Translate this page into:
The anti-diarrheal and anti-inflammatory effects of hydroethanol extracts of ripe Annona muricata fruit pulp in Wistar rats using curative method
Address for correspondence: B. C. Okeke-Nwolisa, Department of Medical Laboratory Science, Faculty of Health Sciences and Technology, Nnamdi Azikiwe University, Nnewi Campus, Nnewi, Nigeria. Phone: +234 8064312596. E-mail: bc.okeke-nwolisa@unizik.edu.ng
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Qassim Uninversity and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective:
The study aimed to determine the anti-diarrheal and anti-inflammatory activities of hydroethanolic extract of ripe Annona muricata fruit pulp (HEAMP) on Wistar rats.
Methods:
Fifty male Wistar rats were divided into five groups, each for the diarrhea and inflammatory phases. Groups A and B 10 mL/kg of distilled water and 2 mL of castor oil, Groups C, D, and E received 2 mL of castor oil + extract (150, 300, and 600 mg/kg). Groups A and B received 10 mL/kg of distilled water and 0.1 mL of egg white. Groups C, D, and E received the (0.1 mL) egg white and extract (150, 300, and 600 mg/kg). Data were analyzed using SPSS (25) using ANOVA followed by post hoc least significant difference, presented as mean ± standard error of the mean, and values considered significant at P < 0.05.
Results:
The acute toxicity test of HEAMP is above 5000 mg/kg and rich in flavonoids, saponins, and proteins, with carbohydrates and tannins, and glycosides at moderate levels. Steroids, alkaloids, amino acids, and triterpenoids were absent. Furthermore, 30 min after diarrhea induction, the treated groups significantly increased (P = 0.012) in fecal count compared to Group A. Compared to Group B, the treated groups had a significant decrease in the fecal count at 60, 90, 120, and 150 min. At 30-min, there was a significant increase (P = 0.000) in paw-size in the treated groups compared to Group A. The treated groups had significantly lower edematous paw-size levels from 60 to 150 min compared to Group B.
Conclusion:
The HEAMP had anti-diarrheal and anti-inflammatory properties and is safe for consumption.
Keywords
Annona muricata pulp
castor oil
diarrhea
hydroethanol
inflammation
phytochemicals
Introduction
Annona muricata is a native green plant to the warmest tropical areas in North-South America and has recently been extensively cultivated in subtropical parts of the globe.[1,2] A. muricata belongs to the Annonaceae family,[3] locally known as soursop.[4] Phytochemistry of the hydroethanolic extract of A. muricata pulp reveals its potential as a reservoir of some essential nutrients such as acetogenins, alkaloids, tannins, triterpenes, flavonoids, and steroids. Medicinal plants garnered much attention in treating and preventing various disease conditions.[5] A. muricata treatment has been scientifically known to diverse diseases,[6] including diarrhea, inflammation, fever, diabetes, pain, liver disorders, and many infections.[7-9]
Diarrhea is a common gastrointestinal disorder caused by bacterial infections.[10] It is characterized by abdominal pains, watery stools, and increased bowel movement frequency.[11] Drugs such as antibiotics are commonly employed as antidiarrheal treatments, though side effects, compliance, and ineffectiveness due to bacterial resistance limit their efficacy.[12] Many medicinal plants like A. muricata were documented to possess positive therapeutic antidiarrheal effects. The fruit and bark of A. muricata were used to treat antidiarrheal complications due to its phytochemical constituents by inhibiting intestinal motility and secretions that cause diarrhea.[12]
A. muricata is an edible tropical fruit which is common, readily available and cheap in the producing country especially Nigeria. The moisture content of fruit is >70%. The approximate nutritional composition of soursop includes carbohydrates, glucose; proteins; lipids; ash; fiber, citrate. The epicarp, mesocarp, and juice of A. muricata fruits contain potassium, sodium, iron, magnesium, calcium, chloride, and bicarbonate. Carbonate is present in the fruit juices. The juices also contained phosphorus, zinc and copper.[13,14]
The previous studies that recommended the use of soursop fruit juice[13,14] and use of various other home remedies such as soup, rice water, yoghurt drink, unsweetened fresh juice, and resistant starch for the treatment of diarrhea[15-17] abound in literature and offers hope for the management of acute watery diarrhea.
With the previous points in mind identifying the specific part of the plant, concentration, duration of action and preparation that is more effective will be of significance to understanding more formulation that is efficacious.
Inflammation is the body’s ability to respond to harmful stimuli such as tissue damage or pathogenic infection. The role of phenolics and flavonoids in the treatment of inflammation is as an anti-oxidant, anti-inflammatory, while alkaloids and flavonoids exert iron-chelating effect.[18] Inflammatory responses generally occur in two phases; acute and chronic inflammation.[19] Phytochemistry of A. muricata pulp has shown it to possess flavonoids, alkaloids, and phenolic compounds such as tannins, saponins, steroids, and triterpenoids. Plants with phenolic compounds are known to be of anti-inflammatory significance; as such, A. muricata have been employed to treat various inflammatory processes.[20] Therapeutic potentials report of A. muricata above prompt the present study exploring the potentials of the hydroethanolic extract of A. muricata on diarrhea and inflammation.
Materials and Methods
Procurement, preparation, and identification of A. muricata fruit
Ripe A. muricata fruits were obtained from Nkwo market, Nnewi, Anambra State, Nigeria and were identified by a Taxonomist – Mr. Iroka, Finian Chisom of the Department of Botany, Nnamdi Azikiwe University, Awka, Anambra State. The herbarium number (NAUH4A) was deposited in the herbarium catalogue. The fruits were washed under a running tap to remove debris and air dried. The fruits were cut open, and the seeds and back were dicscarded. The ripe fruit pulp was mashed with a mortar and pestle. 300 g of the mashed sample was milled using an electronic blender (QLINK Multifunction food processor, QMFP-128, Turinar Corp., Shang-Hai, China) into a coarse form and macerated in 60% distilled and 40% absolute ethanol (JHG Chemicals Guangdong, China) for 48 h. Then, filtration was done using a clean porcelain cloth and further filtered using Whatman Qualitative filter paper No. 1 (Sigma Aldrich, WHA1001040, USA). The filtrate was concentrated using a rotatory evaporator (Digital TT-52, Techmel and Techmel, USA) and dried further using a laboratory oven (DGH-9023A, PEM MEDICAL USA) at 45°C into a gel-like form. The 40% ethanolic (hydroethanolic) extract of ripe A. muricata fruit was preserved in an airtight container and kept in a refrigerator at 8oC for further usage. The extraction method was done with modifications as described according to the method employed by Al-Attar and Abu Zeid (2013).[21]
Location of the study
This study was carried out in the laboratory of Department of Physiology, Nnamdi Azikiwe University, Nnewi Campus, Anambra State, Nigeria.
Animal handling
Ethical approval was obtained from Nnamdi Azikiwe University- Animal Research Ethics Committee (NAU AREC), Awka (Reference number: NAU/AREC/2022/00002). Rats handling and treatments conform to the National Institute of Health guidelines for laboratory animal care and use.[22]
Materials
Fifty-(50) inbreed male Wistar rats, Absolute Ethanol (JHD Chemicals, Guangdong China), Castor oil, Egg-white, distilled water, mortar and pestle, porcelain cloth, Whatman qualitative filter paper no. 1 (Sigma Aldrich; WHA1001042, USA), oral cannula, and Vanier caliper. Automatic Water distiller (SZ-1 Search Tech Instrument), electronic blender (QLINK Multifunction food processor, QMFP-128, Turinar Corp., Shang-Hai, China), Rotary evaporator (Digital) TT-52 (Techmel and Techmel, USA), Thermostat Oven (DHG-9023A, PEC MEDICAL USA), chloroform (Guangdong, China), non-heparinized capillary tube, Electronic weighing balance (M-Metallar M311, Guangdong, China), 2ml hypodermic sterile syringe, animal weighing balance (Camry LB11), Normal laboratory chow (Standard Pellet, Vital Feed, Jos, Nigeria), and standard cage.
Experimental animals
Fifty-(50) inbreed male Wistar rats weighing 120–140 gram were used for the study, and the experimental animals were housed in the Animal house, Department of Physiology, Nnamdi Azikiwe University, Awka. The animals were maintained in standard cages at ambient temperature with standard pellet and distilled water ad libitum, and animals were allowed to acclimatize for period of 2 weeks before commencement of study. Animals were maintained under 12 h light and dark cycles.
Acute toxicity of hydroethanol extract of ripe A. muricata fruit pulp
The median lethal dose (LD50) of the extract was determined using a method of Dietrich Lorke.[23] Thirteen animals were used in the study. The animals received the extract through oral route and were carried out in two phases.
Phase I
Nine rats were employed for this phase I, and three rats were allocated to each group. Group 1 received 10 mg/kg/bw/rat, Group 2 received 100 mg/kg/bw/rat, and Group 3 received 1000 mg/kg/bw/rat. The animals were monitored for 24 h for morbidity and mortality. The rats remained normal after 24 h of observation in Phase 1. Then, the study proceeded to the second phase, whereby four rats were employed for the study comprising one rat per group (N: B b.w. = body weight).
Phase II
Three rats were employed for this phase II, and one rat was allocated to each group. Group 1 received 1600 mg/kg/bw/rat, Group 2 received 2900 mg/kg/bw/rat, and Group 3 received 5000 mg/kg/bw/rat. The animals were monitored for another 24 h for morbidity and mortality.
Phytochemical screening
Phytochemical screening for alkaloids, flavonoids, carbohydrates, saponin, proteins, tannins, amino acids, steroids, triterpenoids, and glycosides was carried out in accordance with method adopted by (Dhawan, 2017; Khamis and Aly, 2017).[24,25]
Experimental design and induction of diarrhoea
Wistar rats were fasted for 18 h and divided into five-(5) groups consisting five-(5) animals each. Rats in Groups I and II received distilled water (10 mL/kg/bw) and 2 mL castor oil respectively, Groups III, IV, and V received the extract (150, 300, and 600 mg/kg, respectively), After 30 min, diarrhea was induced by oral administration of castor oil (2 mL) to each rat.[26] Wistar rats were observed at 30-min interval for a period of 2.5 h (150 min) during which the total number of fecal outputs and the number of diarrheic feces excreted were recorded.
Animal groupings for diarrhea
-
Group A (positive control): Distilled water (10 mL/kg/bw)
-
Group B (negative control): (2 mL) Castor oil
-
Group C (low dose): (2 mL) Castor oil + extract (150 mg/kg/bw)
-
Group D (moderate dose): (2 mL) Castor oil + extract (300 mg/kg/bw)
-
Group E (high dose): (2 mL) Castor oil + extract (600 mg/kg/bw)
Statistical analysis
Data obtained for the total number of fecal outputs, the number of diarrheic feces excreted and the hind paw size were analyzed using one-way Analysis of Variance (ANOVA) using SPSS version 25.0 followed by appropriate post hoc test to compare the effects between control and test groups. Results were expressed as mean ± standard error of the mean. Values with P < 0.05 were considered statistically significant.
Results
Table 1 result revealed that the hydroethanolic extract of A. muricata pulp contains flavonoids, saponins, and proteins were high, with carbohydrates, tannins, and glycosides moderately present. However, steroids, alkaloids, amino acids, and triterpenoids are absent.
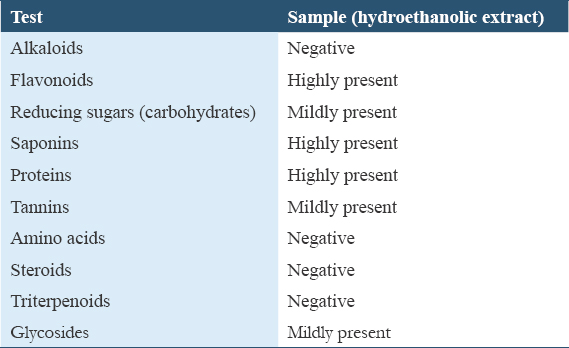
Table 2 result revealed that the acute toxicity (LD50) of hydroethanol extract of ripe A. muricata fruit pulp is above 5000 mg/kg, which is safe for human and animal consumption.
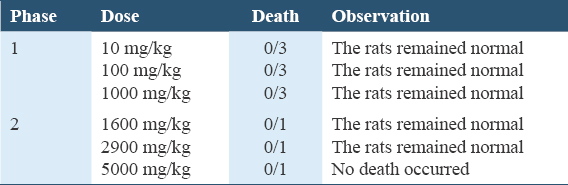
Table 3 results revealed a non-significant increase in the fecal count in-group B and C (P = 0.061; P = 0.145), while Groups D and E had a significant (P = 0.025; P = 0.002) increase compared to Group A at 30 min. However, at 60-min, the fecal count in-group B compared to A had significant (P = 0.000) increase, Groups C, D, and E had significant (P = 0.002; P = 0.004; P = 0.002) decline in fecal count compared to Group B. At 90 min, the fecal count in-group B compared to A had significant (P = 0.001) increase, Groups C, D, and E had significant (P = 0.007; P = 0.013; P = 0.001) decline in fecal count compared to Group B.
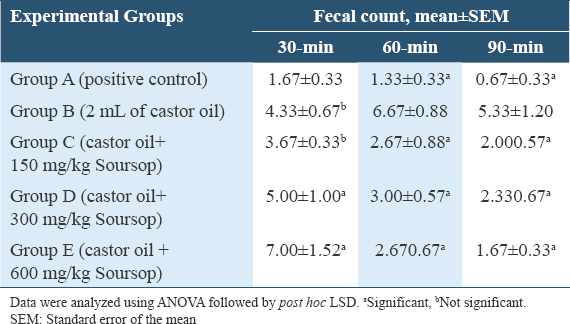
Table 4 results showed the fecal count in-group B compared to A had significant (P = 0.023) increase, Groups C and E had significant (P = 0.044; P = 0.023) decline in fecal count, while Group D had an insignificant decrease (P = 0.156) compared to Group B at 120-min. At 150 min, the fecal count in-group B compared to A had significant (P = 0.004) increase, Groups C, D, and E had significant (P = 0.007; P = 0.007; P = 0.002) decline in fecal count compared to Group B.
Table 5 results revealed a significant increase in the paw-size in Groups B, C, D, and E (P = 0.000; P = 0.000; P = 0.000; and P = 0.000) compared to group A at 30-min. At 60-min, the paw size showed a significant decline in-group D and E (P = 0.018; P = 0.035), while Group C had a non-significant (P = 0.068) decrease compared to group B with Group A having a significant (P = 0.001) increase. At 90-min, the paw size showed a significant decline in-Groups C, D, and E (P = 0.013; P = 0.014; P = 0.002), compared to Group B with Group A having a significant (P = 0.000) increase.
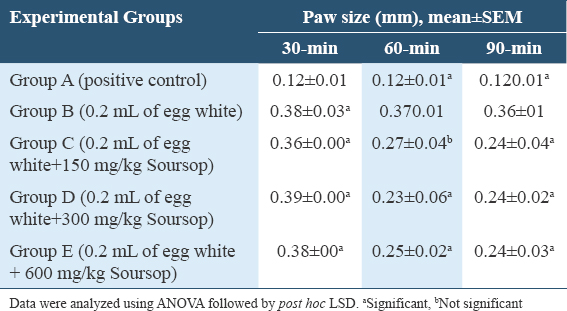
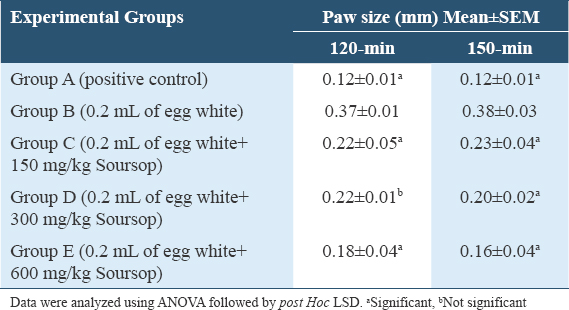
Table 6 results showed that the paw size had a significant decline in-groups C, D, and E (P = 0.010; P = 0.009; P = 0.002), compared to Group B with Group A having a significant (P = 0.000) increase at 120-min. At 150 min, the paw size showed a significant decline in-Groups C, D, and E (P = 0.012; P = 0.004; P = 0.001), compared to Group B with Group A having a significant (P = 0.000) increase.
Discussion
Diarrhea is a gastrointestinal disorder linked to the onset of watery stools that could last for days, and its etiology is linked to infection mostly. However, its etiologies are of different sources, with the most significant being an imbalance of the small and large intestines, such as malabsorption, inflammatory bowel disease, and medication side effects.[27-29] Further, its side effects result in excessive water and electrolytes in the feces. The use of medicinal plants in treating diseases has gained ground in managing diarrhea because of the several unique phytochemicals responsible for altering the process involved. Furthermore, secondary metabolites such as flavonoids, saponins, and polyphenols have significantly attenuated effects seen in anti-diarrhea activity in animal models.[12,30]
The current study investigated the anti-diarrhea activity of hydroethanol extract of ripe A. muricata fruit pulp juice on castor oil-induced diarrhea. The results obtained at the different time intervals revealed that castor oil showed a high fecal count (P < 0.05), which constitutes watery feces, semisolid, and solid feces when comparison was made between the negative and positive control. At 30-min intervals, castor seed oil significantly increased fecal count compared to the treated Groups B to E. However, the mechanism of action linked to these effects may be attributed to the presence of ricinoleic acid, known for its increased intestinal peristaltic movement, irritation and inflammation of the intestinal mucosa, resulting in diarrhea.[31,32] More so, the studies of Fokam Tagne et al.,[32] Cavalcanti et al.,[31] Teferi et al.,[33] and Gabriel et al.[2] are all in agreement with the study findings revealing an increase in the frequency of fecal count following castor-oil induced diarrhea.
Furthermore, at different time intervals (60 min through 150 min), the hydroethanol extract of ripe A. muricata fruit pulp juice showed a significant decline in the fecal count in Groups C, D, and E compared to Group B. The physiology or mechanism of action linked to the decline in the fecal count is attributed to flavonoids and polyphenol compounds present. Afroz et al.[1] reported a significant decrease in the evacuation index (frequency of fecal count) following A. muricata fruit extract in castor oil induced-intestinal disorder, similar to the study findings. Ouachinou et al.,[34] Ngoua-Meye-Misso et al.[35] showed similar results to the study findings revealing that A. muricata possessed an anti-diarrhea effect. Furthermore, Doe et al.[36] reported a significant decrease in fecal frequency and fecal count following A. muricata seed extract on castor oil-induced diarrhea, corroborating the study findings.
Edematous inflammation caused by the allergen is linked to an inflammatory reaction triggered by egg white.[19] Edematous inflammation is excessive fluid accumulation in tissues or within the collagen-mucopolysaccharide matrix with even distribution in interstitial spaces.[37] The inflammatory response is detected through the formation of granuloma, extravasations, and various biochemical exudates.[38] Acute inflammation involves the emigration of leukocytes from the vascular to the extravascular compartment and activation of platelets and their aggregation with the release of proteases and oxidants from phagocytes during acute inflammation.[39]
This study also investigated the anti-inflammatory effect of the hydroethanol extract of ripe A. muricata fruit pulp. The results showed that egg white caused an increase in the paw size of the animal following injection, when positive control was compared to negative control and the Groups C, D, and E. However, the precise mechanism linking edematous egg-white inflammation is not well understood but suggests allergen has been the main potential of the process. The findings of Barung et al.,[19] Sofidiya et al.,[40] and Zhao et al.[38] had a similar report to the study findings, revealing edematous inflammation of the paw-size following egg white injection, which has a similar report as this study. The study revealed a significantly lowered edematous size level at 60 min through 150 min following the administration of the 40% ethanolic extract of A. muricata fruit pulp juice. However, the mechanism of action linked to the decline in paw size is attributed to flavonoids and terpenoids in the extract. Oridupa et al.[41] reported a significant decline in the paw size following administration of methanolic extract of A. muricata leaf extract, which corresponds to the study findings. The report of Abd-Hamid et al.,[42] Chan et al.,[43] Ayun et al.,[20] Ishola et al.[44] had similar reports to the study findings.
The results from this study showed that the hydroethanol extract of ripe A. muricata fruit pulp is rich in flavonoids, saponins, and proteins. More so, these phytonutrients present could be responsible for the anti-diarrhea and anti-inflammatory effects because Hasrat et al.[45] and Mohammed et al.[46] has similar findings to the study result revealing alkaloids being present. Agu and Okolie,[47] Gupta et al.,[48] and Chowdhury et al.[49] have similar report to this study, which revealed the presence of flavonoids, saponins, and proteins. In addition, these phytonutrients contained by the hydroethanol extract of ripe A. muricata fruit pulp, provides an excellent scavenging activity of the free radicals. The study findings also revealed that the acute toxicity of the hydroethanolic extract of A. muricata fruit pulp is >5000 mg/kg, which is safe for both animal and human consumption. Likewise, Gabriel et al.[2] had similar report to the study revealing the acute toxicity test of ethanolic extract of A. muricata to be above 5000 mg/kg.
Conclusion
The study showed that hydroethanol extract of ripe A. muricata fruit pulp juice had anti-diarrhea and anti-inflammatory effects following castor oil-induced diarrhea and edematous egg-white inflammation, respectively. The anti-diarrheal and anti-inflammatory effects of hydroethanol extract of ripe A. muricata fruit pulp juice might be due to the high presence of flavonoids, saponins, and proteins found after the phytochemical analysis of this medicinal plant. It is safe for human and animal consumption because acute toxicity was >5000 mg/kg. In addition, it could be used as a new therapeutic model for treating diarrhea and inflammation in both human and animal models.
Authors’ Declaration Statements
Ethics approval
Ethical approval was obtained from the Nnamdi Azikiwe University-Animal Research Ethics Committee (NAU AREC) Awka (Reference number: NAU/AREC/2022/00002).
Patients consent statement
Not Applicable.
Consent for publication
Not Applicable.
Availability of data and material
Not applicable.
Competing interests
The authors had no conflict of interest among themselves.
Funding statement
Not applicable.
Authors’ Contributions
Ifeoma Bessie Enweani-Nwokelo conceived and supervised the research. Prince Chiazor Unekwe and Ifeoma Egbuonu contributed to the research design. Benedictta Chinweoke Okeke-Nwolisa contributed to experimental and laboratory studies, literature review, and statistical analyses of research data. All authors contributed to manuscript writing and approved the final article for publication.
Acknowledgements
The authors are grateful to Ezeokafor, E.N., Hudu, A.A., and Eyeghre, O.A. for their technical support during the laboratory procedures.
ORCID link of the corresponding author
Orcid ID*: https://orcid.org/0000-0003-1285-0961
References
- Methanol soluble fraction of fruits of Annona muricata possesses significant antidiarrheal activities. Heliyon. 2020;6:e03112.
- [Google Scholar]
- Anti-diarrhea efficacy of Annona muricata L. on animal model. Afr J Biol Med Res. 2020;3:25-32.
- [Google Scholar]
- Annona muricata:Is the natural therapy to most disease conditions including cancer growing in our backyard?A systematic review of its research history and future prospects. Asian Pac J Trop Med. 2017;10:835-48.
- [Google Scholar]
- Phytochemical fractions from Annona muricata seeds and fruit pulp inhibited the growth of breast cancer cells through cell cycle arrest at G0/G1 phase. J Cancer Res Ther. 2020;16:1235-49.
- [Google Scholar]
- Three new anti-proliferative Annonaceous acetogenins with mono-tetrahydrofuran ring from graviola fruit (Annona muricata) Bioorg Med Chem Lett. 2014;24:2773-6.
- [Google Scholar]
- Annona Species (Annonaceae) Oils. In: In:Essential Oils in Food Preservation, Flavor and Safety. Amsterdam: Elsevier Inc; 2016. p. :221-9.
- [Google Scholar]
- Annona muricata Linn. Leaf as a source of antioxidant compounds with in vitro antidiabetic and inhibitory potential against a-amylase, a-glucosidase, lipase, non-enzymatic glycation and lipid peroxidation. Biomed Pharmacother. 2018;100:83-92.
- [Google Scholar]
- Anticancer properties of graviola (Annona muricata):A comprehensive mechanistic review. Oxid Med Cell Longev. 2018;2018:1826170.
- [Google Scholar]
- Ameliorative effect of Annona muricata (Graviola) extract on hyperglycemia induced hepatic damage in Type 2 diabetic mice. Antioxidants. 2021;10:1546.
- [Google Scholar]
- Diarrhea. 2022. In:Statpearls. Treasure Island, FL: StatPearls Publishing; Available from: https://www.ncbi.nlm.nih.gov/books/NBK448082
- [Google Scholar]
- Bacterial gastroenteritis. 2021. In:StatPearls. Treasure Island, FL: StatPearls Publishing; Available from: https://www.ncbi.nlm.nih.gov/books/NBK513295
- [Google Scholar]
- Pharmacological activities of soursop (Annona muricata Lin.) Molecules. 2022;27:1201.
- [Google Scholar]
- Use of Soursop and sweetsop juices in the management of diarrhoea in children. J Diarrhoeal Dis Res. 1998;16:252-3.
- [Google Scholar]
- The biochemical analysis of Soursop (Annona muricata L.) and sweetsop (A. squamosa L.) and their potential use as oral rehydration therapy. J Food Agric Environ. 2004;2:39-43.
- [Google Scholar]
- Home management of diarrhoea among underfives in a rural community in Kenya:Household perceptions and practices. East Afr J Public Health. 2008;5:142-6.
- [Google Scholar]
- 2017. Diarrhoeal Disease:Key Facts. Geneva: World Health Organization; Available from: https//www.who.int/news-room/factsheets/detail/diarrhoeal-disease
- Diarrhea. 2021. In:Encyclopedia of Infant and Early Childhood Development. Cambridge: Academic Press; :1-3. 394-401 Available from: https://www.ncbi.nlm.nih.gov/books/NBK448082/
- [Google Scholar]
- Phytochemical analysis, pharmacological and safety evaluations of halophytic plant, Salsola cyclophylla. Molecules. 2021;26:2384.
- [Google Scholar]
- Egg white-induced inflammation models:A study of edema profile and histological change of rat's paw. J Adv Pharm Technol Res. 2021;12:109-12.
- [Google Scholar]
- Anti-inflammation of soursop leaves (Annona muricata L.) against hemorrhoids in mice induced by croton oil. Pharmacogn J. 2020;12:784-92.
- [Google Scholar]
- Effect of tea (Camellia sinensis) and olive (Olea europaea L.) leaves extracts on male mice exposed to diazinon. Biomed Res Int. 2013;2013:461415.
- [Google Scholar]
- Pain and laboratory animals:Publication practices for better data reproducibility and better animal welfare. PLoS One. 2016;11:e0155001.
- [Google Scholar]
- Comparison of different solvents for phytochemical extraction potential from datura metel plant leaves. Int J Biol Chem. 2017;11:17-22.
- [Google Scholar]
- Preliminary phytochemical screening of different solvent extracts of some medicinal plants. Middle East J Appl Sci. 2017;7:226-31.
- [Google Scholar]
- Evaluation of antidiarrheal activity of aerial parts of vinca major in experimental animals. Middle East J Sci Res. 2011;7:784-8.
- [Google Scholar]
- Diarrhea in chronic inflammatory bowel diseases. Gastroenterol Clin North Am. 2012;41:651-75.
- [Google Scholar]
- Chinese clinical practice guidelines for acute infectious diarrhea in children. World J Pediatr. 2018;14:429-36.
- [Google Scholar]
- BLOG:What Can Annona muricata Fruit Juice do to the Gut Microbiome of Healthy Nigerian Children Under-five, And Those with Acute Watery Diarrhoea? 2021. Available from: https://www.isapp-sfa.com/newblog/2021/9/7/what-can-annona-muricata-fruit-juice-do-to-the-gutmicrobiome-of-healthy-nigerian-children-under-five-and-those-withacute-watery-diarrhoea
- [Google Scholar]
- Antidiarrhoeal potentials of methanol bark extract of Hymenocardia Acida Tul (Euphorbiaceae) in laboratory animals. Bull Natl Res Cent. 2021;45:1-12.
- [Google Scholar]
- Antidiarrheal effect of extract from the bark of Combretum leprosum in mice. An Acad Bras Cienc. 2018;91:1-13.
- [Google Scholar]
- Effect of the hydroethanolic extract of Bixa orellana Linn (Bixaceae) leaves on castor oil-induced diarrhea in swiss albino mice. Gastroenterol Res Pract. 2019;2019:1-9.
- [Google Scholar]
- Evaluation of in vivo antidiarrheal activity of 80% methanolic leaf extract of osyris quadripartita decne (Santalaceae) in swiss albino mice. J Evid Based Integr Med. 2019;24:1-9.
- [Google Scholar]
- National inventory and usage of plant-based medicine to treat gastrointestinal disorders with cattle in Benin (West Africa) S Afr J Bot. 2019;122:432-46.
- [Google Scholar]
- Medicinal plants used in management of cancer and other related diseases in Woleu-Ntem province, Gabon. Eur J Integr Med. 2019;29:100924.
- [Google Scholar]
- Evaluation of the anti-diarrheal activity of the ethanolic seed extract of Annona muricata. J Phytopharm. 2019;8:199-202.
- [Google Scholar]
- Edema and fluid dynamics in connective tissue remodelling. J Mol Cell Cardiol. 2010;48:518-23.
- [Google Scholar]
- Evaluation on analgesic and anti-inflammatory activities of total flavonoids from Juniperus sabina. Evid Based Complement Alternat Med. 2018;2018:1-10.
- [Google Scholar]
- Anti-inflammatory effects of the chloroform extract of Annona muricata leaves on phospholipase A2 and prostaglandin synthase activities. Transl Biomed. 2017;8:137.
- [Google Scholar]
- Investigation of the anti-inflammatory and antinociceptive activities of Hymenocardia acida Tul. (Hymenocardiaceae) Afr J Biotechnol. 2010;9:8454-9.
- [Google Scholar]
- Anti-inflammatory and analgesic properties of methanol extract of Annona muricata leaves in animal models. Trop Vet. 2021;37:203-13.
- [Google Scholar]
- Anti-inflammatory and anti-nociceptive activities of the ethanolic extract of Annona muricata Leaf. J Nat Remedies. 2010;10:97-104.
- [Google Scholar]
- Anti-arthritic activities of Annona muricata L. leaves extract on complete Freund's adjuvant (CFA)-induced arthritis in rats. Planta Med. 2010;76:P166.
- [Google Scholar]
- Mechanisms of analgesic and anti-inflammatory properties of Annona muricata Linn. (Annonaceae) fruit extract in rodents. J Med Food. 2014;17:1375-82.
- [Google Scholar]
- Isoquinoline derivatives isolated from the fruit of Annona muricata as 5-HTergic 5-HT1A receptor agonists in rats:Unexploited antidepressive (lead) products. J Pharm Pharmacol. 2011;49:1145-9.
- [Google Scholar]
- Phytochemical characterization, in vitro anti-inflammatory, anti-diabetic, and cytotoxic activities of the edible aromatic plant;Puicaria jaubertii . Molecules. 2021;26:203.
- [Google Scholar]
- Proximate composition, phytochemical analysis, and in vitro antioxidant potentials of extracts of Annona muricata (Soursop) Food Sci Nutr. 2017;5:1029-36.
- [Google Scholar]
- Annonaceous acetogenins:The unrevealed area for cytotoxic and pesticidal activities. Syst Rev Pharm. 2011;2:104-9.
- [Google Scholar]
- Screening of antidiabetic and antioxidant potential along with phytochemicals of Annona genus:A review. Futur J Pharm Sci. 2021;7:144-54.
- [Google Scholar]







