Translate this page into:
The exploration of therapeutic potential of bioflavonoids in metabolic acidosis and inflammation-associated with acute kidney injury: Therapeutic potential of bioflavonoids in acute kidney injury
Address for correspondence: Fares K. Khalifa, Department of Biochemistry, Faculty of Science, King Abdul Aziz University, Jeddah, Saudi Arabia. Tel.: 00966531569236. E-mail: fkkhalifa@kau.edu.sa
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Qassim Uninversity and was migrated to Scientific Scholar after the change of Publisher.
Abstract
ABSTRACT
Objectives:
The goal of this study was to estimate and compare the prospective therapeutic impacts of different types of bioflavonoids (flavonols, flavanones, and isoflavones) on acute kidney injury (AKI) induced by glycerol in male rats.
Methods:
Fifty adult male albino rats (Sprague-Dawley) were separated into five groups: G1, control; G2, rats injected (i.m.) by glycerol (50%) (10 mL/kg b. w.) to induce AKI; G3, G4, and G5, rats received quercetin (QUR), hesperidin (HSP), and genistein (GEN), respectively, after 24 h of glycerol injection for 42 successive days.
Results:
Treatment with bioflavonoids improved the renal tissue levels of antioxidant biomarkers (superoxide dismutase, glutathione, catalase, nitric oxide, inflammatory cytokines (interleukin-6, interleukin-1 βeta, nuclear factor kappa-B, tumor necrosis factor-alpha), kidney function markers (creatinine, urea, urine albumin creatinine ratio, albumin) as compared to nephrotoxic groups. QUR extract reduced the serum level of kidney function parameters in glycerol-injected rats more significantly (P ≤ 0.01) than HSP and GEN. Results demonstrated that the GEN as a therapeutic natural agent exhibited the greatest advancement in urine values, followed by the QUR and HSP respectively when compared to the AKI group.
Conclusion:
The study results demonstrated the therapeutic effect of bioflavonoids against AKI induced by glycerol. Different types of bioflavonoids could reduce oxidative stress, inhibit the production of inflammatory mediators and cytokines, and improve renal function.
Keywords
Acute kidney injury
bioflavonoids
genistein
hesperidin
inflammation
metabolic acidosis
nephrotoxicity
quercetin
Introduction
Nephrotoxicity is the promotion of structural or functional kidney damage following exposure to one or more of an abundance of medications, other therapies, or exogenous toxins, and can result in abrupt renal failure along with a number of structural abnormalities and functional effects.[1] The kidney is an organ highly vulnerable to damage caused by reactive oxygen species (ROS), likely due to the abundance of long-chain polyunsaturated fatty acids in the composition of renal lipids. In recent years, Oxidative stress has become one of the most popular topics in research of molecular mechanism of renal diseases. Oxidative stress has a critical role in the pathophysiology of several kidney diseases, and many complications of these diseases are mediated by oxidative stress-related mediators and inflammation.[2] Acute kidney injury (AKI), formerly known as acute renal failure, is linked to regular chronic kidney disease, longer hospital admissions, and augmented chronic kidney disease. There have been reports of substantial increases in AKI incidence over the last few decades. AKI mortality is still significant (over 50%), even though it is reversible in those who survive.[3] Efforts to avoid AKI and find efficient treatment to accelerate recovery have garnered a lot of attention in recent years.[4]
Bioflavonoids, also known as flavonoids, are naturally occurring compounds found in most plant families and represented in several edible greens, fruits, and vegetables. They are also present in chocolates and several soft drinks, such as tea and coffee.[5] Based on the tricyclic ring system’s chemical structure, bioflavonoids can be categorized into various subclasses. Flavanols, flavanones, anthocyanins, flavones, and isoflavones are the most significant dietary subclasses.[6] The protective influences of flavonoids against chemically induced kidney toxicity have been extensively studied over recent years. Oxidative stress, inflammatory diseases, and changes in vascular well-being could confront renal health. Flavonoids relieve these effects and have presented promising results in treating acute and chronic nephropathies and renal fibrosis.[7] Flavonoids might also perform directly on the renal parenchyma and interfere with signaling pathways, affecting the progress of renal injury and employing nephroprotective effects in glomerulonephritis and chemically induced kidney failure.[8] Flavonoids apply protective effects by reducing the excessive ROS level or activating renal enzymatic and non-enzymatic antioxidants through different pathways.[9]
Hesperidin (HSP) is a bioflavonoid occurring in extreme concentrations in citrus fruits. Numerous health benefits, including antioxidants, antibacterial, anti-inflammatory, and anticarcinogenic ability have been associated with its consumption.[10] HSP belongs to a subclass of flavonoids called flavanone compounds. It is used to treat cardiovascular disorders, cancer, and Type 2 diabetes and has lately experienced thorough evaluation for its pharmacological and health-promoting benefits.[11]
Quercetin (QUR), a natural flavonoid abundant in fruits and vegetables, this powerful antioxidant lowers oxidative stress and prevents cell aging.[12] QUR also suppressed lipid peroxidation and expression of proinflammatory mediators, On the other hand, it may increase serum’s non-enzymatic antioxidant activity and nitric oxide levels.[13] QUR improves oxidative stress, prevents kidney injury, and restrains renal inflammation in animals with diabetic nephropathy.[14] QUR can be considered a polyphenol with the ability to lower oxidative stress and apoptosis while improving mitochondria mitophagy and biogenesis in the kidney.
Genistein (GEN) is a member of the isoflavone subgroup of the flavonoid family. Legumes including soybeans, fava beans, and lupine are the primary source of this phytoestrogen. Numerous health benefits, including prevention of osteoporosis, decreased risk of cardiovascular disease, and anticancer capabilities, are attributed to GEN.[15] In addition, GEN has clear anti-inflammatory effects on lymphocytes, monocytes, and granulocytes that can serve as a novel source of promise phytotherapeutic agents for anti-inflammatory therapies.[16] The aim of this study was to estimate and compare the prospective therapeutic impacts of different types of bioflavonoids (flavonols, flavanones, and isoflavones) on AKI induced by glycerol in male rats.
Materials and Methods
Chemicals
Glycerol (GLY) was purchased from Sigma-Aldrich, USA. Normal saline was purchased from respective vendors (Baxter Ltd). All chemicals utilized in the study were of analytical grade.
Bioflavonoids
GEN has been used as the food isoflavone purchased from Swanson Health Products Co. (Fargo, ND, USA). QUR has been used as the food flavonols purchased as dietary supplements from Now Foods Co., Bloomingdale, IL., USA. HSP has been used as the food flavanones purchased from Swanson Health Products Co. (Fargo, ND, USA). GEN, HSP, and QUR (were first dissolved in dimethyl sulfoxide (DMSO) then diluted with physiological saline (0.9% sodium chloride). Each rat from the GEN-treated groups received no more than 0.2% DMSO, corresponding to 10 μL. In control and AKI-treated groups, each rat received a physiological saline with 10 μL DMSO.[17]
Laboratory animals and the experimental protocol
Five homogenous groups each of ten adult male Albino rats (Sprague-Dawely) strain, mean weight varied between 105 and 123 g were used. The animals were 6 weeks old at the beginning of the experiment. Animals received human care, housed individually in metabolic stainless-steel cages with wire mesh bottoms and maintained at temperature 22°C ± 5°C, humidity 40% ± 5%, and light-dark cycle held constant 12/12 h. During the experiment, food and water were provided ad libitum.
Group 1: Healthy rats (Control)
Group 2: AKI; AKI was induced with glycerol (GLY) (50%, 10 ml/kg of body wt, i.m.) (Negative control)
Group 3: Flavonols – QUR; Rats received QUR (QUR) extracted from dry scales of onion (250 mg/kg b.w/day) by oral gavage after 24 h of glycerol injection (AKI + QUR) for 6 weeks
Group 4: Flavanones – HSP; Rats received HSP (HPD) extracted from Citrus aurantium skin (250 mg/kg b.w/day) by oral gavage after 24 h of glycerol injection (AKI + HSP) for 6 weeks
Group 5: Isoflavone – GEN; Rats received GEN extracted from Sophora japonica fruit (250 mg/kg b.w/day) by oral gavage after 24 h of glycerol injection (AKI + GEN) for 6 weeks.
Samples collection and preparation of kidney tissue homogenate
At the end of the experiment (6 weeks), rats were fasted for 12 h, then the animals were anesthetized by ethyl ether, and blood samples were taken from hepatic portal vein by syringe and transferred into centrifuge tubes. The tubes centrifuged at 10000 × g for 15 min at 25°C to provide serum needed for the biochemical analysis. Serum samples were taken and kept in dry clean plastic and stored at −20°C till used for the different analysis. The kidneys of each rat were removed by dissection, washed by ice-isotonic saline, and blotted between two filter papers. One gram of the kidney was homogenized in 9 volumes of 0.1 M potassium phosphate-buffered saline at pH 7.4. Homogenates were centrifuged at 5000 rpm for 15 min; aliquots of supernatant were kept at −20°C for the biochemical determinations.
Kidney tissue analysis
Inflammatory cytokines
Kidney tissue levels of tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), nuclear factor kappa-B (NF-κB), and interleukin-1β (IL-1β) were determined by enzyme-linked immunosorbent assay (ELISA) following the instruction of the manufacturer using ELISA kits (Kamiya Biomedical Co. CA. USA).
Serum analysis
Kidney function profile
Urea, creatinine, albumin, glucose, sodium, chlorides, and bicarbonates were evaluated spectrophotometrically using Biodiagnostic Kits. Cairo, Egypt. Anion gap was calculated theoretically by the equation: Anion gap (AG) = ([Na+]) − ([Cl-] + [HCO3-]).
Oxidative stress biomarkers
Reduced glutathione (GSH), superoxide dismutase (SOD), nitric oxide (NO), and catalase (CAT) were evaluated spectrophotometrically using Biovision Kit, CA. USA.
Urine analysis
All animals were kept in individual metabolic cages and 24 h urine samples were collected at the end of the experimental period. The preparation of samples was carried out immediately after urine collection and analyzed for urinary parameters. Urine samples were acidified with 10% hydrochloric acid to block the growth of bacteria and molds and stored below 4°C for subsequent analysis. Urine was analyzed for albumin, ammonia, phosphate, and creatinine (kits were obtained from Biodiagnostic Co. Cairo, Egypt). Urinary pH was measured using a pH meter (Beckman coulter). Urine albumin creatinine ratio (uACR) was calculated as albumin concentration (mg/24 h) divided by creatinine concentration (mmol/24 h).
Statistical analysis
Results are presented as means ± standard error of three technical repetitions per sample.
The recorded data were treated statistically using the one-way analysis of variance. The means were compared by the least significant difference test at P ≤ 0.01. Statistical analyses were performed using Statistical Package for the Social Sciences statistical software (SPSS) (IBM SPSS Statistics, version 20).
Results
Effect on kidney function profile
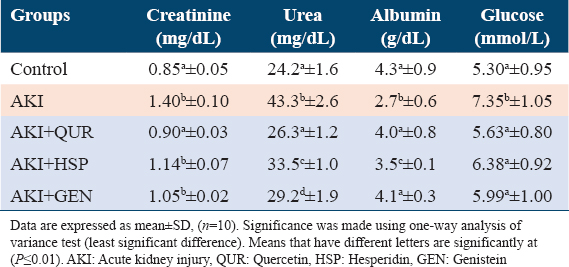
Results illustrated in Tables 1 and 2 showed that the injection to glycerol is followed by AKI as observed by higher creatinine, sodium, glucose, urea, and anion gap levels. Treatment with different types of bioflavonoids caused a significant reduction in urea, creatinine, sodium, glucose, and AG levels when compared to AKI rats (G2). QUR extract reduced the serum level of kidney function parameters in glycerol-injected rats more significantly (P ≤ 0.01) than HSP and GEN groups.
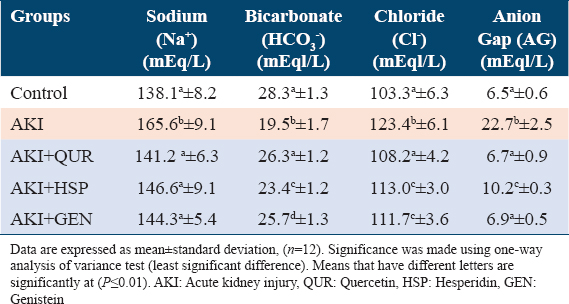
Effect on urine albumin, creatinine, uACR
Glycerol injection induced nephrotoxicity which is displayed by significant changes in biomarkers of urine albumin (A) and creatinine (C) and uACR values as compared to the normal control group. These effects were pronouncedly alleviated by treatment with different types of bioflavonoids [Table 2]. Results demonstrated that the GEN supplemented group (AKI + GEN) exhibited the greatest advancement in urine values, followed by the QUR and HSP, respectively, when compared to the AKI group [Figure 1].
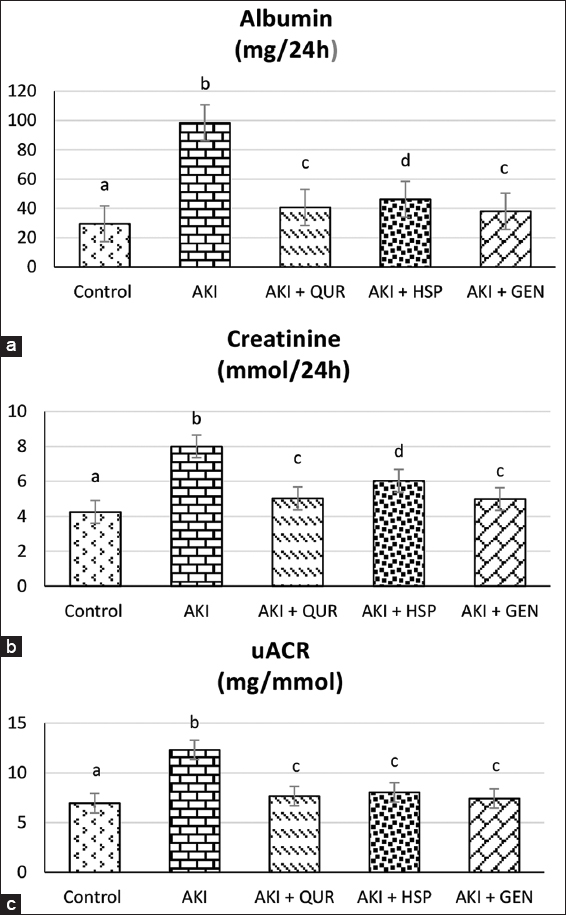
- Effect of different types of bioflavonoids on urine values of albumin (a), creatinine (b), and urine albumin-creatinine ratio (uACR) (1c) Data are expressed as mean ± standard deviation, (n = 10). Significance was made using one-way analysis of variance test (least significant difference). Means that have different letters are significantly at (P ≤ 0.01). AKI: Acute kidney injury, QUR: Quercetin, HSP: Hesperidin, GEN: Genistein
Effect on urine values of pH, ammonia, and inorganic phosphates
Urinary pH value was decreased significantly (P ≤ 0.01) in AKI-induced rats (G2) when compared to all experimental groups. The results of the present study showed that urinary ammonia and phosphate excretion was increased significantly in AKI-induced group (G2) and gradually decreased by bioflavonoids supplementation as therapeutic natural agents. An improvement was observed in QUR-treated rats (AKI + QUR) when compared to AKI rats. All types of bioflavonoids maintain these urinary parameters value near to the levels of control rats [Figure 2].
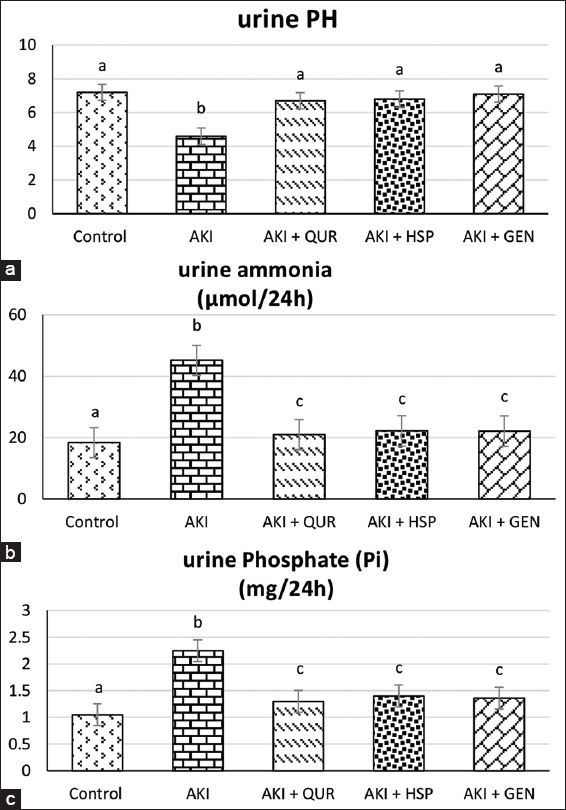
- Effect of different treatments on urine values of pH, ammonia, and inorganic phosphates, Figures (a-c) respectively. Data are expressed as mean ± standard deviation, (n = 10). Significance was made using a one-way analysis of variance test (least significant difference). Means that have different letters are significantly at (P ≤ 0.01). AKI: Acute kidney injury, QUR: Quercetin, HSP: Hesperidin, GEN: Genistein
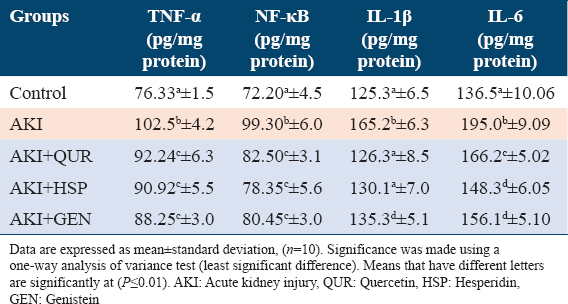
Effect on kidney tissue levels of inflammatory cytokines
Results in Table 3 showed the effect of different treatments on renal tissue levels of TNF-α, NF-kB, IL-1β, and IL-6. The injection of glycerol increased the renal tissue levels of inflammatory cytokines (34.28 %, 37.53%, 31.84%, 42.85% for TNF-α, NF-κB, IL-1β, and IL-6, respectively) as compared to the control group (G1). On the other hand, treatment with different types of bioflavonoids caused a significant reduction in inflammatory cytokines levels when compared to non-therapeutic rats.
Effect on serum glucose and indicators of antioxidant defense status
The results showed that glycerol-induced oxidative stress represented as a significant (P≤0.01) reduction in serum SOD, GSH, and CAT activities and elevation of NO level as compared to control group. Supplementation of bioflavonoids led to a marked increase in GSH, CAT, and SOD activities. In addition, rats treated with bioflavonoids demonstrated a significant reduction in NO levels. HSP-supplemented group, followed by QUR and GEN-supplemented rats, experienced the greatest improvement in oxidative stress biomarkers [Figure 3].
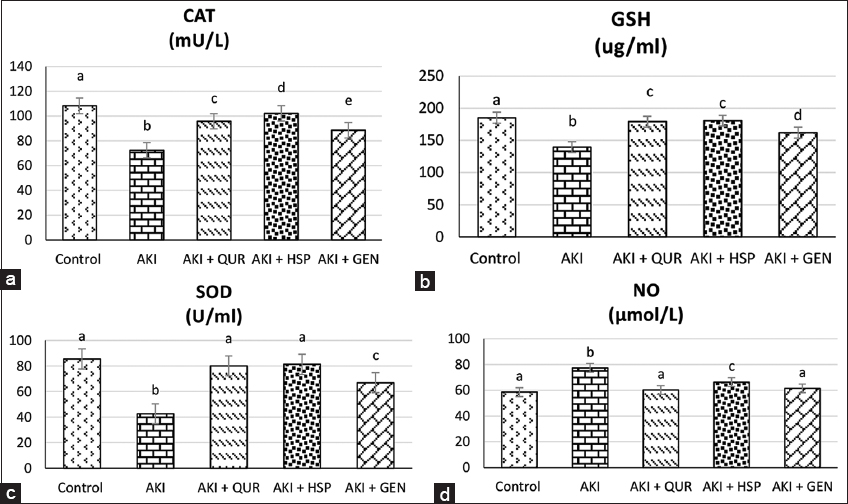
- (a-d) Effect of different types of bioflavonoids on serum levels of indicators of antioxidant defense status. Data are expressed as mean ± standard deviation, (n = 10). Significance was made using a one-way analysis of variance test (least significant difference). Means that have different letters are significantly at (P ≤ 0.01). AKI: Acute kidney injury, QUR: Quercetin, HSP: Hesperidin, GEN: Genistein
Discussion
Nephrotoxicity is defined as an abrupt alteration in renal function that can lead to changes in serum urea and creatinine, glomerular filtration rate, or urine output. Chemicals or medications like glycerol can cause nephrotoxicity as a side effect, which can lead to renal impairment and potentially multi-organ disease.[18] As evidenced by the rise in serum levels of creatinine, urea, glucose, chloride, and anion gap value, the current study’s findings verified that glycerol causes nephrotoxicity. The decrease in GFR, which denotes impairment to glomerular functioning, was associated with an increase in both serum creatinine and urea. One of the most vital organs, the kidney helps maintain the body’s homeostasis by controlling blood pressure, osmotic pressure, electrolyte levels, acid-base balance, and toxin removal.[19]
It has been shown that an increase in urinary ammonia is a common reaction to metabolic acidosis and that urine excretion of ammonia is necessary for maintaining proper acid-base balance in a number of species. The current study’s findings confirmed that after receiving a glycerol injection, rats already had considerable metabolic acidosis, which increased the amount of ammonia excreted in their urine. An increase in renal ammonia production is shown by the rise in urine ammonia in nephrotoxic rats. It has been demonstrated that the primary cause of urine ammonia is glutamine. Glutamate may contribute to renal ammonia through two different mechanisms. Glutamine can be deaminated to produce glutamate and ammonia, or it can be transaminases to remove the amino group, producing α-ketoglutarate and an amino acid.[20]
The results of the present study displayed that, rats given various bioflavonoids showed less acidity than the group given glycerol injections without any kind of treatment. Serum bicarbonate and albumin levels were lower in nephrotoxic animals and greater in anion gap compared to other experimental groups. These alterations were considerably reduced and returned to a value that was close to control when bioflavonoids (QUR, HSP, and GEN) were used as therapeutic agents. According to recent research, bioflavonoids either prevent or minimize the kidney damage brought on by arterial hypertension. The recognized preventive effects of flavonoids in the vascular system and the decrease of blood pressure may take precedence over the action mechanism. It has been discovered that dietary QUR improves kidney glutathione transferase activity, potassium depletion, and oxidative stress while lowering systolic blood pressure and renal hypertrophy.[21]
Numerous experimental animal models have clearly shown the connection between oxidative stress and nephrotoxicity.[22] A specific reference level of NO may be important for preserving normal renal function, according to several animal studies.[23] In the pathophysiology of AKI, recent research has shown that increased NO and its metabolite, peroxynitrite, production is a key factor in oxidative stress and tissue damage.[24]
The results of the present study revealed that glycerol significantly raises blood NO levels, indicating that oxidative stress and NO generation, when triggered by glycerol, contribute to kidney damage brought on by GLY. The present study’s findings showed that, in comparison to the control group, GLY significantly raised the NO level in rat serum. This suggests that GLY administration may cause inducible NO synthase to be induced, which would increase NO production and ultimately result in the formation of toxic peroxynitrite. Oral supplementation of HSP to the GLY-induced group significantly decreased the NO level, which implies that HSP may operate to boost the antioxidant system by preventing the production of NO. The expression of isoforms of inducible NO synthase, cyclooxygenase, and lipoxygenase – which produce NO, prostanoids, and leukotrienes as well as inflammatory mediators such cytokines and chemokines have been shown to be inhibited by bioflavonoids.[25]
Oxidative stress results from a disruption of redox equilibrium caused by an increase in ROS production. Cells use an antioxidant defense system that includes enzyme components, glutathione peroxidase (GSH-Px), CAT, and SOD to protect themselves from ROS-induced cellular damage. Nevertheless, oxidative damage and diseases might result when the antioxidant defense system is unable to neutralize or get rid of the excess ROS.[26] The human body maintains homeostasis using antioxidant defense systems to maintain the balance between oxidants and antioxidants.[27] Bioflavonoids have been shown to lower the levels of reactive oxygen and other free radicals in the human body, and act as exogenous antioxidants by donating electrons to peroxynitrite, hydroxyl, and peroxyl radicals.[28] Flavonoids work through both direct and indirect pathways to produce their antioxidant activity. Eliminating ROS directly is the direct mechanism.[29] Indirect antioxidant effects include inhibiting pro-oxidant enzymes and promoting the synthesis or activation of antioxidant enzymes.
Bioflavonoids have been shown to activate the synthesis of endogenous antioxidants such as GSH, SOD, and CAT via activating intracellular antioxidant signaling pathways.[30] By activating the nuclear factor erythroid 2–related factor 2 (Nrf2) signaling pathway and reducing the spontaneous degradation of the Nrf2 protein, flavonoids like QUR and GEN have been shown to have a protective impact in a number of disorders.[31] Since ROS have been linked to GFR impairment, HSP ’s antioxidant qualities may be responsible for its protective effect on serum urea and creatinine concentration.[32]
It has been proposed that flavonoids’ antioxidant qualities are due to their capacity to donate hydrogen and scavenge free radicals.[33] By chelating metal ions, flavonoids can prevent the production of free radicals and the propagation of free radical reactions. Due to its antioxidant properties, HSP, one of the citrus flavonoids, has a positive effect by raising the activities of CAT and SOD content. The presence of the 3′hydroxy and 4′methoxy groups on the aromatic B ring, which gives hydrogen and an electron to neutralize the hydroxyl and superoxide free radicals, may be the cause of HSP’s ability to scavenge free radicals.[34] Low CAT and SOD activity levels were correlated to GLY-induced nephrotoxicity, suggesting oxidative damage caused by GLY. These antioxidant enzymes may become inactive as a result of the elevated ROS generation in GLY-induced nephrotoxicity.[35] Furthermore, following the GLY injection, the GSH levels were considerably lower than those of the control group. The higher consumption of GSH in the non-enzymatic elimination of oxygen radicals may be the cause of the drop in GSH concentration following the GLY injection.
The immune system uses inflammation as a defense against damage or infection. Inflammation serves to repair tissue structure and physiological function while removing toxic and foreign stimuli.[36] Acute inflammation is an inflammatory reaction that occurs right after an injury and lasts for a few days, whereas chronic inflammation is a reaction that lasts for a longer period of time. If acute inflammation is not resolved, it may develop into chronic inflammation, which may accelerate tissue damage and the resulting functional deficits.[37] Although the physiological roles of acute inflammation in defense and healing are well established, alterations in the inflammatory regulation mechanism can result in a chronic inflammatory process that disrupts homeostasis.[38] Innate immune cells are described as part of the inflammatory response, and they release chemokines and proinflammatory cytokines that attract lymphocytes and cause tissue damage. Chronic inflammation may result from the production of ROS, reactive nitrogen species, and other proteases during the inflammatory immune response.[39]
Bioflavonoids have been shown to exert anti-inflammatory potentials by different mechanisms, including immune cell regulation and transcription factors, and enzyme inhibition. According to research, flavonoids affect the maturation, activation, and signaling transduction of immune cells, which can prevent the synthesis and release of cytokines and chemokines. By suppressing IL-6 and lowering T-cell allogeneic proliferation, QUR has been demonstrated to prevent dendritic cell maturation.[40]
Bioflavonoids could reduce the production of proinflammatory cytokines from mast cells, and other immune cells.[41] Flavonoids from wild blueberries also prevent monocytes from sticking to the human umbilical vein endothelial cells in a proinflammatory setting caused by TNF-α.[42] Renal failure and damage are frequently linked to elevated levels of TNF-α. In rats with renal damage, TNF-neutralization decreased the expression of inflammatory markers and NF-kB activation while increasing NO release. This decreased renal fibrosis and inflammation and had a protective effect on the progression of renal injury.[43]
Recent research has demonstrated that oxidative stress and inflammation caused by hyperglycemia play a key role in the pathogenesis and development of diabetic nephropathy.[44] Flavonoids’ significance in diabetic nephropathy has been evaluated in a number of research, the majority of which have found that they improve renal function. According to certain research, GEN may be a useful preventative measure against diabetic nephropathy in Type 1 and Type 2 diabetes. The three major ways that GEN helps diabetic nephropathy are by lowering renal inflammation, improving fasting blood glucose levels, and preventing the production of advanced glycation end products. By lowering lipid peroxidation and raising SOD and CAT activity, QUR has been shown to support diabetic rats avoid glomerular and tubular damage.[45] In addition, QUR may reduce the excretion of urine microalbumin, hyperglycemia, lipid metabolism problems, and creatinine levels in the serum.[46] By raising the activity of SOD and GSH-Px and lowering MDA, IL-1β, and TNF-α levels, it may also lessen free radicals.[47]
The current study also showed that glycerol resulted in strong inflammatory responses as verified by apparent increment in kidney tissue NF-κB, TNF-α, IFN-γ, and IL-6 as compared to the control group. Significant cellular damage is caused by glycerol, which also increases the expression of many inflammatory chemokines and cytokines, including TNF-α. The transcription factor known as NF-κB is essential for the development and maintenance of inflammation and regulate oxidative stress in pathological settings.[48] By inducing the transcription of several genes in distinct innate immune cells, such as adhesion molecules, cytokines, and chemokines, NF-κB activation directly causes the inflammatory response. In addition, by inducing the regulation of cell proliferation, apoptosis, and differentiation and promoting the formation of inflammatory T cells, NF-κB indirectly contributes to inflammation.[49] NF-κB is essential for numerous immunological and inflammatory response mechanisms. The appropriate regulation of the NF-κB signaling system is one promise therapeutic strategy for the treatment of tissue damage and inflammatory disorders. Therefore, a substance that inhibits NF-κB activation could be used as a novel anti-inflammatory treatment. Previous Studies have recognized the anti-oxidative properties of isoflavones such as GEN.[50]
GEN has been shown to protect cells from over production of ROS by scavenging free radicals. This prevents the activation of NF-κB, which is essential for the production of inflammation and cytokines.[51] GEN has been demonstrated to inhibit the synthesis of cytokines that promote inflammation. TNF-α and IL-6 pharmaceutical inhibitions are excellent examples of how to use a strategy to reduce inflammatory cytokines. It has been demonstrated that GEN works to reduce inflammation through several mechanisms. One of these mechanisms is the downregulation of NF-κB, which lowers TNF-α and IL-6 levels.[52]
Conclusion
The present study has revealed that exposure to subchronic doses of glycerol impairs the kidney function. However, the administration of oral different types of bioflavonoids (GEN, HSP, QUR) for six weeks has a therapeutic effect against nephrotoxicity by reversing and downregulation of inflammatory cytokines and mediators bringing them to near-normal levels. The results demonstrated that bioflavonoids could suppress the production of inflammatory cytokines, reduce the oxidative stress, and improve renal function.
Ethical Approval
This study was approved by the Ethics Committee of Sciences Academy of Experimental Research, Egypt [30 July 2024, No. 44605].
Availability of Data and Materials
The data sets used in this study are available with the corresponding author and will be provided on a reasonable request.
Competing Interest
The authors have no conflicts of interest to declare.
Authors’ Contributions
Prof. Fares Khalifa and Prof. Rasha Hussein supervised the project. Dr. Abeer Banjabi, Dr. Maha Al-Bazi, and Dr. Sahar Alkhodair performed and analyzed the experiments, Dr. Aliaa Sabban, Dr. Bahiya Osrah, and Dr. Hayat M. Albishi reviewed and validated the analysis, statistics, and grammar checking at the final stages and all the authors had written the manuscript, reviewed the manuscript, and agreed to submit it.
Funding Statement
This work was not funded by any institution.
References
- The nephrotoxicity of drugs used in causal oncological therapies. Curr Oncol. 2022;29:9681-94.
- [Google Scholar]
- Oxidative stress in the pathophysiology of kidney disease:Implications for noninvasive monitoring and identification of biomarkers. Oxid Med Cell Longev. 2020;2020:5478708.
- [Google Scholar]
- Recovery of kidney function after acute kidney disease-a multi-cohort analysis. Nephrol Dial Transplant. 2024;39:426-35.
- [Google Scholar]
- Important flavonoids and their role as a therapeutic agent. Molecules. 2020;25:5243.
- [Google Scholar]
- Plant flavonoids:Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022;383:132531.
- [Google Scholar]
- Plant flavonoids bioavailability in vivo and mechanisms of benefits on chronic kidney disease:A comprehensive review. Phytochem Rev. 2023;22:1541-65.
- [Google Scholar]
- Health benefits of cyanidin-3-glucoside as a potent modulator of Nrf2-mediated oxidative stress. Inflammopharmacology. 2021;29:907-23.
- [Google Scholar]
- Hesperidin:A review on extraction methods, stability and biological activities. Nutrients. 2022;14:2387.
- [Google Scholar]
- The role of flavonoids as a cardioprotective strategy against doxorubicin-induced cardiotoxicity:A review. Molecules. 2022;27:1320.
- [Google Scholar]
- Protective effect of quercetin against oxidative stress-induced toxicity associated with doxorubicin and cyclophosphamide in rat kidney and liver tissue. Iran J Kidney Dis. 2017;11:124-31.
- [Google Scholar]
- Dietary antioxidant supplements and uric acid in chronic kidney disease:A review. Nutrients. 2019;11:1911.
- [Google Scholar]
- Anti-invasive effect and pharmacological mechanism of genistein against colorectal cancer. Biofactors. 2020;46:620-8.
- [Google Scholar]
- Genistein:A review on its anti-inflammatory properties. Front Pharmacol. 2022;13:820969.
- [Google Scholar]
- Protective effect of Genistein against N-nitrosodiethylamine (NDEA)-induced hepatotoxicity in Swiss albino rats. J Pharm Anal. 2015;5:51-7.
- [Google Scholar]
- Nephrotic syndrome:Pretibial epidermolysis bullosa in a patient with CD151 tetraspanin defect:A case report. Int J Health Sci (Qassim). 2024;18:35-40.
- [Google Scholar]
- Body fluid compartments, cell membrane ion transport, electrolyte concentrations, and acid-base balance. Semin Nephrol. 2019;39:368-79.
- [Google Scholar]
- Amino acid metabolism in the kidneys:Nutritional and physiological significance. Adv Exp Med Biol. 2020;1265:71-95.
- [Google Scholar]
- Bioflavonoid combination attenuates diabetes-induced nephropathy in rats via modulation of MMP-9/TIMP-1, TGF-b, and GLUT-4-associated pathways. Heliyon. 2024;10:e33217.
- [Google Scholar]
- Renal dysfunction is associated with a reduced contribution of nitric oxide and enhanced vasoconstriction after a congenital renal mass reduction in sheep. Circulation. 2015;131:280-8.
- [Google Scholar]
- Dietary sources, classification, biosynthesis, and mechanism of action of flavonoids in combating oxidative stress. In: Role of Flavonoids in Chronic Metabolic Diseases:From Bench to Clinic. Vol Vol. 18. United States: John Wiley and Sons; 2024. p. :67-114.
- [Google Scholar]
- A brief overview of oxidative stress in adipose tissue with a therapeutic approach to taking antioxidant supplements. Antioxidants (Basel). 2021;10:594.
- [Google Scholar]
- Are flavonoids effective antioxidants in plants?Twenty years of our investigation. Antioxidants (Basel). 2020;9:1098.
- [Google Scholar]
- Plant flavonoids on oxidative stress-mediated kidney inflammation. Biology (Basel). 2022;11:1717.
- [Google Scholar]
- Therapeutic effect of bromelain and papain on intestinal injury induced by indomethacin in male rats. Int J Health Sci (Qassim). 2023;17:23-30.
- [Google Scholar]
- Quercetin attenuated oxidative DNA damage through NRF2 signaling pathway in rats with DMH induced colon carcinogenesis. Life Sci. 2020;253:117584.
- [Google Scholar]
- The flavonoid hesperidin methyl chalcone targets cytokines and oxidative stress to reduce diclofenac-induced acute renal injury:Contribution of the nrf2 redox-sensitive pathway. Antioxidants (Basel). 2022;11:1261.
- [Google Scholar]
- Computational chemistry strategies to investigate the antioxidant activity of flavonoids-an overview. Molecules. 2024;29:2627.
- [Google Scholar]
- Hesperidin, a citrus bioflavonoid, decreases the oxidative stress produced by carbon tetrachloride in rat liver and kidney. BMC Pharmacol. 2005;5:2.
- [Google Scholar]
- Protective effects of N-acetylcysteine and S-adenosy-LMethionine against nephrotoxicity and immunotoxicity induced by ochratoxin A in rats. Int J Health Sci (Qassim). 2024;18:17-24.
- [Google Scholar]
- Overview of Inflammation. In: In Inflammation Resolution and Chronic Diseases. Vol Vol. 1. Berlin, Germany: Springer Nature; 2024. p. :1-18.
- [Google Scholar]
- Assessment of antipsychotic-induced cytotoxic effects on isolated CD1 mouse pancreatic beta cells. Int J Health Sci (Qassim). 2023;17:11-21.
- [Google Scholar]
- Anti-inflammatory activity of natural dietary flavonoids. Food Funct. 2010;1:15-31.
- [Google Scholar]
- Inflammaging:Significance and intervention. Int J Health Sci (Qassim). 2024;18:4-5.
- [Google Scholar]
- Quercetin exerts anti-tumor immune mechanism by regulating IL-6/JAK2/STAT3 signaling pathway to deplete Treg cells. Toxicon. 2024;243:107747.
- [Google Scholar]
- The novel flavone tetramethoxyluteolin is a potent inhibitor of human mast cells. J Allergy Clin Immunol. 2015;135:1044-52.e5.
- [Google Scholar]
- Different effects of anthocyanins and phenolic acids from wild blueberry (Vaccinium angustifolium) on monocytes adhesion to endothelial cells in a TNF-alpha stimulated proin-flammatory environment. Mol Nutr Food Res. 2016;60:2355-66.
- [Google Scholar]
- Neutralization of tumor necrosis factor-alpha reduces renal fibrosis and hypertension in rats with renal failure. Am J Nephrol. 2012;36:151-61.
- [Google Scholar]
- The signaling of cellular senescence in diabetic nephropathy. Oxid Med Cell Longev. 2019;2019:7495629.
- [Google Scholar]
- Quercetin, a plant flavonol attenuates diabetic complications, renal tissue damage, renal oxidative stress and inflammation in streptozotocin-induced diabetic rats. Metabolites. 2023;13:130.
- [Google Scholar]
- Kidney protection effects of dihydroquercetin on diabetic nephropathy through suppressing ROS and NLRP3 inflammasome. Phytomedicine. 2018;41:45-53.
- [Google Scholar]
- Quercetin liposomes ameliorate streptozotocin-induced diabetic nephropathy in diabetic rats. Sci Rep. 2020;10:2440.
- [Google Scholar]
- The pivotal role of NF-kB in the pathogenesis and therapeutics of Alzheimer's disease. Int J Mol Sci. 2022;23:8972.
- [Google Scholar]
- Updates on the chemistry, processing characteristics, and utilization of tea flavonoids in last two decades (2001-2021) Crit Rev Food Sci Nutr. 2023;63:4757-84.
- [Google Scholar]
- Inhibitory activities of genistein on signaling pathways in pulmonary inflammation induced by cisplatin in experimental rats. Ann Clin Anal Med. 2024;15:544-9.
- [Google Scholar]
- Understanding genistein in cancer:The “good“and the “bad“effects:A review. Food Chem. 2016;196:589-600.
- [Google Scholar]







