Translate this page into:
Sulforaphane protects against LPS-induced liver injury in mice by antagonizing oxidative stress and apoptosis through AMPK activation
Address for Correspondence: May M. Alqurashi, Department of Biochemistry, Faculty of Sciences, King Abdulaziz University, Jeddah, Saudi Arabia. E-mail: mmsaqurshi1@kau.edu.sa
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Qassim Uninversity and was migrated to Scientific Scholar after the change of Publisher.
Abstract
ABSTRACT
Objectives:
Given the adverse effect of liver injury on a multitude of body functions, it is vital to understand its underlying mechanism and how to overcome it. In this study, lipopolysaccharide (LPS) was used to induce liver injury, while sulforaphane (SFN), a natural phytochemical, was used as the antagonist to overcome the deleterious effect.
Methods:
Twenty-four mice were divided into three groups: Control group (0.9% saline), LPS induction group (0.75 mg/kg), and SFN treatment (25 mg/kg) followed by LPS induction group (0.75 mg/kg), all with access to food and water ad libitum. Blood samples from retro-orbital sinus were used to measure liver function through two aminotransferases (i.e., alanine transaminase [ALT] and aspartate transaminase [AST]) whereas liver homogenate was used to measure glutathione (GSH), catalase (CAT), and superoxide dismutase (SOD) (antioxidant activity markers); caspase-3 (apoptosis marker); malondialdehyde (MDA) (lipid peroxidation marker); and NO. AMP-activated protein kinase (AMPK), a cellular energy homeostasis and lipid metabolism sensor, was also measured. Statistical analysis including normalization, analysis of variance, Kruskal–Wallis test, and significance of P < 0.05 were applied to all collected data.
Results:
SFN treatment significantly attenuated all tests compared to the induced liver injury by LPS where significant reduction was observed in the levels of hepatic function markers (AST and ALT), lipid peroxidation marker (MDA) as well as apoptosis marker (caspase-3) whereas a marked increase was observed for antioxidant activity markers (SOD, CAT, and GSH) and AMPK.
Conclusion:
These results indicate the protective effect of SFN as it re-instated the levels of antioxidation while decreasing the level of the biomarkers, which were significantly increased during liver injury induction by LPS.
Keywords
Liver injury
lipopolysaccharide
oxidative stress
protective effect
sulforaphane
Introduction
Due to the high morbidity and mortality rates, the liver injury represents a major threat to human health worldwide. Indeed, according to the Centers for Disease Control and Prevention in the United States, liver injury is the ninth leading cause of death,[1] resulting in around two million deaths per year globally.[2] As of 2023, there is no cure for liver injury, whereas the available treatments are only symptomatic.[3] The major hallmark of liver injury is the loss of hepatocyte function, which is characterized by inflammation, oxidative stress, and apoptosis.[4] Therefore, researchers have demonstrated that inhibiting inflammation, oxidative stress, and/or apoptosis could alleviate the development of liver injury.[5]
In recent years, increasing attention has been paid to nutraceuticals due to the potential for use as an alternative or complementary medication with regard to allopathic drugs.[6] Studies have demonstrated that naturally occurring plant phytochemicals show the potential to delay the progress of liver injury through different mechanisms, including neutralizing the detrimental effects of oxidative stress, retarding inflammation, and suppressing apoptosis.[7,8]
Sulforaphane (SFN) (1-isothiocyanato-4-(methylsulfinyl)-butane[SFN]), a natural isothiocyanate, is found in high concentrations within broccoli plants (Brassica oleracea var. Italica) and cruciferous vegetables.[9] SFN has been shown to exert anti-inflammatory,[10,11] anti-oxidative stress, and anti-apoptotic properties in many tissues.[9,12,13] Moreover, the anti-oxidative capability of SFN in liver injury has been demonstrated in a number of previous animal studies.[14,15] For instance, intraperitoneal administration of SFN decreased the malonaldehyde (MDA) and reactive oxygen species (ROS) levels, increased the antioxidant enzyme glutathione (GSH) level, and increased the catalase (CAT) and superoxide dismutase (SOD) activities.[16-18] In addition, SFN pre-treatment has been shown to inhibit apoptosis in the liver through several different mechanisms including the reduction of apoptotic initiator poly (ADP-ribose) polymerase cleavage,[19] decreasing the serum level of the cytokine tumor necrosis factor-α,[20] increasing the activity of Na+–K+-ATPase and Ca2+-ATPase[21] and decrease the levels of certain caspases known to mediate cell survival, such as caspase-1 and caspase-3.[13]
With SNF being the focus of a plethora of publications describing its efficacy in animal models (mainly mice and rats) as well as many clinical trials, its taxological profile was of utmost importance. SFN lethal median dose (LD50) was determined to be around 212.67 mg/kg from a study on mice through intraperitoneal dose administration where they found that different doses <100 mg/kg had no significant effect on the seizure threshold whereas 200 mg/kg significantly decreased it with signs of severe toxicity including deep sedation, ataxia, ptosis, and tremors were noted several minutes post a dose of 300 mg/kg.[22] Most studies on the effect of SNF were chosen way lower than LD50 and proven to have protective, preventive, and ameliorating properties. In addition, SNF was considered safe and well-tolerated by patients in clinical trials with low doses such as 50–150 μmol orally per day for treating children with autism.[23,24] A recent review compared the dosage and means of administration of SNF in animals where they demonstrated that oral administration was found with a median effective dose of 175 μmol/kg body weight whereas intraperitoneal administration was 113 μmol/kg body weight,[25] given the latter a higher probability to elicit toxicity.
The estimated human equivalent dose (HED) is an equation used to help better extrapolate doses for human clinical trials from animal studies to better design them. It states that HED (mg/kg) can be calculated by multiplying the animal dose (mg/kg) with a constant ration depending on the animal species (i.e., 0.081 for mice and 0.162 for rats).[26] With such an equation, the LD50 of SNF is estimated to be 17.23 mg/kg, indicating this phytocompound is relatively safe for human consumption by diet.
Adenosine monophosphate (AMP)-activated protein kinase (AMPK) is a key player in the cellular energy regulatory pathways within multiple organs throughout the body.[27] AMPK may also play a major role as an oxidative stress sensor and redox regulator that is important in terms of maintaining intracellular homeostasis during various stress challenges.[28] Moreover, prior studies have shown that active AMPK exerts an anti-apoptotic effect in multiple types of cells as well as a suppressive effect on caspase-3.[29-31] As liver injury is mainly characterized by elevated levels of oxidative stress and apoptosis, the activation of AMPK signaling to boost the antioxidant capacity and suppress the apoptotic activity has been suggested as a potential therapeutic target in liver injury.
Given the high prevalence of liver disease worldwide and the lack of pre-clinical alternatives, animal models of liver injury are crucial to improving our understanding of the disease’s pathogenesis as well as enabling the identification of therapeutic targets and testing of novel drugs.[32,33] Lipopolysaccharide (LPS), an endotoxin that is among the constituents of the outer membrane of Gram-negative bacteria,[34] can induce inflammation and oxidative stress, leading to liver injury.[35] In addition, LPS is relatively easy to administer and inexpensive to use when compared with other liver-injury-inducing agents.[36] Therefore, the LPS-induced liver injury model has become a widely used animal model due to closely mimicking the clinical symptoms of liver injury.
To the best of our knowledge, only a few studies have investigated the effect of SFN on LPS-induced liver injury. Thus, the present study aims to examine the possible protective effect of SFN against LPS-induced liver injury in mice by evaluating its anti-oxidative and anti-apoptotic properties and determining its possible AMPK-related mechanism of action.
Materials and Methods
Chemicals
The LPS (Escherichia coli, O111: B4) used in this study was purchased from in vivo Gen (San Diego, California, United States). A stock solution of 5 mg/mL (weight/volume [w/v]) of LPS was prepared by dissolving 5 mg of powdered LPS in 1 mL of endotoxin-free water. The SFN was obtained from Santa Cruz Biotechnology (Dallas, Texas, United States). It was dissolved in 3% dimethyl sulfoxide (DMSO) prepared in normal saline. The sucrose, ethylenediaminetetraacetic acid (EDTA), and 3-morpholinopropane-1-sulfonic acid (MOPS) were purchased from SolarbioLife Sciences (Beijing, China). The ethanol was purchased from Thermo Fisher Scientific (Waltham, Massachusetts, United States), whereas the sodium hydroxide (NaOH) was purchased from MyBioSource (San Diego, California, United States).
Animals
The 24 Swiss albino male mice (SWR/J) (18–25 g) used in this study were obtained from the Animal House Unit of King Fahad Medical Research Center (KFMRC), King Abdulaziz University, Jeddah, Saudi Arabia. Three to five mice were housed per cage, and the mice were maintained under a 12-h light/dark cycle at approximately room temperature (23 ± 2°C) and humidity (65%). All the mice had access to food and water ad libitum. Moreover, the mice were treated in accordance with the guidelines of the Animal Unit Committee of KFMRC. All the experiments were performed according to the guidelines of the Biomedical Ethics Research Committee (Reference No. 603–20) of King Abdulaziz University. They also accorded with the rules and regulations of the Animal Care and Use Committee of KFMRC, which complied with the “System of Ethics of Research on Living Creatures” guidelines prepared by the King Abdulaziz City for Science and Technology and approved by Royal Decree No. M/59 dated August 24, 2010.
Experimental design
The mice were randomly divided into three groups (eight mice per group): the control group (0.9% saline vehicle), the LPS induction group (0.75 mg/kg), and the SFN treatment (25 mg/kg) followed by LPS induction (0.75 mg/kg) group. The total duration of the study was 2 weeks [Figure 1]. During the 1st week, the mice in the control and LPS groups were injected daily with intraperitoneal (IP) normal saline, whereas the mice in the SFN group were injected with IP SFN. Disease induction was performed in the 2nd week, with the mice in each group receiving two IP injections daily: Saline + 3% DMSO in the control group, 0.75 mg/kg of LPS + 3% DMSO in the LPS group, and 25 mg/kg of SFN + 0.75 mg/kg of LPS in the SFN+LPS group.

- Illustration of the experimental design
Determination of liver function
Blood samples were drawn from the retro-orbital sinus of mice in all the groups before euthanasia. The samples were centrifuged at 3000 g for 10 min after the pallet was discarded. The levels of two aminotransferases known to be markers of hepatic function – namely, serum aspartate transaminase (AST) and alanine transaminase (ALT) – were determined using enzyme-linked immunosorbent assay (ELISA) kits (MyBioSource, San Diego, California, United States) according to the manufacturer’s protocols. The results were read at 450 nm using a microplate reader (BioTekInstruments, Winooski, Vermont, United States).
Preparation of liver homogenate
The livers obtained from all the groups were weighed, chopped into small pieces, and had lysis buffer added to the tissues. The lysis buffer was prepared by dissolving 17.1 g of sucrose (0.25 M) in 100 mL of distilled water, then 2 mL of EDTA (1 mM), 10 ml of MOPS (5 mM), and 0.2 mL of ethanol (0.1% [v/v]) were, respectively, added, with the pH adjusted to 7.2 using NaOH (1M). The mixture was then homogenized using an ultrasonicator (BioLogics, Cary, North Carolina, United States). Following complete homogenization, the homogenate was centrifuged in a cold centrifuge (4°C) at 5000 g for 5 min. Aliquots were then prepared and stored at −80°C.
Measurement of hepatic antioxidant activity
In the liver homogenate, the SOD, CAT, and GSH activity levels were measured using a colorimetric assay kit (SolarbioLife Sciences, Beijing, China) according to the manufacturer’s protocol. In brief, the CAT activity was measured using the rate of the decrease in the H2O2, the SOD activity was determined using xanthine oxidase methods, and the GSH activity was measured based on its reaction with 5,5’-dithiobis-2-nitrobenzoic acid to form a product that could be detected spectrophotometrically.
Measurement of hepatic MDA and NO content
In the liver homogenate, the MDA and NO contents were measured using a colorimetric assay kit (Solarbio Life Sciences, Beijing, China) according to the manufacturer’s protocol. Briefly put, the MDA content was measured using the thiobarbituric acid method, while the NO content measurement was performed based on the product of the NO2 and diazonium sulfonamide reaction (diazo compounds) under acidic conditions, where the compounds could further couple with naphthyl vinyl diamine to form a product that could be spectrophotometrically detected.
Determination of caspase-3 activity
The hepatic activity of caspase-3 was assessed using liver homogenate through an active caspase-3 ELISA kit (MyBioSource, San Diego, California, United States) according to the manufacturer’s instructions. 100 μL of each sample, standard and blank was added to a well plate in duplicate followed by 100 μL of phosphate-buffered saline (pH 7.0–7.2) added to the blank control well and 10 mL of balance solution added into only the sample wells and mixed. After that, 50 μL of conjugate was added to each well, incubated for 1 h at 37°C, washed 5 times with diluted wash solution, and 50 mL of substrate A and B were added to each well and incubated at 37°C for 20 min. Finally, 50 mL of the stop solution was added to each well to stop the reaction. The absorbance was read at 450 nm using a microplate reader purchased from BioTek Instruments (Winooski, Vermont, United States).
Determination of AMPK activity
The hepatic AMPK activity was determined using a phosphorylated AMPK ELISA kit purchased from MyBioSource (San Diego, California, United States) according to the manufacturer’s instructions. The absorbance was read at 450 nm using a microplate reader purchased from BioTek Instruments (Winooski, Vermont, United States).
Statistical analysis
All the data were expressed as the mean ± standard error of the mean and statistically analyzed using GraphPad Prism 9.1.2 software. Normality was evaluated by means of the Kolmogorov–Smirnov test. The one-way analysis of variance followed by the post hoc Tukey’s test was used for comparisons between the groups in terms of all the results except for the non-normally distributed variables, for which the Kruskal–Wallis test followed by Dunn’s test was used. The differences between the groups were considered statistically significant if P < 0.05.
Results
Serum aminotransferase concentrations
The results demonstrated a significant (P < 0.0001) increase in the AST and ALT concentrations in the LPS group when compared with the control group [Figure 2]. By contrast, SFN administration significantly (P < 0.0001) reduced the concentrations of AST and ALT when compared LPS group [Figure 2].

- Serum aminotransferase concentrations. The LPS endotoxin caused an increase in the AST and ALT concentrations, whereas SFN administration significantly decreased the aminotransferase levels. ****Represents P<0.0001. LPS: lipopolysaccharide, SFN: Sulforaphane, AST: Serum aspartate transaminase, ALT: Alanine transaminase
MDA and NO contents
LPS administration resulted in a significant (P < 0.0001) increase in the MDA and NO contents when compared with the control group [Figure 3]. By contrast, SFN administration caused a significant (P < 0.0001) decrease in the MDA content when compared with the LPS group, although no significant difference in the NO content was observed [P = 0.0706; Figure 3].

- Malondialdehyde and nitric oxide contents. LPS administration increased the MDA and NO contents, whereas SFN decreased the measured MDA content but caused no difference in the NO content. ****Represents P<0.0001. LPS: Lipopolysaccharide, SFN: Sulforaphane, MDA: Malondialdehyde, NO: Nitric oxide
Hepatic antioxidant enzyme activity
Figure 4 illustrates the findings concerning the antioxidant enzymes. Here, LPS administration caused a significant (P < 0.0001) decrease in the SOD and CAT activities and the concentration of GSH when compared with the control group. By contrast, SFN treatment significantly (P < 0.0001) increased the SOD and CAT activities and the GSH concentration when compared with the LPS group.

- Antioxidant enzyme activities and concentrations.LPS administration decreased the antioxidant enzymes, whereas SFN treatment increased the measured antioxidant enzymes. ****Represents P<0.0001. LPS: Lipopolysaccharide, SFN: Sulforaphane, SOD: Superoxide dismutase, CAT: Catalase, GSH: Glutathione
Hepatic caspase-3 activity
LPS administration significantly (P < 0.0001) increased the caspase-3 activity when compared with the control group, whereas SFN treatment significantly (P < 0.0001) decreased the caspase-3 activity [Figure 5].

- Caspase-3 activities. LPS administration increased the caspase-3 activity, whereas SFN treatment decreased it. ****Represents P<0.0001. LPS: Lipopolysaccharide, SFN: Sulforaphane
Hepatic AMPK activity
The findings revealed that LPS administration significantly (P < 0.0001) decreased the AMPK activity when compared with the control group, whereas SFN treatment significantly (P < 0.0001) increased the AMPK activity [Figure 6].
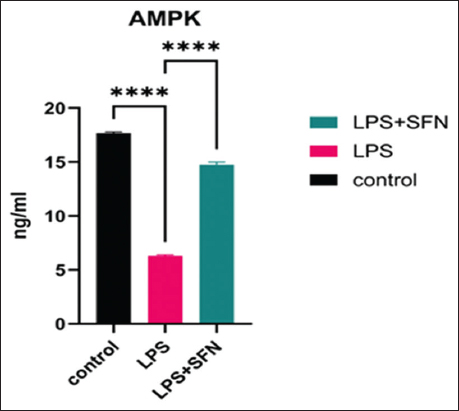
- AMPK activities. LPS administration decreased the AMPK activity, whereas SFN treatment increased the measured AMPK activity. ****Represents P<0.0001. LPS: Lipopolysaccharide, SFN: Sulforaphane, AMPK: AMP-Activated protein kinase
Discussion
Prevention methods against liver injury (be it hepatitis or cirrhosis) mainly focus on vaccines, early screening and detection of the disease, reducing obesity, reducing alcohol consumption level, and monitoring risk factors related to age, medical history, or hygiene[37-40] but there is a lack of methods for healthy individuals to help protecting and preventing the disease. Therefore, finding natural products that can offer potential protective approaches is a very important task. Therefore, this study sought to investigate the possible protective mechanisms of SFN pre-treatment in relation to LPS-induced liver injury in mice. Our results demonstrate a clear pattern regarding the effects of LPS and SFN, whereby SFN administration for 2 weeks significantly protected against LPS-induced liver injury. Moreover, our data demonstrate the anti-oxidative and anti-apoptotic effect of SFN as a possible protective mechanism against LPS-induced liver injury that occurs through AMPK activation.
The two studied aminotransferase enzymes (AST and ALT) are intracellular enzymes (which occur inside cells, not high in blood serum), meaning that their elevation in the blood represents cellular damage.[41] They are predominantly found in the liver and so are routinely used as indicators of abnormal hepatic physiology (i.e., liver injury) because they serve as sensitive biomarkers in the serum.[42] One of the main effects of hepatic injury induced through LPS is the elevation of the AST and ALT enzymes.[43] In our study, the results show that the LPS group had increased AST and ALT activities when compared with the control group, whereas IP injection of SFN for 14 days significantly decreased the AST and ALT activities, indicating SFN to exert a protective and ameliorating effect against LPS in the liver. These results accord with those of several previous studies.[14,20,44-46]
Excessive ROS production that results in oxidative stress is believed to be an early event in the progression of liver injury.[47] Enzymes such as CAT and SOD play a pivotal role in ROS regulation and protection from tissue damage.[48] Moreover, antioxidant compounds such as GSH play an essential role in cell defense against oxidative stress through modulating the physiological levels of ROS. Furthermore, MDA and NO (a lipid peroxidation end product) act as indicators of cell membrane damage and lack of antioxidant defense.[49] One of the major pathological mechanisms of LPS in relation to liver injury entails disturbing the cellular antioxidant defenses and causing the release of mitochondrial ROS.[50] In this study, LPS administration induced oxidative stress in mice liver, as indicated by the increased levels of MDA and NO, the reduced level of GSH, and the reduced CAT and SOD activities when compared with the control group. Similar results have been broadly reported by several prior studies.[3,51-53] By contrast, IP injection of SFN increased the hepatic antioxidants’ effect and reduced the level of oxidative stress, as evidenced by the remarkably mitigated MDA and NO levels, the significantly restored CAT and SOD activities, and the significantly restored GSH levels. These results indicate that SFN’s hepatoprotective effect could be attributed to its antioxidant potential. Data concerning SFN’s hepatoprotective efficacy against oxidative damage using an LPS-induced liver injury model are limited, with only a few previous studies having discussed the exact mechanism involved.[14,17,20,44,45]
Activation of the executioner enzyme (caspase-3) and promotion of hepatic apoptosis are typical pathological features of liver injury.[54,55] Therefore, intervention in hepatic apoptosis has been suggested as an approach to alleviating liver injury. The results of our study demonstrate that caspase-3 was elevated in the LPS group and significantly reduced in the group treated with SFN, indicating that the anti-apoptotic effect of SFN might be an effective hepatoprotective strategy in liver injury. Our results support the findings of certain previous studies that revealed the endotoxin LPS to induce the expression of apoptotic markers such as caspase-3 in the liver and, therefore, contribute to liver injury,[35,56] whereas SFN attenuates the deleterious effect of apoptosis.[13]
The underlying mechanism by which SFN improves hepatic injury is not yet fully understood. However, prior studies have reported that the activation of AMPK has a potential role in modulating oxidative stress[57-60] and inhibiting apoptosis in different cell types, including hepatocytes.[61-63] Conversely, other studies have shown that SFN can fight different disorders through regulating the AMPK signaling pathway.[64,65] Thus, the present study investigated whether SFN can protect against liver injury through AMPK activation mediating its anti-apoptotic and anti-oxidative effects.
Our results concerning AMPK activity reveal opposing patterns regarding the situation post-LPS and post-SFN treatment, with the AMPK activity being reduced following the LPS-induced liver injury but significantly increased following the SFN treatment. This finding indicates, for the 1st time, the effectiveness of SFN as a potent activator of AMPK.
Conclusion
The present study found that 2 weeks of SFN treatment protected against LPS-induced liver injury in mice by increasing its anti-oxidative and anti-apoptosis ability through the activation of AMPK. Taken together, the results suggest that SFN might be an effective prophylactic agent for the treatment of liver injury.
Limitations and future studies
This study had a number of limitations that need to be discussed and further explored. First, the study did not involve histological or pathological examinations of the liver. Moreover, the anti-inflammatory property of SFN was not examined in this study. Therefore, it will be significant to continue examining SFN’s prophylactic effect and its underlying mechanism in liver injury by addressing these two limitations. In addition, elucidation of the mechanism by which SFN activates the AMPK signaling pathway and induces its anti-oxidative and anti-apoptosis properties may provide the opportunity to develop preventive strategies for liver injury in the future.
Ethics Approval and Consent to Participate
The authors certify that the mice used in the experimental design were treated in accordance with the guidelines of the Animal Unit Committee of KFMRC. All the experiments were performed according to the guidelines of the Biomedical Ethics Research Committee (Reference No. 603–20) of King Abdulaziz University. They also accorded with the rules and regulations of the Animal Care and Use Committee of KFMRC, which complied with the “System of Ethics of Research on Living Creatures” guidelines prepared by the King Abdulaziz City for Science and Technology and approved by Royal Decree No. M/59 dated August 24, 2010. Jointly, the authors approved the publication of this manuscript.
Availability of Data and Material
The data supporting the findings of this study are available within the article. More detailed data used to support the findings of the current study are available from the corresponding author upon reasonable request.
Competing Interests
All the authors declared that there was no conflict and/or competing interests.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors’ Contributions
Rasha A. Mansouri; conceptualization, data curation, formal analysis, methodology, and supervision. Huda F. Alshaibi; investigation, methodology, and project administration. May M. Alqurashi; writing, reviewing, and proofreading. Maimoonah M. Shaikh; conceptualization, formal analysis, funding acquisition, investigation, methodology, validation, and writing the original draft. Khulud A. Bahaidrah; investigation, methodology, and resources. Noor A. Alzahrani; investigation, methodology, and resources.
References
- Alanyl-glutamine protects against lipopolysaccharide-induced liver injury in mice via alleviating oxidative stress, inhibiting inflammation, and regulating autophagy. Antioxidants (Basel). 2022;11:1070.
- [Google Scholar]
- Artificial Liver. Berlin: Springer Singapore; 2020. p. :569.
- The potential effect of phytochemicals and herbal plant remedies for treating drug-induced hepatotoxicity:A review. Mol Biol Rep. 2021;48:4767-88.
- [Google Scholar]
- A comprehensive overview of hepatoprotective natural compounds:Mechanism of action and clinical perspectives. Arch Toxicol. 2016;90:39-79.
- [Google Scholar]
- Review of polyphenol-rich products as potential protective and therapeutic factors against cadmium hepatotoxicity. J Appl Toxicol. 2019;39:117-45.
- [Google Scholar]
- Isothiocyanate from Broccoli, sulforaphane, and its properties. J Med Food. 2019;22:121-6.
- [Google Scholar]
- Anti-inflammatory effect of sulforaphane on LPS-stimulated RAW 264.7 cells and ob/ob mice. J Vet Sci. 2020;21:e91.
- [Google Scholar]
- Sulforaphane reduces lipopolysaccharide-induced inflammation and enhances myogenic differentiation of mouse embryonic myoblasts via the toll-like receptor 4 and NLRP3 pathways. Adv Clin Exp Med. 2022;32:457-67.
- [Google Scholar]
- Effects of sulforaphane in the central nervous system. Eur J Pharmacol. 2019;853:153-68.
- [Google Scholar]
- Sulforaphane attenuates nonalcoholic fatty liver disease by inhibiting hepatic steatosis and apoptosis. Nutrients. 2022;14:76-88.
- [Google Scholar]
- Sulforaphane ameliorates ethanol plus carbon tetrachloride-induced liver fibrosis in mice through the Nrf2-mediated antioxidant response and acetaldehyde metabolization with inhibition of the LPS/TLR4 signaling pathway. J Nutr Biochem. 2021;89:108573.
- [Google Scholar]
- Sulforaphane ameliorates cadmium induced hepatotoxicity through the up-regulation of/Nrf2/ARE pathway and the inactivation of NF-κB. J Funct Foods. 2021;77:104297.
- [Google Scholar]
- Protective effects of sulforaphane on exercise-induced organ damage via inducing antioxidant defense responses. Antioxidants (Basel). 2020;9:136.
- [Google Scholar]
- Sulforaphane protects liver injury induced by intestinal ischemia reperfusion through Nrf2-ARE pathway. World J Gastroenterol. 2010;16:3002-10.
- [Google Scholar]
- Sulforaphane prevents microcystin-LR-induced oxidative damage and apoptosis in BALB/c mice. Toxicol Appl Pharmacol. 2011;255:9-17.
- [Google Scholar]
- Sulforaphane increases the survival rate in rats with fulminant hepatic failure induced by D-galactosamine and lipopolysaccharide. Nutr Res. 2014;34:982-9.
- [Google Scholar]
- Sulforaphane reduces apoptosis and oncosis along with protecting liver injury-induced ischemic reperfusion by activating the Nrf2/ARE pathway. Hepatol Int. 2015;9:321-9.
- [Google Scholar]
- Increased seizure susceptibility and other toxicity symptoms following acute sulforaphane treatment in mice. Toxicol Appl Pharmacol. 2017;326:43-53.
- [Google Scholar]
- Sulforaphane treatment in children with autism:A prospective randomized double-blind study. Nutrients. 2023;15:718.
- [Google Scholar]
- Sulforaphane treatment of autism spectrum disorder (ASD) Proc Natl Acad Sci U S A. 2014;111:15550-5.
- [Google Scholar]
- Broccoli or sulforaphane:Is it the source or dose that matters? Molecules. 2019;24:3593.
- [Google Scholar]
- A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7:27-31.
- [Google Scholar]
- AMP-activated protein kinase:An energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25:1895-908.
- [Google Scholar]
- AMP-activated protein kinase, stress responses and cardiovascular diseases. Clin Sci (Lond). 2012;122:555-73.
- [Google Scholar]
- A new constitutively active mutant of AMP-activated protein kinase inhibits anoxia-induced apoptosis of vascular endothelial cell. Hypertens Res. 2009;32:133-9.
- [Google Scholar]
- Activation of AMP-activated protein kinase alpha1 alleviates endothelial cell apoptosis by increasing the expression of anti-apoptotic proteins Bcl-2 and survivin. J Biol Chem. 2010;285:15346-55.
- [Google Scholar]
- Effects of aging on cardiac and skeletal muscle AMPK activity:Basal activity, allosteric activation, and response to in vivo hypoxemia in mice. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1270-5.
- [Google Scholar]
- Mouse models of liver parenchyma injuries and regeneration. Front Cell Dev Biol. 2022;10:903740.
- [Google Scholar]
- Preclinical models of acute liver failure:A comprehensive review. PeerJ. 2021;9:e12579.
- [Google Scholar]
- Innate immune sensing and its roots:The story of endotoxin. Nat Rev Immunol. 2003;3:169-76.
- [Google Scholar]
- Deletion of TLR4 attenuates lipopolysaccharide-induced acute liver injury by inhibiting inflammation and apoptosis. Acta Pharmacol Sin. 2021;42:1610-9.
- [Google Scholar]
- Adiponectin protects LPS-induced liver injury through modulation of TNF-α in KK-Ay obese mice. Hepatology. 2004;40:177-84.
- [Google Scholar]
- Type A viral hepatitis:A summary and update on the molecular virology, epidemiology, pathogenesis and prevention. J Hepatol. 2018;68:167-84.
- [Google Scholar]
- Burden of liver disease in Europe:Epidemiology and analysis of risk factors to identify prevention policies. J Hepatol. 2018;69:718-35.
- [Google Scholar]
- Changing prevalence of anti-hepatitis A virus in adolescents in a rural township in Taiwan. Chang Gung Med J. 2010;33:321-6.
- [Google Scholar]
- Hepatitis A:Epidemiology and prevention in developing countries. World J Hepatol. 2012;4:68-73.
- [Google Scholar]
- Correlation of serum alanine aminotransferase and aspartate aminotransferase with coronary heart disease. Int J Clin Exp Med. 2015;8:4399-404.
- [Google Scholar]
- New insights into the pathogenesis of non-alcoholic fatty liver disease:Gut-derived lipopolysaccharides and oxidative stress. Nutrients. 2020;12:2762.
- [Google Scholar]
- Hepatic protective effects of sulforaphane through the modulation of inflammatory pathways. J Asian Nat Prod Res. 2020;22:386-96.
- [Google Scholar]
- The protective effect of broccoli seed extract against lipopolysaccharide-induced acute liver injury via gut microbiota modulation and sulforaphane production in mice. Foods. 2023;12:2786.
- [Google Scholar]
- Sulforaphane, an Nrf-2 agonist, modulates oxidative stress and inflammation in a rat model of cuprizone-induced cardiotoxicity and hepatotoxicity. Cardiovasc Toxicol. 2023;23:46-60.
- [Google Scholar]
- Oxidative stress as a crucial factor in liver diseases. World J Gastroenterol. 2014;20:8082-91.
- [Google Scholar]
- Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell Physiol Biochem. 2017;44:532-53.
- [Google Scholar]
- Role of lipid peroxidation as a mechanism of liver injury after acetaminophen overdose in mice. Toxicol Sci. 2003;76:229-36.
- [Google Scholar]
- Reactive oxygen species in metabolic and inflammatory signaling. Circ Res. 2018;122:877-902.
- [Google Scholar]
- Modulatory role of lipoic acid on lipopolysaccharide-induced oxidative stress in adult rat Sertoli cells in vitro. Chem Biol Interact. 2009;182:112-8.
- [Google Scholar]
- Oridonin protects LPS-induced acute lung injury by modulating Nrf2-mediated oxidative stress and Nrf2-independent NLRP3 and NF-κB pathways. Cell Commun Signal. 2019;17:62.
- [Google Scholar]
- Artemisitene alters LPS-induced oxidative stress, inflammation and ferroptosis in liver through Nrf2/HO-1 and NF-kB pathway. Front Pharmacol. 2023;14:1177542.
- [Google Scholar]
- Caspase 3 role and immunohistochemical expression in assessment of apoptosis as a feature of H1N1 vaccine-caused drug-induced liver injury (DILI) Electron Physician. 2017;9:4261-73.
- [Google Scholar]
- Sophocarpine attenuates LPS-induced liver injury and improves survival of mice through suppressing oxidative stress, inflammation, and apoptosis. Mediators Inflamm. 2018;2018:5871431.
- [Google Scholar]
- Sipjeondaebo-tang alleviates oxidative stress-mediated liver injury through activation of the CaMKK2-AMPK signaling pathway. Evid Based Complement Alternat Med. 2018;2018:8609285.
- [Google Scholar]
- Tryptanthrin prevents oxidative stress-mediated apoptosis through AMP-activated protein kinase-dependent p38 mitogen-activated protein kinase activation. Arch Pharm Res. 2017;40:1071-86.
- [Google Scholar]
- AMPK activation by isorhamnetin protects hepatocytes against oxidative stress and mitochondrial dysfunction. Eur J Pharmacol. 2014;740:634-40.
- [Google Scholar]
- Upregulation of mitochondrial uncoupling protein-2 by the AMP-activated protein kinase in endothelial cells attenuates oxidative stress in diabetes. Diabetes. 2008;57:3222-30.
- [Google Scholar]
- Activation of AMPK and inactivation of Akt result in suppression of mTOR-mediated S6K1 and 4E-BP1 pathways leading to neuronal cell death in in vitro models of Parkinson's disease. Cell Signal. 2014;26:1680-9.
- [Google Scholar]
- Endogenous AMPK acts as a detrimental factor in fulminant hepatitis via potentiating JNK-dependent hepatocyte apoptosis. Cell Death Dis. 2017;8:e2637.
- [Google Scholar]
- AMPK activation regulates apoptosis, adipogenesis, and lipolysis by eIF2alpha in adipocytes. Biochem Biophys Res Commun. 2006;340:43-7.
- [Google Scholar]
- Sulforaphane attenuates obesity by inhibiting adipogenesis and activating the AMPK pathway in obese mice. J Nutr Biochem. 2014;25:201-7.
- [Google Scholar]
- Modulation of endoplasmic reticulum stress via sulforaphane-mediated AMPK upregulation against nonalcoholic fatty liver disease in rats. Cell Stress Chaperones. 2022;27:499-511.
- [Google Scholar]







