Translate this page into:
The synergistic effect of oxaliplatin and punicalagin on colon cancer cells Caco-2 death
Address for correspondence: Hadeil Muhanna Alsufiani, Department of Biochemistry, Faculty of Sciences, King Abdulaziz University, Jeddah, Saudi Arabia/Experimental Biochemistry Unit, King Fahd Medical Research Center, King Abdulaziz University, Jeddah, Saudi Arabia. E-mail: halsufiani@kau.edu.sa
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Qassim Uninversity and was migrated to Scientific Scholar after the change of Publisher.
Abstract
ABSTRACT
Objectives:
The objectives of the study are to investigate the synergistic effect of oxaliplatin (oxa) and punicalagin (pun) on the death of colon cancer cells (Caco-2) by apoptosis and autophagy.
Methods:
The effects of the combined treatments (5 μM oxa + 50 μM pun, 5 μM oxa + 75 μM pun, 20 μM oxa + 50 μM pun, and 5 μM oxa + 75 μM pun) were compared with untreated Caco2 cells (control) or cells treated with oxa alone. Apoptosis was detected using an Annex in V FITC flow cytometry assay and poly (ADP-ribose) polymerase cleavage by western blotting. Light chain 3 was detected by western blotting as an autophagy marker.
Results:
The combined treatments significantly increased the number of apoptotic cells in comparison to untreated cells or cells treated with oxa alone. By contrast, the combined treatments had no significant effect on autophagy.
Conclusion:
The combined treatment significantly promoted cell death through apoptosis while maintaining a basal level of autophagy.
Keywords
Apoptosis
autophagy
Caco2
colon cancer
oxaliplatin
punicalagin
synergetic effect

Introduction
Colon cancer, also known as colorectal cancer, is a type of cancer that originates in the large intestine or rectum. It is the third most common cancer worldwide, accounting for around ten percent of all cancer cases. It is also the second leading cause of cancer-related deaths worldwide.[1] Colon cancer can be treated in various ways, including immunotherapy, targeted therapy, and chemotherapy. Chemotherapy involves the use of anti-cancer drugs, either singly or in combination, to destroy cancer cells. The most common chemotherapy drugs used to treat colon cancer are fluorouracil (5-FU), capecitabine, irinotecan, and oxaliplatin (oxa), among others.[2,3]
Oxa is an alkylating agent that contains platinum metal. The modes of action by which oxa induces cell death are through apoptosis and autophagy.[4] Oxa is efficient at treating colon cancer; however, it causes a number of adverse side effects, such as diarrhea, hair loss, allergic reactions, loss of fertility, fatigue, tingling, and decreases in blood cell numbers. Furthermore, cancer cells can become resistant to oxa treatment in some cases.[5,6] Therefore, the development of new strategies to reduce the side effects and overcome this resistance is important for the effective treatment of colon cancer.
Several studies have investigated the synergetic effect of oxa and natural products on colon cancer cell lines. In general, natural products can enhance the efficacy of oxa used as a colon cancer treatment.[7] For instance, resveratrol and oxa combinations can inhibit the growth of Caco-2 cells by activating caspase-3 and promoting the cleavage of poly-ADP ribose polymerase (PARP).[8] Similarly, a combination of blueberry extracts and oxa was able to induce apoptosis in HCT-116 cells by activating caspases-3 and -9.[9] Cao et al. found treatment of colon cancer cells (HCT-116 and RKO) with alantolactone and oxa-induced apoptosis.[10] Another study found nobiletin chemosensitized HT29 and SW480 colon cancer cells to oxa treatment.[9]
One interesting natural product is punicalagin (pun), a natural product belonging to the ellagitannin family. Pun is abundant in pomegranate peel, juice, and seeds, with the highest content in pomegranate peel.[11] Pun has significant antioxidant, anti-inflammatory, antifungal, and anti-cancer properties.[12-14] It is also known to affect multiple signaling pathways involved in cancer cell viability and proliferation through actions on apoptotic, autophagic, and senescence machineries in various cancer cell lines, including colon cancer cells.[12,15] For example, Larrosa et al. reported treating Caco-2 cells with pun-induced apoptosis through the intrinsic pathway through mitochondrial release of cytochrome c into the cytosol and activation of initiator caspase 9 and effector caspase 3.[16] Similarly, Omar et al. showed treating Caco-2 with pun-induced apoptosis through the activation of caspases-3, -8, and -9 and by PARP cleavage.[17] However, no previous studies have examined the effectiveness of combinations of pun and oxa as treatments for colon cancer. The mode of action is still largely unexplored and needs investigation. Thus, the aim of the current study was to determine the synergistic effect of pun and oxa on colon cancer (Caco-2) cell death by examining their effects on apoptosis and autophagy.
Methods
Cell culture and treatment
The human colorectal carcinoma (Caco-2) cell line was obtained from the European collection of cell cultures, Salisbury, UK. Cells were grown in Dulbecco’s modified Eagle’s medium containing 50 U/mL penicillin/streptomycin, 20% fetal bovine serum, 1% non-essential amino acids, and 1% 2 mM L-glutamine (complete culture medium). The cells were seeded in T75 cm2 flasks and maintained at 37°C in air containing 5% CO2. Every 4 days, the cells were sub-cultured using a complete culture medium. The cells were used for experiments when they had reached 80–90% confluence. Before each experiment, the cells were seeded at a density of 1 × 106 cells/mL, washed with PBS, and then detached using 2 mL of trypsin/ethylenediaminetetraacetic acid. The cells were treated with oxa alone (5 μM or 20 μM) or with a combination of both oxa and pun as follows: 5 μM oxa + 50 μM pun, 5 μM oxa + 75 μM pun, 20 μM oxa + 50 μM pun, and 5 μM oxa + 75 μM pun.
Cell apoptosis assay
Annexin V-FITC
An Annexin V-FITC apoptosis detection kit (PF032, Merck Millipore) was used according to the manufacturer’s protocol to detect apoptotic cells with a BD FACSCanto flow cytometer (California, USA). In normal viable cells, phosphatidylserine (PS) is located on the cytoplasmic surface of the cell membrane. Upon induction of apoptosis, PS is translocated to the cell surface. FITC-conjugated annexin V binds to PS on the outer surface of cells undergoing apoptosis.
Propidium iodide (PI) was used to detect cells with compromised cell membranes to allow differentiation of viable cells (Annexin V negative, PI negative), early apoptotic cells (annexin V positive, PI negative), and late apoptotic/necrotic cells (annexin V positive, PI positive). Due to the necrotic-like disintegration of cells in late apoptosis, these cells will be positive for Annexin V and PI, and since membrane permeability is increased in necrotic cells, those cells will also bind Annexin V.
PARP cleavage
PARP is an enzyme involved in DNA repair and plays an essential role in maintaining cell viability. Cleavage of this protein by caspase-3 facilitates cellular disassembly and is a recognized hallmark of cell apoptosis.[18] In this assay, Caco2 cells were lyzed and fractionated using Invitrogen NuPAGE 4–12% Bis-Tris gel electrophoresis according to the manufacturer’s protocol. The proteins were then electro-transferred onto polyvinylidene difluoride (PVDF) membranes, blotted with PARP antibody (#9532, Cell Signaling Technology, UK), and loading was confirmed with beta-actin antibody (#3700, Cell Signaling Technology, UK). A chemiluminescent western blot immunodetection kit with Amersham film was used according to the manufacturer’s protocol to develop the protein bands on the membrane.
Cell autophagy assay
Light chain 3 (LC3) was originally identified as a subunit of microtubule-associated proteins 1A and 1B. Cleavage of LC3, immediately after its synthesis, yields the cytosolic LC3-1 form. The conversion of this form to LC3-11 has been used as an indicator of autophagy. In this assay, Caco2 cells were lyzed and fractionated by Invitrogen NuPAGE 4–12% Bis-Tris gel electrophoresis according to the manufacturer’s protocol. Next, the proteins were electro-transferred onto PVDF membranes and blotted with LC3A/B antibody (#4108, cell singling technology, UK) and confirmed loading with beta-actin antibody (#3700, cell signaling Technology, UK). A chemiluminescent western blot immunodetection kit with Amersham film was then used according to the manufacturer’s protocol to develop the protein bands on the membrane.
Statistical analyses
All statistical analyses were performed using GraphPad Prism version 5.0 for Mac OS X software. The data were presented as mean ± standard error of mean (SEM). One-way analysis of variance (ANOVA), followed by Bonferroni’s multiple comparison test, was used to determine the significant differences between untreated cells, cells treated with different concentrations of oxa alone, and cells treated with different combinations of oxa and pun. The statistical significance threshold was taken as P < 0.05.
Results
The effect of oxa and pun on Caco2 cell apoptosis
The induction of cell death by apoptosis was investigated by flow cytometry examination of the effect of oxa alone or in combination with pun on PS externalization. Figure 1 shows that apoptosis was significantly induced in Caco2 cells treated with oxa alone or in combination with pun when compared to untreated cells (control). The percentage of cells in early apoptosis was 12% in untreated cells (control) and significantly increased to 21%, 18%, and 24% in cells treated with 5 μM oxa alone, 5 μM oxa + 50 μM pun, and 5 μM oxa + 75 μM pun, respectively. These percentages were also higher when the cells were treated with higher doses of oxa, reaching 25%, 32%, and 29% in cells treated with 20 μM oxa alone, 20 μM oxa + 50 μM pun, and 20 μM oxa + 75 μM pun, respectively. The combined treatments showed significant increases in the percentage of early apoptotic cells when compared with cells treated with oxa alone.
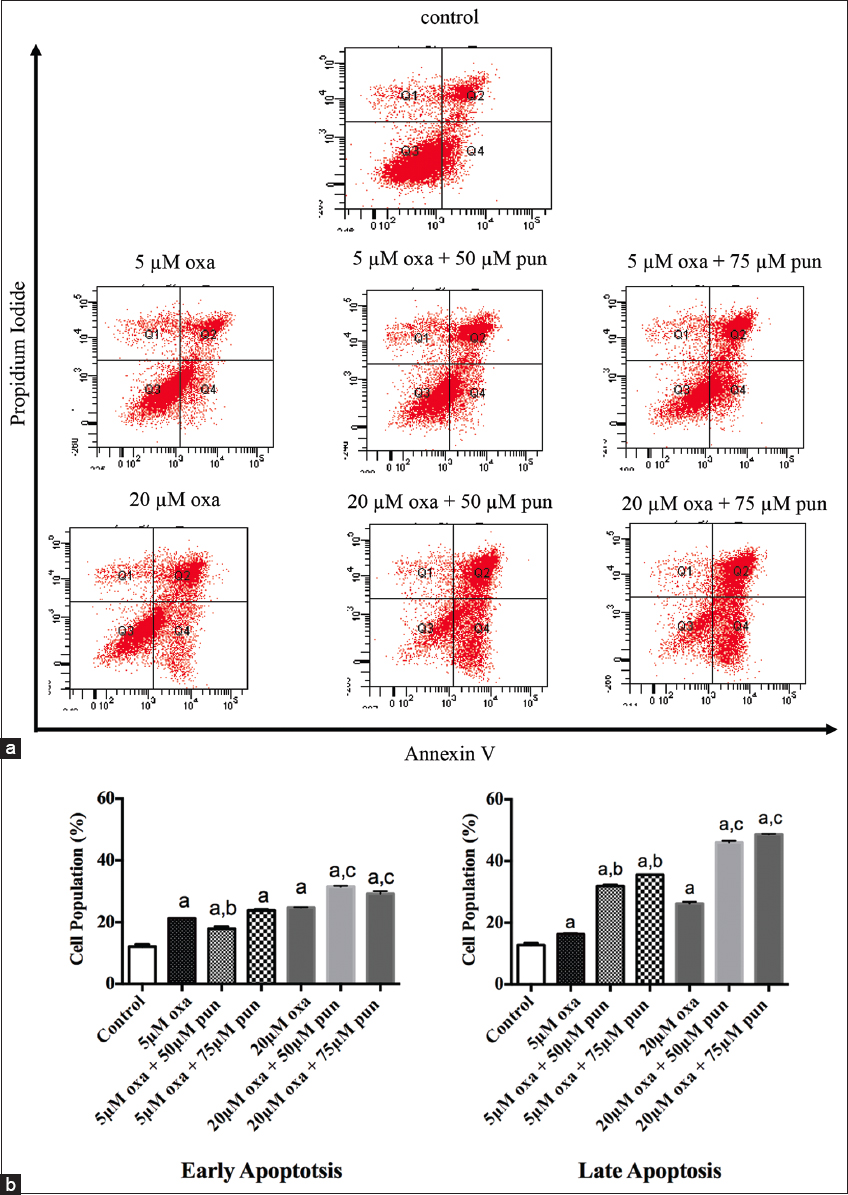
- Detection of apoptosis in untreated Caco2 cells and cells treated with different concentrations of oxa, alone or in combination with pun. (a) Flow cytometry analysis of Caco2 cells harvested and stained with Annexin V and propidium iodide. This combination allows the differentiation of viable cells (Q3; Annexin V negative, PI negative), early apoptotic cells (Q4; annexin V positive, PI negative), and late apoptotic/necrotic cells (Q2; Annexin V positive, PI positive). (b) Proportions of Caco2 cells in the early apoptotic and late apoptotic/necrotic stages. Data are presented as mean ± standard error of mean. A lowercase ‘a’ denotes a significant difference with untreated cells (control). A lowercase ‘b’ denotes a significant difference with cells treated with 5 μM oxa alone. A lowercase ‘c’ denotes a significant difference with cells treated with 20μM oxa alone. Comparisons between means were conducted by one-way analysis of variance followed by Bonferroni’s multiple comparison test (P < 0.05)
The combined treatments also significantly increased the number of cells in the late apoptosis/necrosis stage in comparison to untreated cells (control) or cells treated with oxa alone. Cells treated with 5 μM oxa + 50 μM pun or 5 μM oxa + 75 μM pun showed significantly higher proportions of apoptotic/necrotic cells (32% and 36%, respectively) compared with untreated cells or cells treated with oxa alone (13% and 16%, respectively). A similar trend was observed when cells were treated with higher doses of oxa (20 μM) in combination with pun (50 μM or 75 μM), with the proportion of apoptotic/necrotic cells reaching 46% and 49% with the 20 μM oxa + 50 μM pun and 20 μM oxa + 75 μM pun treatments, respectively. By contrast, the proportion of apoptotic/necrotic cells was only 26% in cells treated with 20 μM oxa only.
Insights into the mechanism of apoptosis were obtained by determining the PARP cleavage levels by western blotting. Cells treated with oxa alone showed a dose-dependent induction of PARP cleavage. Combining the higher dose of oxa (20 μM) with different concentrations of pun (50 μM or 75 μM) induced more cleavage in PARP [Figure 2].
![Western blot of PARP cleavage in untreated Caco2 cells (control), cells treated with oxa alone or combined with pun for 48 h. ([3] untreated cells (control), [2] 5 μM oxa, [3] 5 μM oxa + 50 μM pun, [4] 5 μM oxa + 75 μM pun, [5] 20 μM oxa, [6] 20 μM oxa + 50 μM pun, [7] 5 μM oxa + 75 μM pun). Equal protein loading (20 μg) per lane was determined using ß-actin expression](/content/195/2024/18/2/img/IJHS-18-33-g002.png)
- Western blot of PARP cleavage in untreated Caco2 cells (control), cells treated with oxa alone or combined with pun for 48 h. ([3] untreated cells (control), [2] 5 μM oxa, [3] 5 μM oxa + 50 μM pun, [4] 5 μM oxa + 75 μM pun, [5] 20 μM oxa, [6] 20 μM oxa + 50 μM pun, [7] 5 μM oxa + 75 μM pun). Equal protein loading (20 μg) per lane was determined using ß-actin expression
The effect of oxa and pun on Caco2 cell autophagy
The conversion of the autophagy marker LC3-I to LC3-II was detected by western blotting as a marker of autophagy. No significant difference was noted in the conversion of LC3-I to LC3-II in untreated cells (control), cells treated with oxa alone, or cells treated with oxa combined with pun [Figure 3].
![Western blot of Light Chain 3 expression in untreated caco2 cells (control), cells treated with oxa alone or combined with pun for 48 h. ([1] untreated cells (control), [2] 5 μM oxa, [3] 5 μM oxa + 50 μM pun, [4] 5 μM oxa + 75 μM pun, [5] 20 μM oxa, [6] 20 μM oxa + 50 μM pun, [7] 5 μM oxa + 75 μM pun). Equal protein loading (20μg) per lane was determined using ß-actin expression](/content/195/2024/18/2/img/IJHS-18-33-g003.png)
- Western blot of Light Chain 3 expression in untreated caco2 cells (control), cells treated with oxa alone or combined with pun for 48 h. ([1] untreated cells (control), [2] 5 μM oxa, [3] 5 μM oxa + 50 μM pun, [4] 5 μM oxa + 75 μM pun, [5] 20 μM oxa, [6] 20 μM oxa + 50 μM pun, [7] 5 μM oxa + 75 μM pun). Equal protein loading (20μg) per lane was determined using ß-actin expression
Discussion
The purpose of this study was to investigate the synergistic effect of oxa and pun on colon cancer cell death, including apoptosis and autophagy. Interestingly, the findings are the first to reveal that treating colon cancer cells with oxa combined with pun significantly increases the stimulation of apoptosis over that observed in untreated cells or cells treated with oxa alone. This finding was confirmed at a mechanistic level by the cleavage of PARP, a hallmark of apoptosis. These results are consistent with previous studies reporting that treating colon cancer cells with either oxa or pun alone induces apoptosis.[4,14,17,19]
Two main apoptotic pathways are recognized: the extrinsic or death receptor pathway and the intrinsic or mitochondrial pathway. Both pathways converge into the same execution pathway, which is the activation of caspase-3.[20] Pun has been reported to induce intrinsic apoptosis through several pathways in colon cancer cells.[17,21] One of these pathways involves the release of cytochrome C from mitochondria, causing the activation of caspase-9, which in turn activates caspase-3. Pun can also induce extrinsic apoptosis by activating caspase-8, which also causes the activation of caspase-3. The activation of caspase-3 then promotes the cleavage of PARP and subsequently leads to apoptosis.[17]
The present findings also confirmed that treating colon cells with oxa, either alone or in combination with pun, did not significantly promote autophagy above the levels seen in untreated cells, meaning that autophagy is maintained at a basal level following exposure to oxa and/or pun. Recent studies have shown that apoptosis and autophagy have a crosslinked relationship in anti-cancer therapy, whereby apoptotic signals and products can promote or inhibit autophagy and autophagy can promote or inhibit apoptosis.[20,22,23] This relationship has been reported to be complex in colon cancer, involving several signal transduction pathways and regulators.[20] One possible explanation for our results is that apoptosis inhibits autophagy through caspases, which digest several essential autophagic proteins, thereby inactivating the autophagy program.[20]
It is important to note that this study was limited by the different mechanisms underlying the effects of the combination of oxa and pun on colon cancer cell death by apoptosis and autophagy and the interplay between them. Thus, future research should aim to address this limitation.
Conclusion
This research investigated the synergistic effects of oxa and pun on colon cancer (Caco2) cell death, including apoptosis and autophagy. The combined oxa and pun treatments significantly induced apoptosis but had no significant effect on autophagy.
Funding
This research received no funding.
Conflicts of Interest
There are no conflicts to declare.
Data Availability
Data are available upon reasonable request.
References
- 2023. Colorectal Cancer. Available from: https://www.who.int/news-room/fact-sheets/detail/colorectal-cancer#:~:text=Colon%20cancer%20is%20the%20second,and%20mortality%20rates%20were%20observed
- 2023. Drugs Approved for Colon and Rectal Cancer. Available from: https://www.cancer.gov/about-cancer/treatment/drugs/colorectal
- Current and emerging therapeutic approaches for colorectal cancer:A comprehensive review. World J Gastrointest Surg. 2023;15:495-519.
- [Google Scholar]
- Enhancement of oxaliplatin-induced cell apoptosis and tumor suppression by 3-methyladenine in colon cancer. Oncol Lett. 2015;9:2056-62.
- [Google Scholar]
- Oxaliplatin resistance in colorectal cancer enhances TRAIL sensitivity via death receptor 4 upregulation and lipid raft localization. Elife. 2021;10:67750.
- [Google Scholar]
- Coexisting molecular determinants of acquired oxaliplatin resistance in human colorectal and ovarian cancer cell lines. Int J Mol Sci. 2019;20:3619.
- [Google Scholar]
- The synergism of natural compounds and conventional therapeutics against colorectal cancer progression and metastasis. Front Biosci (Landmark Ed). 2022;27:263.
- [Google Scholar]
- Resveratrol-induced potentiation of the antitumor effects of oxaliplatin is accompanied by an altered cytokine profile of human monocyte-derived macrophages. Apoptosis. 2014;19:1136-47.
- [Google Scholar]
- Combinatorial effect of blueberry extracts and oxaliplatin in human colon cancer cells. J Cell Physiol. 2019;234:17242-53.
- [Google Scholar]
- Enhancement of oxaliplatin-induced colon cancer cell apoptosis by alantolactone, a natural product inducer of ROS. Int J Biol Sci. 2019;15:1676-84.
- [Google Scholar]
- Characterisation of pomegranate-husk polyphenols and semi-preparative fractionation of punicalagin. Phytochem Anal. 2017;28:433-8.
- [Google Scholar]
- Punicalagin in cancer prevention-via signaling pathways targeting. Nutrients. 2021;13:2733.
- [Google Scholar]
- The dietary hydrolysable tannin punicalagin releases ellagic acid that induces apoptosis in human colon adenocarcinoma Caco-2 cells by using the mitochondrial pathway. J Nutr Biochem. 2006;17:611-25.
- [Google Scholar]
- Effect of punicalagin on human colon cancer Caco-2 cells. Mal J Nutr. 2016;22:125-36.
- [Google Scholar]
- Role of poly(ADP-ribose) polymerase (PARP) cleavage in apoptosis. Caspase 3-resistant PARP mutant increases rates of apoptosis in transfected cells. J Biol Chem. 1999;274:22932-40.
- [Google Scholar]
- In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J Nutr Biochem. 2005;16:360-7.
- [Google Scholar]
- The interaction mechanism between autophagy and apoptosis in colon cancer. Transl Oncol. 2020;13:100871.
- [Google Scholar]
- Granatin B and punicalagin from Chinese herbal medicine pomegranate peels elicit reactive oxygen species-mediated apoptosis and cell cycle arrest in colorectal cancer cells. Phytomedicine. 2022;97:153923.
- [Google Scholar]
- Interplay between apoptosis and autophagy in colorectal cancer. Oncotarget. 2017;8:62759-68.
- [Google Scholar]
- The role of interaction between autophagy and apoptosis in tumorigenesis (review) Oncol Rep. 2022;48:208.
- [Google Scholar]






