Translate this page into:
Synthesis, characterization, and applications of iron oxide nanoparticles
Address for correspondence: Sulaiman Faisal, Institute of Integrative Biosciences, CECOS University Peshawar, Pakistan. E-mail: sulaiman@cecos.edu.pk
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Qassim Uninversity and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective:
The green synthesis method for nanoparticles is getting more attention globally, due to its lesser cost, non-hazardous, and eco-friendly nature. The novelty of the present work is to investigate the anti-bacterial and degradation activity of the green synthesized Iron Oxide NPs.
Methods:
In this study, the Iron Oxide NPs were synthesized through a green synthesis route from leaves of Ficus Palmata. UV-Vis confirmed Iron Oxide NP’s peaks between (230–290 nm), while Fourier transforms infrared spectroscopy analysis showed that several groups were involved in reduction and stabilization.
Results:
Results indicated that the highest photo thermal activity was shown in light and it was almost 4 folds greater than the control. Similarly, Iron Oxide NPs showed excellent antimicrobial potential against bacterial species “Salmonella typhi” “Xanthomonas Oryzae” and “Lactobacillus” at low concentrations (150 μg/mL). Hemolytic assay results showed that the toxicity was lesser than 5% at both dark and light conditions. Moreover, we also evaluated the photo-catalytic potential of Iron Oxide NPs against methylene orange. Results indicated that almost complete degradation was noted after 90 min in the presence of continuous light. All tests were performed in triplicates. All the data was subjected to P-test (P < 0.5) using Excel and graph pad (V.5.0).
Conclusion:
Iron Oxide NPs holds a promising future and could be used in treating diseases, and microbial pathogenesis and also could be used as a vector in drug delivery. Moreover, they can also eradicate persistent dyes and could be used as an alternative to remediate pollutants from the environment.
Keywords
Biocompatibility
ficus palmata
green synthesis
microbial pathogenesis
nanotechnology
photothermal
Introduction
Nanotechnology is an emerging field that has got a phenomenal interest in the previous few decades. The word “nano” is used as a prefix for one billionth part 10-9[1,2] Due to their extraordinary physical and chemical properties, nanoparticles have been used in various fields of science.[3] Among other nano structures, metallic nanoparticles have also got significant interest, it has been used in medical, sensing, electronics, and imaging devices.[4] Metallic nanoparticles distinctive physical and chemical properties and could be used in several biomedical applications such as drug delivery, treatment of diseases such as Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis, bone inflammation, skin infections, tuberculosis (TB)[5,6] and also used in biological sensors.[7] Various methods have been used for the synthesis of metal and other NP’s. Such as chemical and Physical methods which include thermal decomposition, hydrothermal synthesis, sol-gel synthesis, sonochemical reactions, and colloidal chemistry-based methods etc.[8] As such routes for the synthesis of NPs produce toxic waste and have many environmental and health concerns such as irritation, stomach pain, allergic reactions, and inflammation.[9-11] Both methods are expensive because of the high usage of energy and other factors.[12] Due to number of drawbacks of the physical and chemical methods, it limits their uses in biological applications. Currently scientists and researchers have found cost effective and environmental friendly method called the green synthesis method to address these concerns. There are various sources for the green synthesis method such as plants, microbes, and other biological products which are used as reducing and stabilizing agents. Bio-mediated synthesis of nanoparticles is a simple process. Simply a metal salt in the required amount is taken and mixed with plant extract and the reaction takes minutes to a few hours and will complete at ordinary room temperature. The metallic salt solution functions as a reducing agent and can form respective nanoparticles.[13-19] This method of synthesis of nanoparticles have got more attention during the last decade, especially green synthesis of iron nanoparticles NP’s which are considered as safe as compared to other metallic NP’s. Green synthesized nanoparticles have numerous applications in the field of biology, physics, and chemistry. Due to their biocompatible nature, green synthesized iron nanoparticles have been used in disease diagnostics such as bone inflammation, skin infections, TB, tumor, atherosclerosis, cardiovascular, pulmonary diseases, and hepatitis antibodies detection,[20] and drug delivery system. Iron NPs kills diseased cells selectively by either production of Reactive Oxygen Species (ROS) or inhabiting any particular gene involved in disease onset without hampering normal cell functioning.[21-24] It has also been used in antimicrobial, anticancer, and antifungal therapies.[25] Iron nanoparticles kill microbes via production of ROS, obstructing membrane structure and changing macromolecules chemistry which leads to death of microbial pathogens. Green synthesized nanoparticles have also been shown to have excellent photo thermal activities, which means they can gain heat from normal visible light and could be used in sensing, disease control, and treatment at the earliest stages. Previously, the photothermal potential of iron nanoparticles has been reported and shows a lot of promise in the biomedical field.[26] Moreover, besides their medical applications they can also be used in the degradation of persistent pollutants. Iron oxide nanoparticles have also been reported to degrade various dyes and have also shown better activities when compared to their counterparts. Using Iron Oxide NPs can successively degrade the synthetic organic dye methylene blue (MB) by sodium borohydride (MB by NABH4). As MB is widely used as a redox indicator in analytical chemistry and have a long life period and is a potential pollutant.[27,28] Thus herein, we aim to synthesize Iron Oxide NPs using a green chemistry approach, and will be used to evaluate their biological and degradation applications.
Materials and Methods
Chemicals
Iron (III) Oxide, Para Sintesis, (Fe2O3, M = 159.70), Copper (II) nitrate trihydrate extra pure. Methyl Orange dye, Safranin dye, Ethanol, Barium Chloride, Sulfuric Acid, Triton X–100, Phosphate buffer saline (PBS), Yeast, Peptone, Agar, and Sodium Chloride were procured from Sigma Aldrich or local vendors.
Machines and other devices
Electronic Balance (ALE-223), Heating Oven (UF110), Incubator (MCO-18ACL-PA), Autoclave (Hirayama HV-110), Shaking Incubator (FS-50B/70B), Water Bath (Vt-Lsi02), Magnetic stirrer (ARE 6), Vortex Mixer (VM-96A), Sonicator (AU33-10), Spectrophotometer (VT-UV1900), PH Meter (Jenway 3510), Centrifuge Machine (75004240), and Class II Biological Safety Cabinet (LA2-6A2-E) were used in our study.
Synthesis of iron oxide NPs
The fresh and healthy leaves were taken from ficus palmata plant and washed properly with dH2O, to remove dusts and impurities. Leaves were kept at dark to become dry upon complete, dry leaves were grinded through blander to fine power, the fine powder was then stored in an air tight container for future use. For synthesis briefly 5 g of powder leaves were taken and added into 100 mL of dH2O, and stirred for about 10 min in order to mix properly. After that solution was placed in beaker and heated 20°C for 4 min in oven, to extract all the phytochemicals. Later the extract was filtered via filter paper; supernatant obtained was stored at 4°C. Later 10 mL of the leaves extract was added into 90 mL of an aqueous solution of 1 mM Iron (III) Oxide salt. The mixture was placed on magnetic stirrer for 30 min. later it was left at dark for a period of 1 h, the color of the mixture was noted, which changed to dark brown indicating the formation of NPs. After that in order to obtain the pure NP’s, reaction mixture was centrifuged at 9000 rpm for 10 min, to get the precipate. Later centrifugation obtained precipate washed 3 times. The first two rounds with dH2O while the 3rd round washed with ethanol 70% for each round 9000 rpm was used for 10 min. Obtained NPs were put in incubator at 60°C for 24 h to obtain pure and highly crystalline Iron Oxide NP’s. The whole process of NPs are shown in Figure 1 step by step.
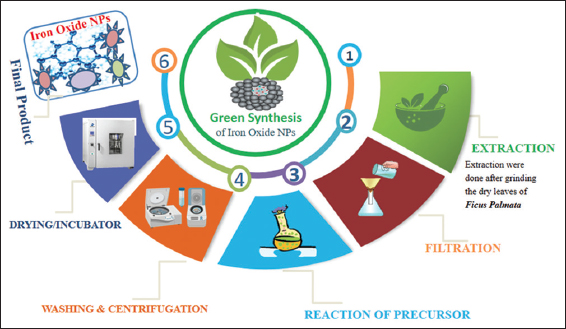
- Diagrammatic explanation of the green synthesis method
Characterization
UV-VIS spectrophotometer
A double beam UV-Vis Spectrophotometer (VT-UV1900, Thermo Scientific, Finland) was used to detect the presence of Iron Oxide NPs in the reaction mixture. The reaction mixtures were scanned in the wavelength (λ) ranging between (230 and 290) nm.
Fourier transform infrared spectroscopy (FTIR) spectroscopy
Major functional groups which assist in the reduction and stabilization of nanoparticles are defined by FTIR. The functional groups (plant specific) are found in association with synthesized nanoparticles. The facility used for their detection is which utilizes potassium bromide (KBr) pellets spanning the specific spectral range of 400–4000 cm-1.
Photothermal activity
The photothermal activity was evaluated as previously described by[29] with slight modification. Photo thermal activity of Iron Oxide NPs was measured by using of pH meter probe. Briefly (0.03 g) NP’s were added to 50 mL of dH2O and sonicated in an ultrasonicator for a period of 30 min. Later sonication 1 mL of Iron Oxide NP’s was mixed with 4 mL of dH2O and initial reading was taken by spectrophotometer. First Iron Oxide NPs were placed in light and values were noted periodically after each minute till 10 min. The whole process was performed in a replicate. Similarly, same process was conducted in the dark to check the Photothermal activity of Iron Oxide NPs.
Minimum inhibitory concentration (MIC)
MIC is used to check, the smallest concentrations of the test sample (NPs, drugs, etc.) that could stop bacterial growth. In the present study, different cultured bacteria (Salmonella typhi, Lactobacillus, Xanthomonas Oryzae) were used. McFarland solution 0.5% is used as standard to balance the turbidity of bacterial culture. And then added 5 mL of sterile media in all the dilutions to get 10 mL of the total solution. After that, the NP’s dilution was prepared set standard of 1000 uL. In the present study the 96 wells plate contained and labeled as, first two columns (1st, 2nd) contained bacteria “Lactobacillus and Salmonella typhi”, the next two columns (3rd, 4th) were taken as control, followed by columns (5th, 6th, and 7th taken blanks) and again the columns (8th, 9th) contained bacterial species “X. Oryzae and Salmonella typhi), 10th column blanked and again (11th, and 12th) were taken as Positive and Negative control. After filling all the wells, the initial reading was taken via spectrometer. Then the plate was exposed to continuous light for 15 min, and then placed in the incubator for 24 h, and after the 24 h the result was checked by spectrometer on 600 nm microplate reading, which showed that the growth of all the bacteria species was completely inhibited at higher concentrations of NP’s.[30-32]
Hemolytic assay/biocompatibility assay
To evaluate Biocompatibility the Hemolytic assay was performed as previously reported by[33] with minor modification. Briefly, 1cc of fresh blood was taken from a healthy donor and mixed with 20 mL PBS solution. The hemolytic assay for the Iron Oxide NPs was performed in two conditions, in Light and dark. For this using different concentrations, and each solution was diluted up to 1 mL. For the positive control, 500 μL of Triton X-100 and also 500 μL of blood were taken. Negative control only blood (1000 μL) was used. After that, each set of samples (light and Dark) were placed in the light box and dark room for a period of 15 min respectively. Then, all the samples were placed in the incubator for half an hour at 37°C temperature. All the samples were centrifuged for 15 min at 1500 rpm. Later centrifugation, the supernatant was collected without disturbing the pellet and the OD for each sample was checked via spectrometer at 576 nm.
The results were collected using the formula which is explained by Nadhman et al.[34]
Hemolysis percentage = OD at 576nm of NP’s/OD at 575nm in 0.1% Triton X–100*1.
Photo catalytic activity
The photo-catalytic activity of Iron Oxide NPs was performed by using Methyl Orange (MO) dye under continuous light irradiation and dark condition. A stock solution was made by adding 10 mg MO dye to 1000 ml dH2O. Later, 10 mg Iron Oxide NP’s was added to 50 mL of mentioned stock of MO dye above. Falcon tubes were covered with aluminum foil initially and initial value was taken. Later the falcon tubes were placed under visible light and dark. Data was collected periodically (0 min, 10 min, 20 min, 30 min, 40 min, 60 min, and 90 min) and the values were noted respectively.
Results
UV-spectrophotometery
UV Vis spectrophotometry is an important tool for the initial confirmation of nanoparticle biosynthesis. Our results revealed peaks between 230 and 290 nm, which confirms the presence of Iron Oxide nanoparticles. Our results are in accordance with[35,36] who revealed the presence of iron nanoparticles at a similar range as shown in Table 1.

FTIR
Several phytochemicals are involved in the capping and stabilization of biosynthesized nanoparticles. FTIR is a powerful tool to analyze the functional groups which are involved in capping nanoparticles. The current study revealed FTIR peaks at 1087.29 cm-1, 520.34 cm-1, and 435.70 cm-1. The peaks in FTIR which are shown in Figure 2 confirmed the capping of Iron Oxide NPs by the phytochemicals present in the plant extract. The peaks at 520.34 cm-1 and 435.70 cm-1 denoted the presence of Fe-O bond which is in agreement with the data reported by,[37] Papaver somniferum L. mediated novel bioinspired lead oxide (PbO) and iron oxide (Fe2O3) nanoparticles.[38] Apart from this, a peak at 1087.29 cm-1was also obtained which can be attributed to the presence of phytochemicals possessing C-O bonds.
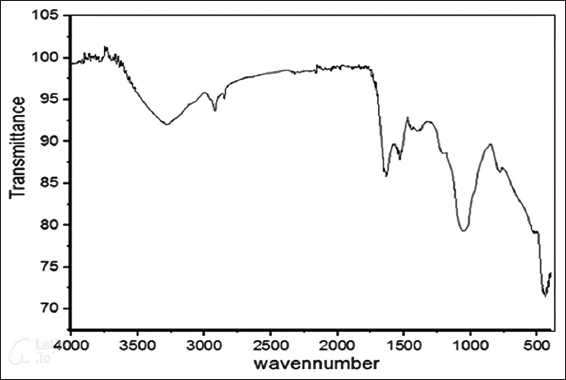
- FTIR graph of iron oxide NP’s
Photothermal activity
Photothermal activity is a phenomenon related to magnetic radiation, it is produced when materials are exposed to continuous light, as a result of photo-excitation. In this study photo thermal activity of Iron Oxide nanoparticles was performed via pH meter probe, in light and dark conditions. The result showed that NPs exhibited better activity in the light increase was noted and an almost 4 times increase was noted as compared to control as shown in Figure 3a and b. Similarly, when checked the same procedure in the dark it showed no significant increase and at least 2 fold increase was noted only as compared to the control. Iron Oxide has shown excellent photothermal activities due to: Good biocompatible nature, and better optical and physical properties.[39-42] From the literature study it is cleared that Iron Oxide nanoparticles have good photothermal activity in light, which could be due to their better super magnetic activity, NIR absorption and itself a photothermal agent.[39]
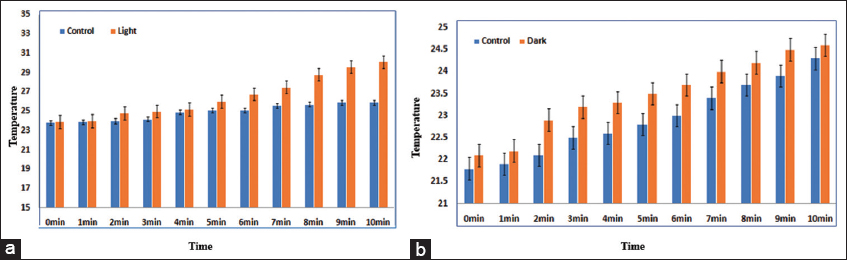
- (a and b) Photothermal activity of Iron Oxide NPs under visible light and dark conditions, respectively
MIC
MIC is the least concentration of a chemical usually drug, that requires stopping or eliminating the bacterial growth. As in the literature, various types of methods used for MIC activity like “Broth dilution assay, Micro plate-based method (96 well plates), etc have been used among the MIC has been most successful”.[43,44] Iron Oxide NPs have great potential against various pathogenic bacteria including both gram Negative as well as Gram-positive bacteria.[40] Furthermore, recent studies of Iron Oxide NPs showed significant result against one of the pathogenic bacteria known as “Pseudomonas aeruginosa.”[45] Moreover, studies have also shown Iron Oxide NP’s can be used to eradicate of the Biofilms.[46]
In the present study, MIC for 3 different types of bacterial strains such as “Salmonella typhi” “X. Oryzae” are (Gram Negative bacteria) and one bacteria strain was also taken as Gram-positive i.e., “Lactobacillus”. Lactobacillus are not pathogenic bacteria, these normally live in our digestive, urinary, and genital systems without causing diseases, using different doses of Iron Oxide nanoparticles our MIC result indicated that the Iron Oxide nanoparticles have excellent potential against the inhabitation of these pathogenic bacteria. As from the highest concentration i.e., 950 μL, to the concentration 150 μL showed the complete killing of bacteria in wells and below these concentrations up to some instant bacterial growth recorded in wells of the plate. Iron Oxide nanoparticles mostly kill bacterial cells by various mechanisms, such as ROS production, cellular disruption, and hampering gene experiments.[14] Iron Oxide NPs have been reported to have excellent Antimicrobial potential which could be the same here.[40,47]
Hemolysis
Hemolysis is the breakdown of the red blood cells under diverse stimuli. To evaluate the toxicity of Iron Oxide nanoparticles synthesized by the green synthesis method, hemolytic assay was performed on freshly isolated blood taken from a healthy donor. In this study, the hemolytic assay was performed at different concentrations (500 μL, 250 μL, 125 μL, 62 μL, and 31 μL). Our result showed that Iron Oxide nanoparticles synthesized by the green synthesis method are biocompatible and completely non-hemolytic at high concentrations in both light and dark conditions, as shown in Table 2. Materials with >5% hemolysis are hemolytic, 2–5% slightly hemolytic while <2% non-hemolytic and biocompatible for RBCs.[48] The lower toxicity of iron oxide NPs synthesized here could be due to their functional groups coated on their surfaces, it reduces the production of ROS and minimal usage of toxic solvents make them an excellent vehicle for drug delivery.[40,47,49]
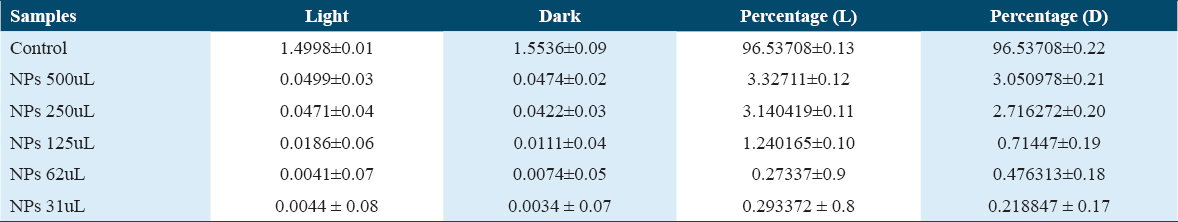
Photo catalytic activity
In this study, the photo catalytic degradation of MO dye was evaluated in the presence of continuous light using Iron Oxide nanoparticles. The result showed a continuous decline in MO dye under continuous light as time passed as shown in [Figure 4a]. Moreover, the color of the dye was also changed from orange to transparent in continuous visible light as shown in [Figure 5a]. However, in dark conditions, no significant degradation and color change was noted as shown in [Figures 4b and 5b], respectively. The previous study also cleared that Iron Oxide NPs used as a good photocatalyst for the degradation of different hazardous dyes in less time compared to other NP.[50] Most interestingly, in recent studies have shown that Iron Oxide NPs synthesized by the green method were able to decolorize the modern and most hazardous dye (MO), with more than 70% efficiency in less time,[51] which is similar to our study results.
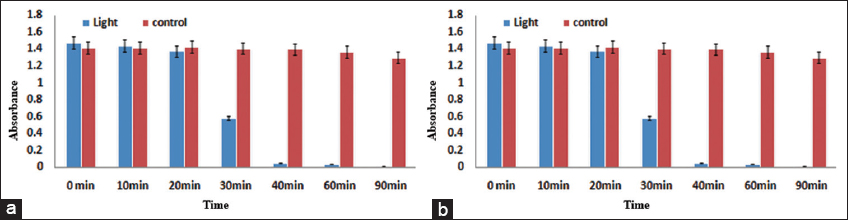
- (a and b) Photocatalytic activity of Iron Oxide NPs in continuous visible light and dark, respectively
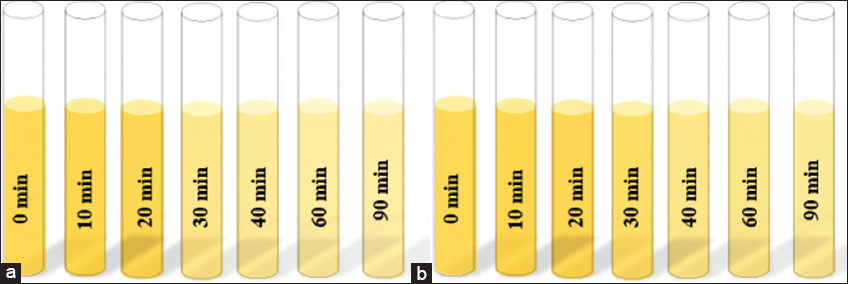
- (a) and (b) showing color changes of MO with Iron Oxide NP’s in the presence of “Light and dark” respectively
Discussion
In the last decades, nanotechnology has become a cutting-edge tool for scientists. Nanoparticles are produced using a variety of synthesis techniques, including chemical precipitation, laser pyrolysis, sol-gel, chemical deposition, electrochemistry, and greener method are used for the synthesis of NPs.[52] Current research and development in materials science and technology is giving a lot of attention to a new era of “green synthesis” approaches and technologies mostly, green synthesis of nanomaterials.[53] Plant-based nanoparticle synthesis has been called a “green” method of producing nanoparticles. Since it uses biologically acceptable solvents and little to no toxic chemicals. It is more efficient and practical. Therefore, various attempts were undertaken to synthesize metallic nanoparticles using plant extracts, such as silver, gold, palladium, zinc, copper, iron, cobalt, nickel, etc.[54] In this work we synthesized plant-mediated Iron Oxide NPs, from the plant of ficus palmate. UV Vis spectroscopy results revealed that the Iron Oxide NPs have peaked between 230 and 290 nm, which is the confirmation of synthesized Iron Oxide nanoparticles. Several phytochemicals are involved in the capping and stabilization of biosynthesized nanoparticles. The peaks of FTIR confirmed the capping of Iron Oxide NPs by the phytochemicals present in the plant extract. The peaks at 520.34 cm-1 and 435.70 cm-1 denoted the presence of a Fe-O bond which is in agreement with the data reported by,[37] P. somniferum L. mediated novel PbO and iron oxide (Fe2O3) nanoparticles.[38] Apart from this, a peak at 1087.29 cm-1 was also obtained which can be attributed to the presence of phytochemicals possessing C-O bonds.
There are two categories in which metallic nanoparticle applications fall. Applications of nanoparticles studied for their anti-bacterial, anti-fungal, anti-viral, and anti-cancer properties are included in biological applications. Non-biological uses include the reduction of 4-nitrophenol and photocatalysis of pollutant dyes like MB and photothermal activity.[55] Herein the photothermal activity of Iron Oxide NPs shows that NPs exhibited better activity in the light increase was noted and almost 4 times increase as compared to control. Similarly, in the dark, it showed no significant increase and at least 2 fold increase was noted only as compared to control. Iron Oxide has shown excellent photothermal activities due to: Good biocompatible nature, and better optical and physical properties. From the literature study, it is clear that Iron Oxide nanoparticles have good photothermal activity in light, which could be due to their better super magnetic activity.[39]
Biocompatibility test showed that Iron Oxide nanoparticles synthesized by green synthesis method are biocompatible and completely non-hemolytic at high concentrations in both light and dark conditions Materials with >5% hemolysis are hemolytic, 2–5% slightly hemolytic while <2% non-hemolytic and biocompatible for RBCs. The lower toxicity of iron oxide NPs synthesized here could be due to their functional groups coated on their surfaces, it reduces the production of ROS and minimal usage of toxic solvents make them excellent vehicle for drug delivery.[40,47,49] Muhammad et al. results also revealed that the synthesized Iron Oxide NPs cytotoxic activity was examined, and it was discovered that they had good biocompatibility and exhibited lower toxicity in a brine shrimp fatality assay. These nanoparticles can therefore be used in a variety of medical applications and are harmless for the environment.[56]
As in the literature, various types of methods used for MIC activity like ‘Broth dilution assay, Micro plate-based method (96 well plates) etc. have been used among the MIC have been most successful.[43,44] Iron Oxide NP’s have great potential against various pathogenic bacteria including both Gram-negative as well as Gram-positive bacteria.[40] In the present study MIC for 3 different types of bacterial strains such as “Salmonella typhi” “Xanthomonas oryzae” are (Gram Negative bacteria) and one bacteria strain was also taken as gram Positive i.e., “Lactobacillus.” Lactobacillus are not pathogenic bacteria, MIC results indicated that the Iron Oxide nanoparticles have excellent potential against the inhabitation of these pathogenic bacteria. Iron Oxide nanoparticles mostly kill bacterial cells by various mechanisms, such as ROS production, cellular disruption, and hampering gene experiments.[14] Iron Oxide NPs have been reported to have excellent Antimicrobial potential which could same here.[40,47] Furthermore, recent studies of Iron Oxide NPs showed significant results against one of the pathogenic bacteria known as “P. aeruginosa.”[45] Moreover, studies have also shown Iron Oxide NPs can be used to eradicate of the Biofilms.[46]
In this study, we also evaluated the photo-catalytic degradation of MO dye in the presence of continuous light using Iron Oxide nanoparticles. A continuous decline in MO dye under continuous light as time passed. Moreover, the color of the dye was also changed from orange to transparent in continuous visible light. However, in dark conditions no significant degradation and color change was noted. From the previous study also cleared that Iron Oxide NP’s used as a good photo catalyst for the degradation of different hazardous dye in less time compared to other NP.[50] Most interestingly in recent studies have shown that Iron Oxide NPs synthesized by green method were able to decolorize the modern and most hazardous dye (MO), with more than 70% efficiency in less time,[51] which is similar to our study results. So With such potential applications, Iron Oxide NPs holds a promising future and could be used in treating diseases, and microbial pathogenesis and also could be used as a vector in drug delivery. Moreover, they can also eradicate persistent dyes and could be used as an alternative to remediate pollutants from the environment.
Conclusion, future perspectives
Nanotechnology is a modern field that has got a remarkable interest in the previous few decades. The biological synthesis (Green synthesis) of NPs is cost-effective, dynamic, and eco-friendly approach. In our study, we synthesized green Iron Oxide NPs, characterized them using a variety of spectroscopic techniques, and assessed their biological potential, photothermal potential, and photocatalytic potential under various circumstances, including (Light and Dark). Results indicated that continuous visible light increased photothermal and photocatalytic activities. The biocompatibility assay also resulted that both in light and dark the synthesized NPs were safe and can be used for various biological activities. Moreover, the MIC of the Green synthesized Iron Oxide NPs showed outstanding results against various bacterial strains. The synthesized NPs have shown better Photo-thermal, photo-catalytic activity, and Biocompatible and also showed great potential to kill microbes, so they could be used in treating various diseases in the future. Though we studied the NPs for various biological and degradation purposes, however, it should be elucidated properly with proper mechanisms.
Authors’ Declaration Statements
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
All relevant data are within the paper.
Competing interests
All authors of this work have declared that there is no conflict of interest.
Funding statement
This work don’t receive any funding.
Authors’ contributions
Dr.Sulaiman Faisal, Dr. Farida Anjum, Dr. Dawood Ahmad and Dr. Muhammad Nadeem supervised the project. Abrar Hussain, Muhammad Ijaz, Gulzar Ahmad, Muhammad Yasar, Abdul Aziz, Hamza Iqbal, Muhammad Gharib Nawaz and Faraz Ahmad Khan perform and analyze the experiments, Wajeeha Shakeel, Hashmat Momand, Rukhsar Ali, Sidra Ahmad, Hafsa Shah review and validate the analysis, statistics and grammar checking at the final stages all the authors have written the manuscript, reviewed the manuscript and agreed to submit it.
Acknowledgment
I would like to thank the Institute of Integrative Biosciences Cecos University Peshawar which provided us its all facility and research materials. I also want to express my gratitude to all of my colleagues, whose support allowed us to accomplish this study.
References
- Gold nanoparticles:Synthesis and applications in drug delivery. Trop J Pharm Res. 2014;13:1169-77.
- [Google Scholar]
- Biosynthesis of nanoparticles:Technological concepts and future applications. J Nanopart Res. 2008;10:507-17.
- [Google Scholar]
- Applications of nanoparticles in biology and medicine. J Nanobiotechnology. 2004;2:3.
- [Google Scholar]
- Application of iron oxide nanoparticles in the diagnosis and treatment of neurodegenerative diseases with emphasis on Alzheimer's disease. Front Cell Neurosci. 2020;14:21.
- [Google Scholar]
- Expression of Bcl-2 and clinicopathological variables in salivary glands mucoepidermoid carcinoma. Int J Health Sci (Qassim). 2022;16:26-31.
- [Google Scholar]
- Synthesis of iron oxide nanoparticles coated sand by biological method and chemical method. Procedia Technol. 2016;24:210-6.
- [Google Scholar]
- Green synthesis of Fe and Fe/Pd bimetallic nanoparticles in membranes for reductive degradation of chlorinated organics. J Memb Sci. 2011;379:131-7.
- [Google Scholar]
- Nanoparticle synthesis, applications, and toxicity. In: In:Applications of Nanobiotechnology. London, UK: IntechOpen; 2019. p. :1-16.
- [Google Scholar]
- Association of smoking, p53 and Ki-67 immunomarkers with bladder neoplasms in tribal region of India. Int J Health Sci (Qassim). 2022;16:11-7.
- [Google Scholar]
- Green synthesis of ZnO nanoparticles by Aspalathus linearis:Structural and optical properties. J Alloys Compd. 2015;646:425-30.
- [Google Scholar]
- Noble metal nanoparticles:Plant-mediated synthesis, mechanistic aspects of synthesis, and applications. Ind Eng Chem Res. 2016;55:9557-77.
- [Google Scholar]
- The current trends in the green syntheses of titanium oxide nanoparticles and their applications. Green Chem Lett Rev. 2018;11:492-502.
- [Google Scholar]
- Green nanobiotechnology:Factors affecting synthesis and characterization techniques. J Nanomater. 2014;2014:219.
- [Google Scholar]
- Green synthesis of CuO nanoparticles with leaf extract of Calotropis gigantea and its dye-sensitized solar cells applications. J Alloys Compd. 2015;632:321-5.
- [Google Scholar]
- A cross-tabulated analysis for the influence of climate conditions on the incidence of dengue fever in Jeddah City, Saudi Arabia during 2006-2009. Int J Health Sci (Qassim). 2022;16:3-10.
- [Google Scholar]
- Cardiotoxicity in cancer patients treated with chemotherapy:A systematic review. Int J Health Sci (Qassim). 2022;16:39-46.
- [Google Scholar]
- Factors affecting the choice of dermatology as a specialty by medical students:Data from 28 KSA medical schools. Int J Health Sci (Qassim). 2022;16:18-25.
- [Google Scholar]
- The role of nanoparticles in the diagnosis and treatment of diseases. Sci Inq Rev. 2020;4:14-26.
- [Google Scholar]
- Some interesting properties of metals confined in time and nanometer space of different shapes. Acc Chem Res. 2001;34:257-64.
- [Google Scholar]
- Imaging and nanomedicine for diagnosis and therapy in the central nervous system:Report of the eleventh annual Blood-brain Barrier Disruption Consortium meeting. AJNR Am J Neuroradiol. 2006;27:715-21.
- [Google Scholar]
- Applications of nanotechnology to atherosclerosis, thrombosis, and vascular biology. Arterioscler Thromb Vasc Biol. 2006;26:435-41.
- [Google Scholar]
- Peroxynitrite induced cytotoxicity and detection in cardiovascular, neurodegenerative and inflammatory disorders. Int J Health Sci (Qassim). 2022;16:1-2.
- [Google Scholar]
- Duality of iron oxide nanoparticles in cancer therapy:Amplification of heating efficiency by magnetic hyperthermia and photothermal bimodal treatment. ACS Nano. 2016;10:2436-46.
- [Google Scholar]
- Green synthesis of iron oxide nanoparticles and their catalytic and in vitro anticancer activities. J Clust Sci. 2017;28:245-57.
- [Google Scholar]
- Picrasma quassioides mediated cerium oxide nanostructures and their post-annealing treatment on the microstructural, morphological and enhanced catalytic performance. Ceram Int. 2016;42:6610-8.
- [Google Scholar]
- Visible-light-responsive ZnCuO nanoparticles:Benign photodynamic killers of infectious protozoans. Int J Nanomedicine. 2015;10:6891-903.
- [Google Scholar]
- Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc. 2008;3:163-75.
- [Google Scholar]
- Prevalence and antibiotic resistance of Salmonella species isolated from chicken liver in Peshawar. J Clin Med Res. 2021;3:1-11.
- [Google Scholar]
- Isolation and identification of antibiotics susceptible Staph aureus from unprocessed milk. J Clin Med Res. 2021;3:1-11.
- [Google Scholar]
- A full-length protocol to test hemolytic activity of palytoxin on human erythrocytes. Invertebr Surviv J. 2007;4:92-4.
- [Google Scholar]
- PEGylated silver doped zinc oxide nanoparticles as novel photosensitizers for photodynamic therapy against Leishmania. Free Radic Biol Med. 2014;77:230-8.
- [Google Scholar]
- Appropriate size of magnetic nanoparticles for various bioapplications in cancer diagnostics and therapy. ACS Appl Mater Interfaces. 2016;8:3092-106.
- [Google Scholar]
- External magnetic field-enhanced chemo-photothermal combination tumor therapy via iron oxide nanoparticles. ACS Appl Mater Interfaces. 2017;9:16581-93.
- [Google Scholar]
- The impact of the hybrid platform of internet of things and cloud computing on healthcare systems:Opportunities, challenges, and open problems. J Ambient Intell Humaniz Comput. 2019;10:4151-66.
- [Google Scholar]
- Papaver somniferum L mediated novel bioinspired lead oxide (PbO) and iron oxide (Fe2O3) nanoparticles:In-vitro biological applications, biocompatibility and their potential towards HepG2 cell line. Mater Sci Eng C Mater Biol Appl. 2019;103:109740.
- [Google Scholar]
- Biosynthesis of pure hematite phase magnetic iron oxide nanoparticles using floral extracts of Callistemon viminalis (bottlebrush):Their physical properties and novel biological applications. Artif Cells Nanomed Biotechnol. 2018;46(1):693-707.
- [Google Scholar]
- Biomedical nanomagnetics:A spin through possibilities in imaging, diagnostics, and therapy. IEEE Trans Magn. 2010;46:2523-58.
- [Google Scholar]
- Magnetite nanoparticles for cancer diagnosis, treatment, and treatment monitoring:Recent advances. Mater Today (Kidlington). 2016;19:157-68.
- [Google Scholar]
- Extraction optimization of medicinally important metabolites from Datura innoxia Mill.:An in vitro biological and phytochemical investigation. BMC Complement Altern Med. 2015;15:376.
- [Google Scholar]
- An innovative microplate assay to facilitate the detection of antimicrobial activity in plant extracts. J Rapid Methods Autom Microbiol. 2009;17:519-34.
- [Google Scholar]
- Antibacterial activity of biochemically capped iron oxide nanoparticles:A view towards green chemistry. J Photochem Photobiol B. 2017;170:241-6.
- [Google Scholar]
- Superparamagnetic iron oxide-encapsulating polymersome nanocarriers for biofilm eradication. Biomaterials. 2017;119:78-85.
- [Google Scholar]
- Biosynthesis of iron oxide (Fe2O3) nanoparticles via aqueous extracts of Sageretia thea (Osbeck.) and their pharmacognostic properties. Green Chem Lett Rev. 2017;10:186-201.
- [Google Scholar]
- Biological interactions in vitro of zinc oxide nanoparticles of different characteristics. Mater Res Express. 2014;1:035041.
- [Google Scholar]
- Biological synthesis of metallic nanoparticles:Plants, animals and microbial aspects. Nanotechnol Environ Eng. 2017;2:18.
- [Google Scholar]
- Green synthesis of a-Fe2O3 nanoparticles for photocatalytic application. J Mater Sci Mater Electron. 2014;25:3572-7.
- [Google Scholar]
- One-step green synthesis and characterization of iron oxide nanoparticles using aqueous leaf extract of Teucrium polium and their catalytic application in dye degradation. Adv Nat Sci Nanosci Nanotechnol. 2019;10:015007.
- [Google Scholar]
- Toxicity and safety assessment of green nanomaterials. In: In:Green Nanomaterials for Industrial Applications. Amsterdam: Elsevier; 2022. p. :509-22.
- [Google Scholar]
- Green synthesis of nanomaterials. In: In:Synthesis of inorganic nanomaterials. Amsterdam: Elsevier; 2018. p. :169-84.
- [Google Scholar]
- Ficus carica assisted green synthesis of metal nanoparticles:A mini review. Biotechnol Rep (Amst). 2020;28:e00569.
- [Google Scholar]
- Green synthesis of metal nanoparticles and their applications in different fields:A review. Z Phys Chem. 2019;233:1325-49.
- [Google Scholar]
- Comparison of characteristics and biocompatibility of green synthesized iron oxide nanoparticles with chemical synthesized nanoparticles. Environ Res. 2021;201:111585.
- [Google Scholar]







