Translate this page into:
Safety and efficacy of elbasvir/grazoprevir in patients infected with hepatitis C virus genotype 4 in Qassim region of Saudi Arabia
Address for correspondence: Dr. Sumaira Ahmed, MBBS, FCPS, Department of Gastroenterology and Hepatology, King Fahad Specialist Hospital, General Directorate of Health Affairs, Buraydah, Qassim, Saudi Arabia. E-mail: drsumaira_ahmed@hotmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Qassim Uninversity and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective:
Untreated hepatitis C virus (HCV) infection can lead to cirrhosis, hepatocellular cancer, and even death and also increases liver diseases. The elbasvir/grazoprevir (EBR-GZR) treatment regimen when given in HCV genotype (GT) 1 and GT4 infection for 8 or 12 weeks showed a high sustained virolgical response (SVR) rates in different populations. This study was assessed the effectiveness as well as safety of EBR-GZR in 12 week treatment regimen in HCV GT4-infected treatment-naïve Saudi patients.
Methods:
This study conducted from June 2017 and December 2020 on Saudi HCV patients infected with GT4. Treatment protocol was given for 12 weeks in treatment-naive HCV GT4 infected cirrhotic and non-cirrhotic participants which were later followed for a total of 24 weeks for safety and efficacy of EBR-GZR.
Results:
We analyzed data of 54 participants with HCV GT 4 infection. Mean age was (53.46 ± 14.94) Treatment regimen was given to 14 cirrhotic (F4) and 40 non-cirrhotic (F0–F3). SVR was seen in 98.1% of participants with tolerable side effects and improved model for end stage liver disease (MELD) scores as fall in percentage seen from 18.5% to 14.8% in participants with MELD > 10.
Conclusion:
This retrospective study confirms that EBR-GZR for 12 weeks is a safe and effective treatment regimen in HCV GT4 patients in studied Saudi population. Treatment completion was followed by high SVR12 rates with improvement in prognostic markers of liver disease in participants with compensated cirrhosis. In short, the EBR-GZR combination showed efficacy in achieving the SVR12 in Child-Pugh B cirrhotic and non-cirrhotic population with a favorable safety profile.
Keywords
Cirrhosis
elbasvir
grazoprevir
hepatitis
sustained virological response
Introduction
Hepatitis C virus (HCV) infection is one of a pre-eminent cause of chronic liver disease across the globe. HCV associated chronic hepatitis if not timely treated can progress to potentially life-threatening complications of liver disease that leads to cirrhosis, hepatocellular carcinoma (HCC), liver failure, and death. Several extra hepatic manifestations are associated with HCV infection and elimination of HCV infection reduces morbidity and all-cause mortality. There are six prevalent HCV genotypes globally and include genotype 1 (1a and 1b), 2, 3, 4, 5, and 6.
Exact data on the prevalence of HCV infection in Saudi Arabia are currently unavailable, as most HCV research was conducted over 10 years ago. The rate of prevalence recorded for Saudi Arabia in various studies ranges from 0.22% to 1.1%.[1,2] The most prevalent genotype (GT) is HCV GT4, followed by HCV GT1.[3] The objective of HCV therapy is to cure the infection by achieving sutained virolgical response (SVR), that is, undetectable HCV RNA after 12 weeks of treatment completion. Newly approved direct-acting antivirals (DAAs) which inhibit HCV non-structural proteins are associated with high SVR rates.
Elbasvir (EBR) is an NS5A inhibitor that prevents HCV RNA replication and virion assembly, while Grazoprevir (GZR) is a protease inhibitor of HCV NS3/4A that prevents cleavage of the polyprotein necessary for replication for weeks after the end of therapy.[4] These inhibitors are administered as a single fixed dose combination, which has been approved for the treatment of HCV GT1 and GT4 infections in treatment-naive compensated cirrhotic and non-cirrhotic patients and patients for whom prior therapy has failed.[5]
Meta-analysis data from six clinical trials of treatment with EBR-GZR, with or without ribavirin, shows 12-week post-treatment SVR (SVR12) rates of 89% to 100% in patients with compensated cirrhosis who were infected with HCV GT1, GT4, or GT6 and there was no virological failure.[6] Integrated pooled analysis revealed that the SVR12 rate was 88.6% for cirrhotic and non-cirrhotic patients infected with HCV GT4, for whom treatment was administered for 12 or 16 weeks with EBR-GZR (with or without ribavirin), and the SVR12 rate was 87.5% among participants who received 12 weeks of EBR-GZR without ribavirin.[7] The overall response rate for the fixed-dose combination regimen used in the treatment of HCV GT4 for 12–16 weeks (with or without ribavirin) was >95% SVR12 in treatment -naïve and treatment-experienced patients.[8,9] This study aims to assess the effectiveness and tolerability of EBR-GZR in cirrhotic and non-cirrotic participants with HCV GT 4 with a sample size of 54 patients. The study novelty is that there is no published data available with 12-week treatment duration with EBR-GZR in Saudi Arabia in a large sample size.
Methods
Study design
This retrospective study which was conducted at King Fahad specialist Hospital, Buraidah between May 2017 and December 2020, in accordance with Good Clinical Practice guidelines and approved by regional institutional review boards and regulating agencies (Registration No. 1442-1477174). Informed consents were obtained from all participants for the initiation of treatment. The final manuscript was reviewed and approved by all research authors. Data were collected using medical record review. A fixed-dose combination tablet containing EBR 50 mg and GZR 100 mg was given to all participants for 12 weeks, and all participants were followed up for another 12 weeks to track their SVR status and monitor side effects or adverse effects related to the treatment. Dose modifications were not allowed and the study was terminated after 12 weeks of completion of treatment.
Participants
The participants included male and female patients 18 years and older with HCV infection, any level of detectable HCV RNA, and a documented HCV GT4 infection. As data were collected retrospectively, patients with missing data were excluded from the study. All cirrhotic participants were assigned a child pugh (CP) score based on clinical and laboratory data and model for end stage liver disease (MELD) score based on laboratory data. Cirrhosis and non-cirrhosis was defined by clinical, radiological features, and liver stiffness assessed by transient elastography with a value >12.5 kpa and <12.5 kpa, respectively, within 6 months of screening.[10-12] Patients with decompensated cirrhosis were excluded as GZR is contraindicated in this population. HBV coinfected patients are of risk of reactivation of HBV and fulminant liver failure including death with DAA treatment. For treatment experienced, a different study protocol may be needed and hence not included in this study. None of our participants were found to have HCC, HIV coinfection, HBV coinfection, and past history of treatment with interferon and DAA and hence, they were not included in this study.
Assessment and analysis
The primary end point was to achieve SVR after 12 weeks of completion of treatment (SVR12), which is defined as HCV RNA <15 IU/mL (lower limit of quantitation; 15 IU/mL, lower limit of detection: 15 IU/mL). Efficacy is presented for the intention-to-treat population, which includes all participants who received ≥1 dose of study medication. The model for end-stage liver disease (MELD) score and CP score was analyzed at baseline and at follow-up during week 24. Evaluation of safety was done by monitoring for adverse events (AEs) and serious AEs, with serious AEs defined as events that may lead to treatment discontinuation or death. Analysis of laboratory abnormalities included grade 3/4 laboratory abnormality defined as alanine aminotransferase (ALT) or aspartate aminotransferase (AST) 5 times the upper limit of normal (ULN), and total bilirubin level >2.6 mg/dL and hemoglobin value of <8.9 g/dL. Statistical analysis was done using Statistical program for social analysis (SPSS statistic version 22.0). Baseline patient characteristics are presented in number and percentages. A 95% confidence interval plot was plotted using Clopper-Pearson method to assess the baseline patient’s characteristics that may affect SVR 12. On-treatment and off-treatment viral response rates are presented in numbers and percentages. Percentages were calculated for changes in MELD score, and the safety profile summary is presented in numbers and percentages. Mean change in MELD and CP scores was calculated.
Results
Sample demography
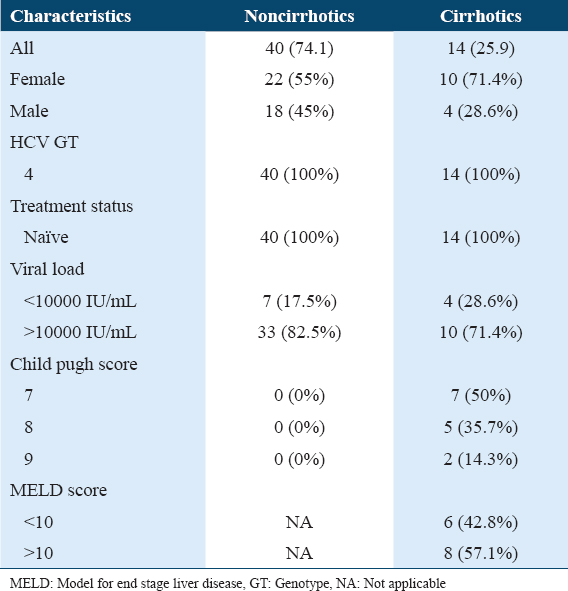
In our study, we assessed data from 54 participants with HCV GT4 infection. Of the 54 participants, 32 were female and 22 were male [Table 1]. The mean age was 53.46 ± 14.94 for all sample subjects, with a predilection for a higher mean age in the cirrhotic subjects at 62.00 ± 16.29. There were 14 cirrhotic participants (25.9%) with predominant CP class B7 (50%) and 40 non-cirrhotic participants (74.1%). In cirrhotic participants, approximately 6 participants (42.9%) had a pretreatment MELD score of <10 and a pre-treatment MELD score of >10 was found in 8 participants (57.1%). Pre-treatment HCV RNA levels were >10000 IU/mL in 43 participants (79.6%), and only 10 (18.5%) of the participants had HCV RNA levels of <10000 IU/mL.
Treatment regimens
EBR 50 mg + GZR 100 mg was administered once daily were given to 53 patients for 12 weeks. Only one patient received the EBR 50 mg+ GZR 100 mg once daily along with ribavirin 200 mg twice daily for 12 weeks. Twelve weeks after completion of the treatment, 98.1% of the participants had undetectable HCV RNA levels. All patients underwent treatment for 12 weeks and were followed up for 24 weeks, except one patient with cirrhosis and CP class B who dropped out of follow-up early in the study during follow-up week 4. Treatment was not discontinued in any patient due to adverse event or serious adverse event.
Efficacy
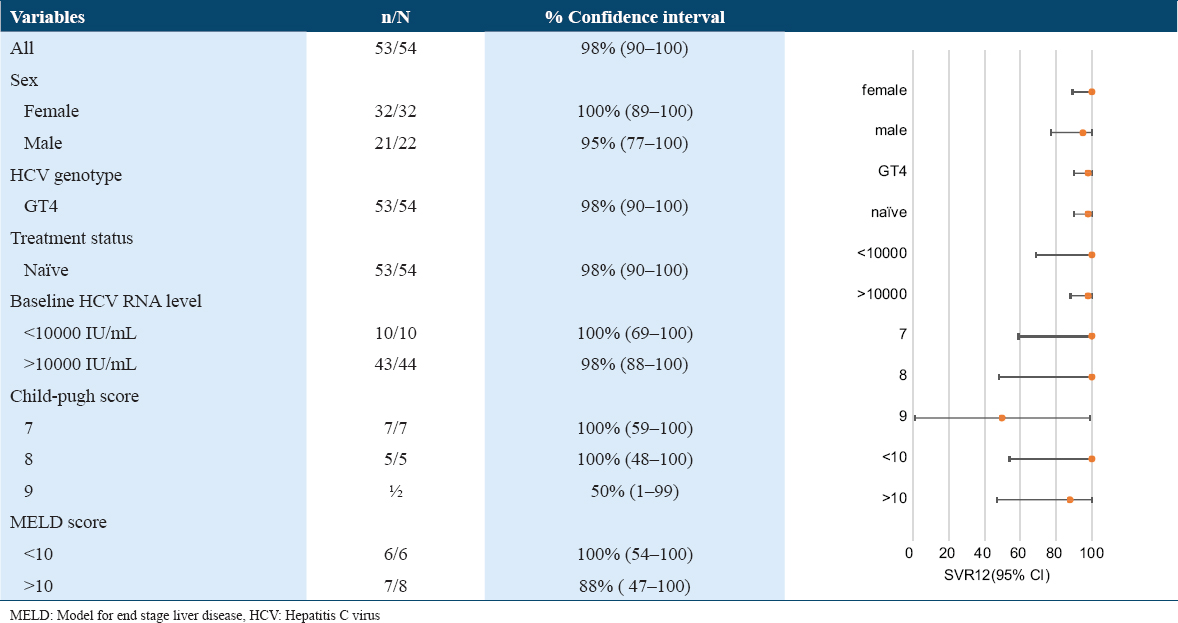
SVR was observed in 98.1% of participants [Table 2]. One cirrhotic patient dropped out of follow-up before study completion during follow-up week 4. No treatment failure was observed during follow-up week 12. High SVR rates was seen across the all sub-populations [Table 2].
Change in MELD score and CP score in Cirrhotic participants
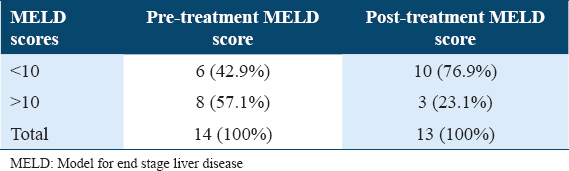
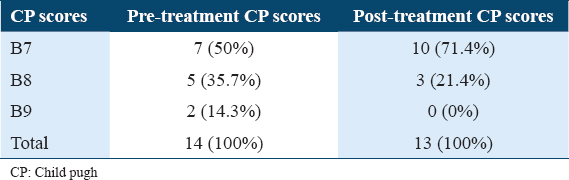
At follow-up week 24, post-treatment MELD scores showed improvement in total of four patients by decrease in MELD score by 2 points in two participants and by 3 points in another two participants. Out of I3 cirrhotics 10 (79.6%) participants had scores of <10, while only 3 (23.1%) maintained a score of >10 [Table 3]. Overall, participants with CP-B cirrhosis had a mean change in MELD score from baseline to follow-up week 24 of −0.769,with a range of (−3)–(−2) [Figure 1].
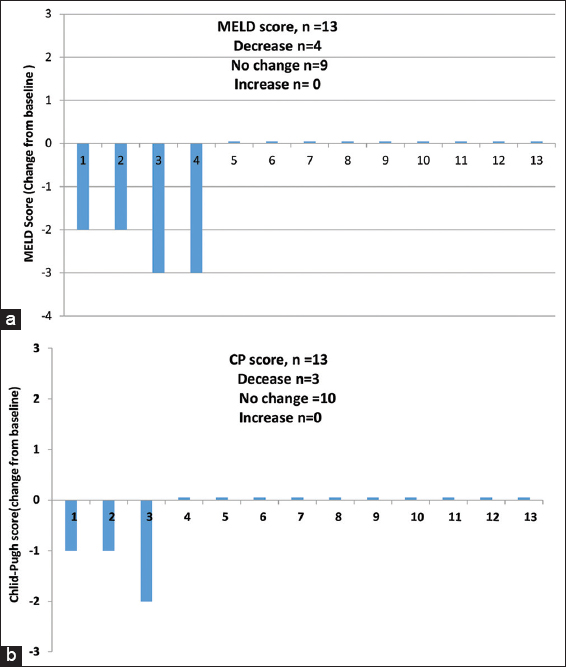
- Change in (a) model for end stage liver disease (MELD) and (b) Child-pugh (CP) scores from baseline to the Follow up week 24 (CP-B participants). At follow-up week 24, 4/13 (30.8%) participants with CP-B class experienced improvement in MELD score, 4 (30.8%) partcipants showed decrease in MELD score (by 2 points in two participants and by 3 points in another two participants), there was no change in MELD score in 9 (69.2%) participants and none showed increase in MELD scores. Overall, participants with CP-B cirrhosis had a mean change in MELD score from baseline to follow up week 24 of −0.769, with a range of (−3)–(−2). At follow up week 24, 3/13 (23.1%) participants with CP-B class had improvement in the CP score when compare with baseline, 3 (23%) participants had decrease in CP score (by 1 point in two participants and by 2 points in another participant), no change was seen in 10 participants (76.9%) and no increase in score was observed in any participant. The mean change in CP score in CP-B cirrhosis from baseline to follow-up week 24 was −0.31, with a range of (−2)–(−1)
CP score was decreased by one point in two participants (CP score decreased from 8 to 7) and by 2 points in one participant (CP score decreased from 9 to 7), at follow-up week 24, about 10 (71.4%) of participants had CP score of B7 and 3 (21.4%) had a score of B8 [Table 4]. The mean change in CP score in CP-B cirrhosis from baseline to follow-up week 24 was −0.31, with a range of (−2)–(−1) [Figure 1].
Safety and tolerability
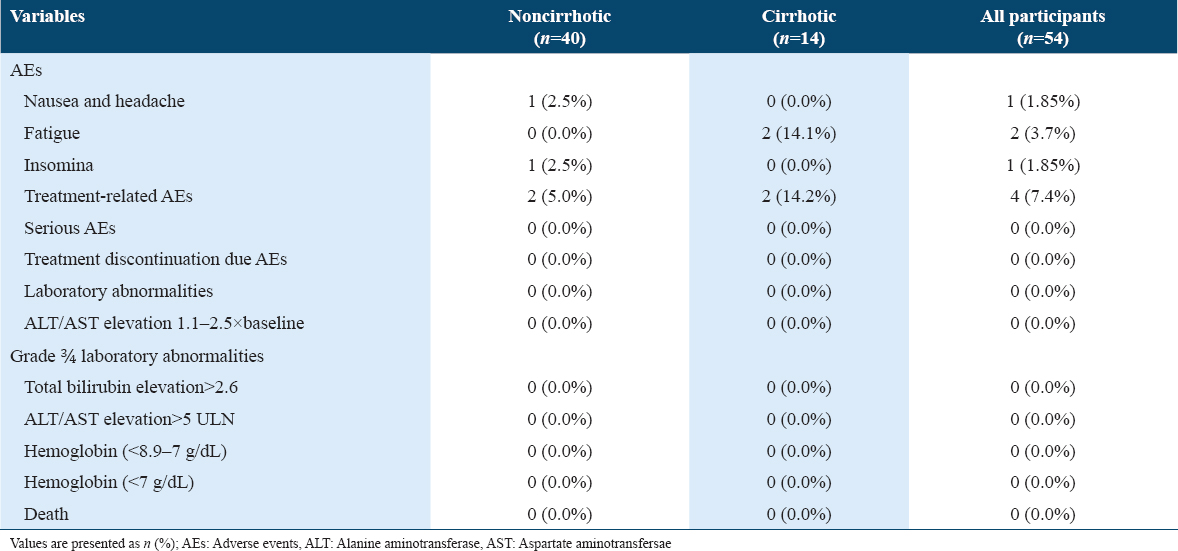
Treatment-related AEs were noted in 7.4% of participants, while no serious adverse event or laboratory abnormality that could result in treatment discontinuation was observed in any participant [Table 5]. There was no death or hepatic decompensation during treatment and on follow-up. About 14.1% of cirrhotic participants reported fatigue. Insomnia was reported by 2.5% of non-cirrhotic participants and 0% of the cirrhotic participants, while about 2.5% of non-cirrhotic participants experienced headache and nausea. All treatment related AEs were subsided on treatment discontinuation.
Discussion
In this open label and retrospective study, EBR-GZR efficacy was demonstrated, with 98.1% of the study sample achieving SVR12 after completing the treatment course in HCV GT4 infection in child B cirrhosis where dose of GZR is 100 mg used with no serious AEs. Both cirrhotic and non-cirrhotic participants were equally responsive to the treatment regimen with recompensation seen in CP class B. Only 7.4% of participants reported treatment-related adverse effects which disappeared on treatment completion. These findings provide evidence that the regiment is safe and effective.
The observed efficacy of the treatment with regard to SVR12 correlates with the study of 117 participants who were enrolled, 80 were treatment-naive with F0-F2 liver fibrosis. A total of 53 participants were randomized to receive 8 weeks of therapy 27 were randomized to receive 12 weeks of therapy. SVR was achieved by 94% (50/53) and 96% (26/27) of those receiving EBR/GZR for 8 or 12 weeks, respectively.[13] In total of 48 patients with chronic HCV-HIV confection with mild liver fibrosis (liver stiffness <8 kPa) treatment with EBR-GZR (8 weeks in GT1b or 12 weeks in GT1a or GT4) SVR was 98%.[14] The SVR rate achieved also correlates with findings of the C-EDGE treatment-naïve trial, in which 100% of the total of 18 participants with GT4 achieved SVR12.[8] Furthermore, the C-SCAPE study also found that 90% of participants with GT4 achieved SVR12.[15] The cirrhotic population is where the efficacy is truly evident, with 100% of the population achieving SVR12. This is consistent with the findings of the C-SALT study, which had 97% of a similar population achieving SVR12. However, it should be considered that the regimen used in the C-SALT study was slightly different, as the GZR dose was only 50 mg in CP class B cirrhotics with HCV GT1 infection, unlike in our study protocol in which 100 mg GZR was tolerated in CP class B participants with HCV GT4 infection.[6] This offers an interesting research area. Furthermore, we had similar findings on the MELD score, with the score improving after treatment, which is evident in the cirrhotic populations of both studies. Our study results are comparable to a recently published Phase 3 clinical trial (ELEGANT-4) but the treatment regimen was given for 8 weeks.[16]
Adverse effects noted in CP Class B participants includes fatigue in 30%, nausea in 10%, headache in 10% and arthralgia in 16.7% of participants. Our study has a more exclusive area of interest, as we assessed HCV GT4 responsiveness to a treatment protocol with a sample size of 54 patients for 12 weeks unlike ELEGANT-4 trial in which 8-week eradication regimen was given in a sample size of 30. Large, randomized, and controlled trials are needed with long and short duration treatment arms in treatment-experienced with prior failure to DAAs, solid organ transplant recipients, hemodialysis patients, HIV-HCV coinfected, can further simplified HCV treatment with this regimen. This study has limitation that it has a small sample size with no control group and being a non-randomized control trial. The study is a single-center and retrospective study conducted in treatment naïve subjects only and reflects treatment effectiveness/safety in a specific population. Other limitations include that liver fibrosis was assessed using non-invasive transient elastography (fibros can only) rather metavir liver biopsy scores. Participants were not followed for long-term complications such as relapse and HCC.
Conclusion
In this retrospective study, the EBR-GZR combination is effective in achieving SVR12 in cirrhotics and non-cirrhotics treatment naive patients. The study results confirms high efficacy and tolerability of EBR-GZR in individuals with HCV GT4 infection. Furthermore, improvement in MELD and CP scores (prognostic markers of liver disease) after eradication of HCV infection favors concept of recompensation in compensated cirrhotic participants where HCV infection was the underlying etiology of liver disease.
Authors Declaration
Ethics approval and consent to participate
This is a retyrospective study for which informed consents are not required. The study has been approved by regional institutional review boards and regulating agencies for implementation and publication. (Registration No. 1442-1477174).
Availability of data and material
The authors confirmed that the data supporting the findings of this study are available within the article.
Competing interests
None.
Funding statement
None.
Authors’ contributions
All the authors have approved the final draft before submission with following contribution: Shivananda Murgod: Conceptualiztion, data curation, methodology, writing-reviewing and editing, and formal analysis; Sumaira Ahmed: Data curation, software, writing-original draft preparation, and formal analysis; Nawaf Almutairi: Project administration, investigation, validation, supervision, visualization, and resources; Abdullah Alqifari: Validation, supervision, investigation, visualization, formal analysis; Data Curation, investigation, and software; Ahmed Shoqueer and Majed Alharbi: Data curation, investigation, software, and resources.
Acknowledgments
We appreciate and thankful to the patients, their families, investigators, and all the participants in this study.
References
- Epidemiology of viral hepatitis in Saudi Arabia:Are we off the hook? Saudi J Gastroenterol. 2012;18:349-57.
- [Google Scholar]
- Is there a need to include HIV, HBV and HCV viruses in the Saudi premarital screening program on the basis of their prevalence and transmission risk factors? J Epidemiol Community Health. 2010;64:989-97.
- [Google Scholar]
- HCV genotypes among 1013 Saudi nationals:A multicenter study. Ann Saudi Med. 2013;33:10-2.
- [Google Scholar]
- Elbasvir/Grazoprevir (Zepatier) for hepatitis C virus Infection. Am Fam Physician. 2017;95:393-4.
- [Google Scholar]
- Safety and efficacy of Elbasvir/Grazoprevir in patients with hepatitis C virus infection and compensated cirrhosis:An integrated analysis. Gastroenterology. 2017;152:1372-82e2.
- [Google Scholar]
- Efficacy of elbasvir and grazoprevir in participants with hepatitis C virus genotype 4 infection:A pooled analysis. Liver Int. 2018;38:1583-91.
- [Google Scholar]
- Grazoprevir-elbasvir combination therapy for treatment-naive cirrhotic and noncirrhotic patients with chronic hepatitis C virus genotype 1, 4, or 6 infection:A randomized trial. Ann Intern Med. 2015;163:1-13.
- [Google Scholar]
- Effectiveness of Elbasvir and Grazoprevir combination, with or without ribavirin, for treatment-experienced patients with chronic hepatitis C infection. Gastroenterology. 2017;152:164-75e4.
- [Google Scholar]
- Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-50.
- [Google Scholar]
- Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41:48-54.
- [Google Scholar]
- Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48:835-47.
- [Google Scholar]
- Efficacy and safety of elbasvir/grazoprevir for 8 or 12 weeks for hepatitis C virus genotype 4 infection:A randomized study. Liver Int. 2020;40:1042-51.
- [Google Scholar]
- Efficacy of Elbasvir/Grazoprevir in early chronic G1/G4 hepatitis C infection in HIV/HCV co-infected patients with mild fibrosis. Gastroenterol Hepatol. 2021;44:191-7.
- [Google Scholar]
- Efficacy and safety of 12 weeks of elbasvir ±grazoprevir ±ribavirin in participants with hepatitis C virus genotype 2, 4, 5 or 6 infection:The C-SCAPE study. J Viral Hepat. 2018;25:457-64.
- [Google Scholar]
- The efficacy of Elbasvir/Grazoprevir fixed-dose combination for 8 weeks in HCV treatment and health-related quality of life (HRQoL) in treatment-naïve, non-cirrhotic, genotype 4-infected patients (ELEGANT-4):A single-center, single-arm, open-label, phase 3 trial. Saudi J Gastroenterol. 2022;28:225-32.
- [Google Scholar]







