Translate this page into:
The usefulness of B-cell lymphoma-2 immunohistochemical stain in the differentiation between reactive atypia and dysplasia/carcinoma in the gallbladder
Address for correspondence: Dr. Abdullah Saleh Alkhamiss, Department of Pathology, College of Medicine, Qassim University, Qassim, Saudi Arabia. Mobile: 00966506139201. E-mail: 3772@qu.edu.sa
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Qassim Uninversity and was migrated to Scientific Scholar after the change of Publisher.
Abstract
ABSTRACT
Objective:
The differentiation between reactive atypical changes and dysplasia/carcinoma in the daily cases of cholecystectomies is a routine histopathological challenge. Up to our knowledge, no immunohistochemical marker can definitely differentiate between these two changes. Many promising markers have been proposed to be helpful tools in this situation. One of them is B-cell lymphoma-2 (BCL-2) immunohistochemical stain. Therefore, this study aims to evaluate its usefulness as a marker that might be helpful in such challenging cases.
Methods:
From the archive of the histopathology laboratories of Qassim University Medical City and King Fahad Specialist Hospital in Qassim, five dysplastic/neoplastic gallbladder cases were collected (in the shape of formalin-fixed, paraffin-embedded blocks) as well as five cholecystitis with reactive atypical changes cases. Two slides from each block were prepared: One was stained with H&E and the other was stained immunohistochemically with BCL-2. The slides were evaluated by two histopathologist consultants in the same sitting using multiheaded microscope to confirm the original diagnosis and to evaluate the BCL-2 staining.
Results:
Five dysplastic/carcinoma cases and five cholecystitis with reactive atypia were collected. The original diagnoses were confirmed by two pathologists. They also confirmed that all the BCL-2 stained slides (with the exception of one reactive case) were negative for BCL-2 immunohistochemical stain.
Conclusion:
BCL-2 immunohistochemical stain is not a promising marker in the differentiation between reactive epithelium and dysplasia/carcinoma in the gallbladder.
Keywords
B-cell lymphoma-2
gallbladder carcinoma
gallbladder dysplasia
gallbladder reactive atypia
Introduction
Although generally it is rare, gallbladder malignancy is the most common type of malignancy that affects the biliary tree.[1,2] In 1777, this type of malignancy had been described for the first time.[3] It has a very poor prognosis, with <10% 5-year survival rate.[3] The incidence rate of this type of malignancy is variable worldwide, in which it is low in some European countries and the USA, relatively higher in some Eastern European countries, and extremely higher in countries of Latin America and South Asia.[1,3,4] Based on the cell origin, carcinoma is the most common type of cancer that is arising in the gallbladder because it represents more than 80% of all the malignant cases arising from the biliary tree.[3,5,6]
B-cell lymphoma-2 (BCL-2) gene is a proto-oncogene that is coding for BCL-2 onco-protein. This protein affects the proliferating cells by prolonging the cell survival because it is blocking the programmed cell death, that is, apoptotic process. Therefore, it plays a major role in some malignancies like follicular lymphoma, that is, BCL-2 overexpression increases the lifespan of cells.[7,8] BCL-2 immunohistochemical stain (clone: BCL-2/100/D5) is a mouse anti-human monoclonal antibody that is used for the qualitative identification by light microscopy of human BCL-2 onco-protein in formalin-fixed, paraffin-embedded tissue. Overexpression of BCL-2 immunohistochemical stain in gallbladder carcinoma has been observed by many authors.[9,10] Not only that, they also observe and describe its pattern of expression. They found that the better the differentiation of the tumor, the more intense staining and overexpression of BCL-2.[10]
Gallstones and cholecystitis are the primary risk factor for gallbladder carcinoma.[3,5] The inflammatory process in the gallbladder (especially with the association of gallstones) may irritate the lining epithelium of the gallbladder. This irritation may cause reactive atypia in this epithelium. The reactive atypia mimics the dysplasia (which can happen in these cases), making it difficult for the pathologist to differentiate between them.[3,11,12] From the last point, the aim of this study is to evaluate the usefulness of using BCL-2 immunohistochemical stain in the differentiation between reactive atypia (that had been sometimes found in the cholecystitis) and the dysplasia/carcinoma in the gallbladder.
Aim
The aim of the study was to evaluate the usefulness of using BCL-2 immunohistochemical stain in the differentiation between reactive atypia and dysplasia/carcinoma in the gallbladder.
Materials and Methods
After obtaining the ethical approval from the Qassim University Committee (number: 2024051506), all the cases of dysplasia/carcinoma of the gallbladder from January 01, 2020 to July 01, 2024 were pulled out form the archive of the histopathology lab of Qassim University Medical City Hospital and King Fahad Specialist Hospital in Qassim. We found 7 cases (in the form of formalin-fixed, paraffin-embedded tissue). Two of them were excluded based on the exclusion criteria (see later). Hence, we had only five dysplastic/carcinoma cases. As a comparison group, we pulled out the first five cases of cholecystitis with reactive changes in consecutive retrograde method, starting from the date of July 01, 2024. We end up with 10 cases, half of them are reactive and the other half are dysplastic/carcinoma. From each case, we chose the most representative formalin-fixed paraffin-embedded blocks (based on the result of the examination of one Hematoxylin and Eosin [H&E] glass slide section that was made from it). From each of the chosen blocks, two 4-μm thickness unstained glass slides were made. Then, one slide was stained with H&E stains, whereas the other slide was immunohistochemically stained with BCL-2. Both stains were done according to the standard protocols (H&E protocol and BCL-2 immunohistochemical protocol). Each glass slide was examined by two consultant pathologists together on the same sit – by using a multihead microscope – in order to: (A) confirm the diagnosis (whether it is a reactive atypia or dysplasia/carcinoma) in a blind way of the original report, and (B) evaluate the immunohistochemical BCL-2 stain pattern in a blind way of the H&E counterpart slides, so the pathologists did not know the result of the corresponding H&E stained slide. The main inclusive criterion is the presence of reactive atypia and/or dysplasia/carcinoma. The exclusion criteria were: (A) absence of reactive atypia and/or dysplasia/carcinoma and (B) presence of a confirmed metastatic tumor in the gallbladder.
Results
Table 1 summarizes the clinical data of the patients. We end up with five carcinoma cases and five cholecystitis cases with reactive changes as a comparison group. The male cases of the carcinoma cases are 3, female cases are 2, and male: female ratio is 3:2. The age of carcinoma patients ranges from 24 to 78 years with an average age of 59.4 years. The average age of male carcinoma cases is 47.7 years, whereas in females it is 77 years.
All the carcinoma patients were presented with signs and symptoms of gallstone-like picture as right upper quadrant abdominal pain, nausea, and vomiting. Two of these cases showed obstructive jaundice. None of them had any signs of metastasis radiologically at the first time diagnosis.
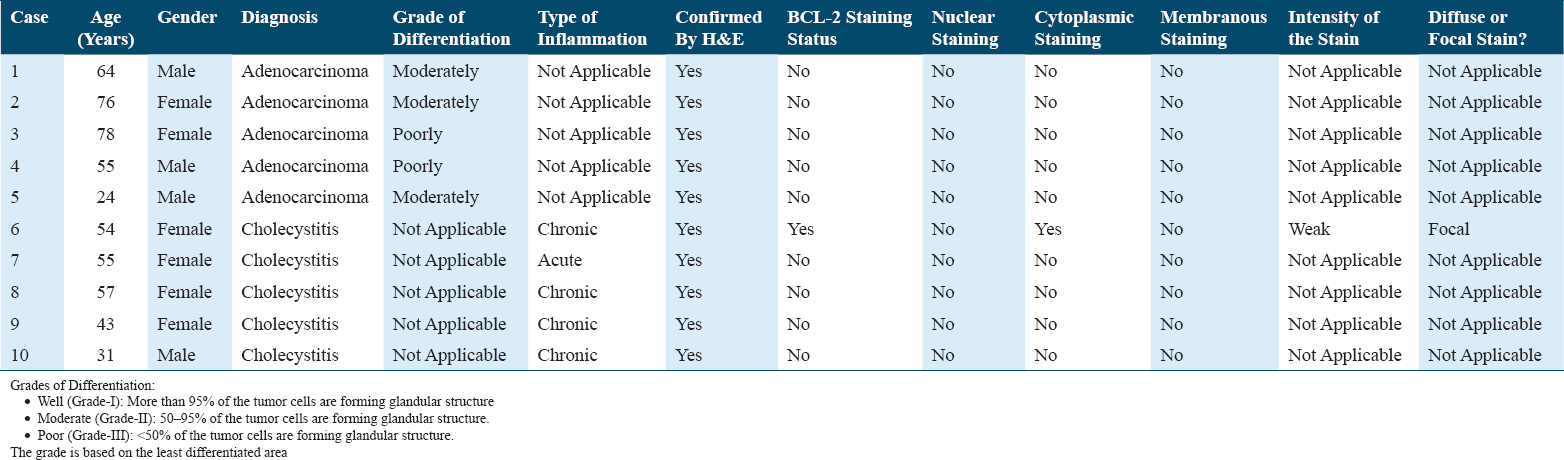
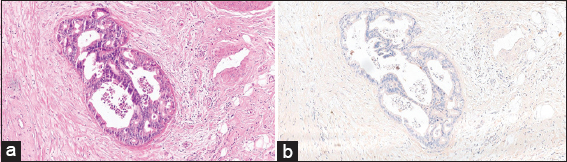
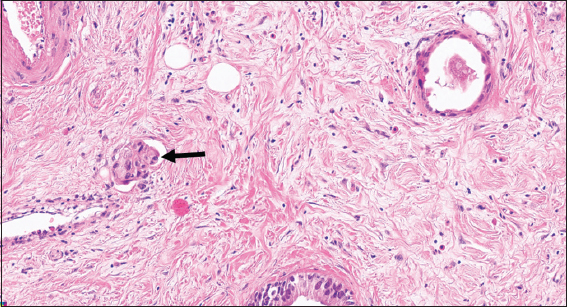
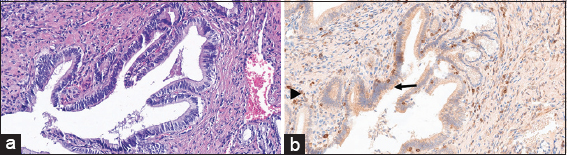
The microscopic examination of the H&E slides of the carcinoma cases shows a typical picture of adenocarcinoma [Figure 1]. Some of the cases show lymphovascular invasion [Figure 2], and others show perineural invasion [Figure 3]. Regarding to the histological grade of these carcinomas, 3/5 of the cases are moderately differentiated, whereas the remaining 2/5 are of poorly differentiated adenocarcinoma [Figure 4]. None of them is well differentiated. There is no clear relation between the gender of the patient and the degree of differentiation.
- (a) (H&E stained ×100) shows moderately differentiated carcinoma, in which the invasive neoplastic cells forming glandular structures. (b) (BCL-2 Immunohistochemical stained ×100) shows the same neoplastic cells do not take the BCL-2 staining. BCL-2: B-cell lymphoma-2
- (H&E stained ×100) shows malignant cells invading the blood vessels (arrow)
- (B-cell lymphoma-2 immunohistochemical stained ×200) shows that the negatively stained neoplastic cells (arrow) invading the positively stained nerve (arrowhead)
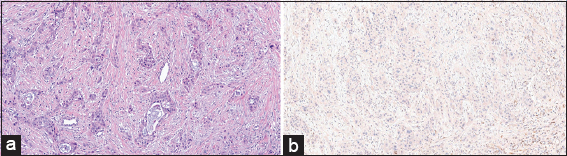
- (a) (H&E stained ×100) shows poorly differentiated carcinoma, in which the neoplastic cells arranged mainly in cords with few glandular structures. (b) (BCL-2 Immunohistochemical stained ×100) shows the same cells do not take the BCL-2 staining. At the same time, lymphocytes are taken this stain in the background. BCL-2: B-cell lymphoma-2
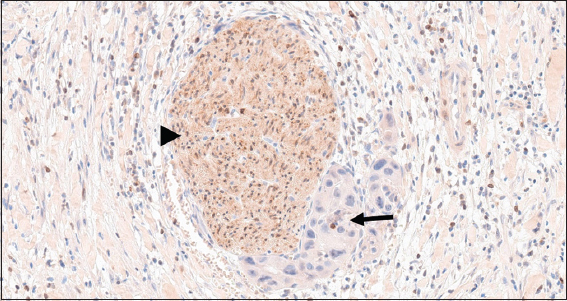
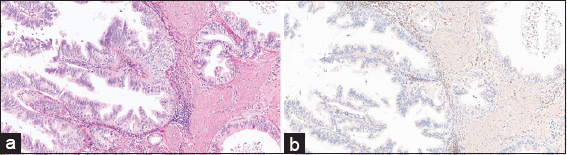
Although all the reactive and neoplastic cases in this study were stained immunohistochemically with BCL-2 stain, only one cholecystitis case with reactive changes stained positively with this stain [Figure 5]. This case shows focal weak cytoplasmic positivity for BCL-2. The rest of the normal looking adjacent epithelium, adjacent dysplasia [Figure 6], and the invasive carcinoma part are negative for BCL-2 [Figures 1, 3, 4].
- (a) (H&E stained ×200) shows reactive changes of the epithelial cells of an inflamed gallbladder. (b) (BCL-2 Immunohistochemical stained ×200) shows the cytoplasm of the same reactive cells stained focally and weakly by the BCL-2 staining (arrow). At the same time as an internal control, scattered lymphocytes in the connective tissue stains strongly by BCL-2 (arrowhead). BCL-2: B-cell lymphoma-2
- (a) (H&E stained ×100) shows dysplastic epithelial cells of the gallbladder. (b) (BCL-2 Immunohistochemical stained ×100), these cells are negative for BCL-2 immunohistochemical stain. The connective tissue shows few lymphocytes that are positive for BCL-2 immunohistochemical stain. BCL-2: B-cell lymphoma-2
Discussion
The early diagnosis and management of gallbladder dysplasia/carcinomas are the key to a successful curing of such diseases. However, due to the late presentation of these cases, the management and curing is difficult.[13] That is why discovering the early dysplastic changes in the gallbladder is crucial. The idea of this research is to evaluate the usefulness of BCL-2 immunohistochemical staining in this challenging area based on its overexpression that had been reported in some articles.[9-11] However, unfortunately, none of our dysplastic/carcinoma cases stains positively for BCL-2. In contrast to our findings, Doval et al. found that 8% of their cases were stained with BCL-2,[9] and Mikami et al. found that 18.4% of their cases are also positive for BCL-2 immunohistochemical stain.[10] This can be explained by the limited number of cases in our research (in comparison to theirs) and by the fact that BCL-2 overexpression is affected by the differentiation of the tumor.[10] To elaborate more, Mikami et al. and Başak et al. found that BCL-2 expression is affected by the tumor differentiation, that is, the more differentiation of the carcinoma, the more overexpression and staining of the BCL-2 and vice versa.[10,14] In our research, all of our neoplastic cases were of moderately and poorly differentiated carcinomas. None of them was well differentiated. This may explain why none of them stains with BCL-2. However, the relationship between the tumor differentiation and BCL-2 staining does not explain the reason of the negative staining of dysplastic epithelium adjacent to the invasive part in our cases. The same observation was also noted by Başak et al.[14]
In contrast to the dysplastic changes in the gallbladder, the dysplastic changes in many other GIT organs are relatively positive for BCL-2.[8,15,16] For example, in the oral cavity, around 30% of the dysplastic squamous cells are positive for BCL-2 immunohistochemical staining.[15] In the gastric dysplasia, 81% of the cases are positive.[8] In the colorectal dysplasia, 45% of the cases are positive.[16]
Regarding the expression of BCL-2 in the reactive atypia, in our study, only one case is stained focally and weakly by BCL-2. The rest are not (similar to the normal as well as the dysplastic/neoplastic epithelium). This finding is also supported by the one that had been described by Başak et al., in which there was no significant difference in the expression of BCL-2 between normal gallbladder epithelium and the dysplastic one.[14]
Therefore, in conclusion, BCL-2 immunohistochemical stain is a not a helpful tool in the differentiation between reactive epithelium and dysplasia/carcinoma in the gallbladder.
Conclusion
BCL-2 immunohistochemical stain is a not a promising tool in the differentiation between reactive epithelium and dysplasia/carcinoma in the gallbladder. At the end of this study, still there is no clear answer about wither Bcl-2 immunohistochemical stain will be a useful tool in the differentiation between well-differentiated adenocarcinoma and reactive atypia of the gallbladder.
Funding
This study is a self-funded by the author.
Conflicts of Interest
The author declared no conflicts of interests.
Acknowledgment
The author gratefully acknowledged the efforts from Dr. Ahmed Alsolai and Mr. Saleh Mohammed Almozaini for their support and recommendations during the preparation of this article.
References
- Epidemiology and molecular pathology of gallbladder cancer. CA Cancer J Clin. 2001;51:349-64.
- [Google Scholar]
- Gallbladder cancer worldwide:Geographical distribution and risk factors. Int J Cancer. 2006;118:1591-602.
- [Google Scholar]
- Bcl-2 initiates a new category of oncogenes:Regulators of cell death. Blood. 1992;80:879-86.
- [Google Scholar]
- Immunohistochemical evidence of aberrant bcl-2 protein expression in gastric epithelial dysplasia. Cancer. 1994;73:2900-4.
- [Google Scholar]
- Expression of epidermal growth factor receptor, p53, Bcl2, vascular endothelial growth factor, cyclooxygenase-2, cyclin D1, human epidermal receptor-2 and Ki-67:Association with clinicopathological profiles and outcomes in gallbladder carcinoma. J Carcinog. 2014;13:10.
- [Google Scholar]
- Association of Bcl-2 protein expression with gallbladder carcinoma differentiation and progression and its relation to apoptosis. Cancer. 1999;85:318-25.
- [Google Scholar]
- Study on Salmonella Typhi occurrence in gallbladder of patients suffering from chronic cholelithiasis-a predisposing factor for carcinoma of gallbladder. Diagn Microbiol Infect Dis. 2013;77:69-73.
- [Google Scholar]
- Incidence of Salmonella typhi infection in symptomatic cholelithiasis in an endemic area. A prospective study. Gastroenterol Hepatol. 1986;9:121-4.
- [Google Scholar]
- Immunohistochemical evaluation of apoptosis and multidrug resistance-related markers in gallbladder dysplasia and carcinoma. South Clin Ist Euras. 2022;33:423-8.
- [Google Scholar]
- Immunohistochemical expression of Bcl-2 in oral epithelial dysplasia and oral squamous cell carcinoma. Indian J Cancer. 2015;52:505-10.
- [Google Scholar]
- Immunohistochemically detectable bcl-2 expression in colorectal carcinoma:correlation with tumour stage and patient survival. Br J Cancer. 1995;72:981-5.
- [Google Scholar]







