitial fibrosis (TIF).[46] A critical interstitial hypoxia potentiates progressive loss of kidney function. Inoue et al. reported a correlation between hypoxia and TIF with reduced GFR in CKD patients.[47] According to a study, chronic hypoxia can cause increased production of powerful vasoconstrictor endothelin-1 with its type A receptor by the renal cortex, which raises RVR.[48]
In the current study, treatment with N. sativa resulted in significant decrement of serum creatinine and BUN and attenuation of proteinuria as well as improvement of hemodynamic parameters of kidneys in DNP + N. sativa rats. Similar to our results, it was reported that pretreatment with N. sativa significantly decreased the levels of creatinine, BUN, and uric acid and resulted in a significant improvement in the tubular renal cells and hemodynamics parameters following ischemia-reperfusion injury in the rat.[38] In addition, renal hemodynamics improving effect of black cumin was reported by Yusuksawad and Chaiyabutr who revealed an increased GFR, effective RPF, and effective RBF, while decreased RVR in streptozotocin-induced diabetic rats.[49] This might be explained by Cherkaoui-Tangi et al., who reported a vasorelaxant activity of essential oil of N. sativa seeds on isolated rat aorta.[50] This study has a few limitations, such as sample size: the number of rats used in the study might be limited. This is due to limitations of funding and facility; duration of the study: the study might have had a relatively short duration. Renal hypoxia is a complex condition, and its progression and effects may require longer observation periods. However, due to death rate in animal models adds a limitation to our study.
Conclusion
The findings of this study suggest that N. sativa may have therapeutic potential in attenuating hypoxia-induced renal injury by modulating oxidative stress and inflammation, and improving kidney functions. Further research is warranted to elucidate the underlying molecular mechanisms and to explore the clinical implications of N. sativa in the prevention and treatment of kidney diseases associated with hypoxia.
Ethics Approval and Consent to Participate
Taif University Laboratory Animal Resources Commission’s recommendations and standards for the care and use of laboratory animals were followed during the animal experiment and Helsinki regulations. The Taif University College of Medicine’s Ethical Committee gave its approval to this project (approval No: 8208).
Availability of Data and Material
All data generated or analyzed in this study are included in this published article. The data are available on request.
Competing Interests
None.
Funding Statement
None.
Author Contributions
Asmaa F. Hassan: Principal investigator, data collection and tabulation, writing - review and editing. Amal F. Gharib: Biochemistry analysis of biomarkers, writing - review and editing. Howaida M. Hagag: Pathology part, writing - original draft, review, and editing. Ola M. Omran: Pathology part writing and tabulation, data extraction and tabulation - review and editing.Enshrah M. Elamin: Assisting in pathology part writing and tabulation.Khadiga A. Ismail: Data analysis support, data extraction, and tabulation, writing - a review. Hebatallah Husseini Atteia: writing - review and editing.
Acknowledgment
Researchers would like to thank Taif University and Qassim University for providing the facilities and the support during the performance, tenure and publication of this project.
References
- Hypoxia, HIF-1, and the pathophysiology of common human diseases. Adv Exp Med Biol. 2000;475:123-30.
- [Google Scholar]
- Hypoxia:Molecular pathophysiological mechanisms in human Diseases. J Physiol Biochem. 2022;78:739-52.
- [Google Scholar]
- Implications of oxidative stress in chronic kidney disease:A review on current concepts and therapies. Kidney Res Clin Pract. 2021;40:183-93.
- [Google Scholar]
- Why is erythropoietin made in the kidney?The kidney as a critmeter. Am J Kidney Dis. 2001;38:415-25.
- [Google Scholar]
- Functional evidence confirmed by histological localization:Overlapping expression of erythropoietin and HIF-2alpha in interstitial fibroblasts of the renal cortex. Kidney Int. 2010;77:269-71.
- [Google Scholar]
- The role of hypoxia-inducible factor-1 alpha in renal disease. Molecules. 2022;27:7318.
- [Google Scholar]
- Impaired angiogenesis in the aging kidney:Vascular endothelial growth factor and thrombospondin-1 in renal disease. Am J Kidney Dis. 2001;37:601-11.
- [Google Scholar]
- Protective effect of Nigella sativa seed extract and its bioactive compound thymoquinone on streptozotocin-induced diabetic rats. Cardiovasc Hematol Agents Med Chem. 2024;22:51-9.
- [Google Scholar]
- Protective effects of Nigella sativa against ischemia-reperfusion injury of kidneys. Renal Fail. 2010;32:126-31.
- [Google Scholar]
- Evaluation of the antiepileptic effect of curcumin and Nigella sativa oil in the pilocarpine model of epilepsy in comparison with valproate. Epilepsy Behav. 2012;24:199-206.
- [Google Scholar]
- Evaluation of antimicrobial and anti-inflammatory activities of seed extracts from six Nigella species. J Med Food. 2009;12:408-15.
- [Google Scholar]
- Antinociceptive effects of Nigella sativa oil and its major component, thymoquinone, in mice. Eur J Pharmacol. 2000;400:89-97.
- [Google Scholar]
- Relaxant effects of different fractions from Nigella sativa L. On guinea pig tracheal chains and its possible mechanism(s) Indian J Exp Biol. 2008;46:805-10.
- [Google Scholar]
- Potential role of Nigella Sativa and its constituent (Thymoquinone) in ischemic stroke. Curr Mol Med. 2024;24:327-34.
- [Google Scholar]
- Kidney hypoxia, attributable to increased oxygen consumption, induces nephropathy independently of hyperglycemia and oxidative stress. Hypertension. 2013;62:914-9.
- [Google Scholar]
- 2,4-dinitrophenol (DNP):A weight loss agent with significant acute toxicity and risk of death. J Med Toxicol. 2011;7:205-12.
- [Google Scholar]
- The effect of hydroalcoholic extract of Nigella Sativa seed on dehydroepiandrosterone-induced polycystic ovarian syndrome in rats:An experimental study. Int J Reprod Biomed. 2021;19:271-82.
- [Google Scholar]
- Nigella sativa prevented liver and renal tissue damage in lipopolysaccharide-treated rats. Saudi J Kidney Dis Transpl. 2018;29:554-66.
- [Google Scholar]
- Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chem Acta. 1978;90:37-43.
- [Google Scholar]
- Microtiter plate assay for superoxide dismutase using MTT reduction by superoxide. Indian J Biochem Biophys. 1998;35:184-8.
- [Google Scholar]
- Catalase. In: Bergmeyer HU, ed. Methods of Enzymatic Analysis. Weinheim, New York: Verlag Chemie, Academic Press Inc; 1974. p. :673-80.
- [Google Scholar]
- Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemic myocardium. J Pharmacol Methods. 1985;14:157-67.
- [Google Scholar]
- Biochemical and histopathological changes in Wistar rats after consumption of boiled and un-boiled water from high and low disease prevalent areas for chronic kidney disease of unknown etiology (CKDu) in north Central Province (NCP) and its comparison with low disease prevalent Colombo, Sri Lanka. BMC Nephrol. 2020;21:38.
- [Google Scholar]
- Protein measurement using bicinchoninic acid:Elimination of interfering substances. Anal Biochem. 1989;180:136-9.
- [Google Scholar]
- Principle of Renal Physiology. New York: Oxford University Press; 1962. p. :196-217.
- Hypoxia-inducible factors and tubular cell survival in isolated perfused kidneys. Kidney Int. 2006;70:60-70.
- [Google Scholar]
- Regulation of hypoxia-inducible factor in kidney disease. Clin Exp Pharmacol Physiol. 2013;40:148-57.
- [Google Scholar]
- Hypoxia pathway proteins are master regulators of erythropoiesis. Int J Mol Sci. 2020;21:8131.
- [Google Scholar]
- Thymoquinone therapy improves hyperglycemia, erythrocyte indices, erythropoietin production and erythrocyte osmotic resistance in rat model of streptozotocin-induced diabetes. Br J Med Med Res. 2015;5:350-61.
- [Google Scholar]
- The role of oxidative stress and hypoxia in renal disease. Kidney Res Clin Pract. 2019;38:414-26.
- [Google Scholar]
- Molecular mechanisms of oxidative stress in acute kidney injury:Targeting the loci by resveratrol. Int J Mol Sci. 2024;25:3.
- [Google Scholar]
- Study on the effect of black cumin (Nigella sativa Linn.). on experimental renal ischemia-reperfusion injury in rats. Acta Cir Bras. 2015;30:542-50.
- [Google Scholar]
- Hypoxia increases expression of CXC chemokine receptor 4 via activation of PI3K/Akt leading to enhanced migration of endothelial progenitor cells. Eur Rev Med Pharmacol Sci. 2017;21:1820-27.
- [Google Scholar]
- Effect of Nigella sativa supplementation on kidney function, glycemic control, oxidative stress, inflammation, quality of life, and depression in diabetic hemodialysis patients:Study protocol for a double-blind, randomized controlled trial. Trials. 2022;23:111.
- [Google Scholar]
- Renal function protection and the mechanism of ginsenosides:Current progress and future perspectives. Front Pharmacol. 2023;14:1070738.
- [Google Scholar]
- Pharmacological functions of salidroside in renal diseases:Facts and perspectives. Front Pharmacol. 2024;14:1309598.
- [Google Scholar]
- GSTA3 attenuates renal interstitial fibrosis by inhibiting TGF-beta-induced tubular epithelial-mesenchymal transition and fibronectin expression. PLoS One. 2016;11:e0160855.
- [Google Scholar]
- Effects of aqueous-ethanolic extract of Nigella sativa seeds (Black Cumin) and Vitamin E on cisplatin-induced nephrotoxicity in rat. Res J Med Plants. 2016;10:295-302.
- [Google Scholar]
- Progressive renal disease:The chronic hypoxia hypothesis. Kidney Int Suppl. 1998;65:74-8.
- [Google Scholar]
- Noninvasive evaluation of kidney hypoxia and fibrosis using magnetic resonance imaging. J Am Soc Nephrol. 2011;22:1429-34.
- [Google Scholar]
- Progressive endothelin-1 gene activation initiates chronic/end-stage renal disease following experimental ischemic/reperfusion injury. Kidney Int. 2013;84:703-12.
- [Google Scholar]
- Restoration of renal hemodynamics and functions during black cumin (Nigella sativa) administration in streptozotocin-induced diabetic rats. J Exp Pharmacol. 2012;4:1-7.
- [Google Scholar]
- Vasorelaxant effect of essential oil isolated from Nigella sativa L. Seeds in rat aorta:Proposed mechanism. Pak J Pharm Sci. 2016;29:1-8.
- [Google Scholar]







