Translate this page into:
Vitamin D deficiency in pediatric sickle cell disease patients without crisis – A cry to investigate it on priority
Address for correspondence: Shahida Aziz Khan, King Fahd Medical Research Center, King Abdulaziz University, Jeddah 21589, Kingdom of Saudi Arabia. Phone: 966126952000. E-mail: sakhan01@kau.edu.sa
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Qassim Uninversity and was migrated to Scientific Scholar after the change of Publisher.
Abstract
ABSTRACT
Objective:
The alarming increase in vitamin D deficiency (VDD) has been shown to result in compounded risks of major health problems globally. As sickle cell disease (SCD) children are already health compromised, the co-morbidities escalate early in life, demanding an early detection, to minimize the adverse effects. This study determined vitamin D levels in children with SCD without a crisis to check for probable associations with inflammation and infections if any.
Methods:
SCD children aged 5–16 years, in a steady state, were enrolled in the study after taking necessary consent and ethical clearance. Hb, serum calcium, vitamin D, and high-sensitivity C-reactive protein (hsCRP) levels were analyzed.
Results:
VDD was seen in most of the children with SCD irrespective of gender and age. Males aged 5–10 years showed significance (P = 0.0375) with vitamin D and white blood cell (WBC) (P = 0.0015) but males aged 11–16-year age group exhibited a very strong-positive correlation with vitamin D (r = 0.9862) and a very strong-negative correlation with Hb (r = −0.9819) and hsCRP (r = −0.9907). Among females, the 11–16-year age group patients exhibited a significant association with vitamin D (P = 0.0487), Ca (P = 0.0118), Hb (P = 0.0007), and hsCRP (P = 0.0001) levels. Correlation “r” values in this age group show a strong-negative correlation with WBC levels (r = −0.6525) as well as hsCRP (r = − 0.6550).
Conclusion:
The increased deficiency of vitamin D in SCD children should be addressed at early ages of life, to reduce the occurrence and severity of associated comorbidities.
Keywords
Diagnostic marker
early intervention
vitamin D
Introduction
The essential role of vitamin D in bone health, immune function, and overall cell health links it to several health problems. Vitamin D deficiency (VDD) has been shown to result in an increased risk of skeletal fractures, infections, autoimmune disease, and cancer.[1]
VDD is considered a major health problem globally with people residing in places receiving more sun, also affected with it.[2] VDD has also been shown to be highly prevalent in different regions of Saudi Arabia across different age groups in both genders.[3-5] VDD prevalence in sickle cell disease (SCD) is 65–100% in countries such as the USA, Spain, and the UK. This is due to the increased frequency of hospitalizations, inadequate nutrition, and an increased energy expenditure, resulting in several comorbidities.[6,7] SCD is indicated by repeated episodes of unbearable pain called crisis, which may require hospitalization at times, with either medication such as hydroxyurea, or in certain cases transfusion or even bone marrow transplantation. As Saudi Arabia fights against the prevention of genetic diseases, they yet pose extreme emotional as well as economic drain to the family as well as the kingdom. As symptoms of pain along with comorbidities start expressing in childhood itself, the need to attend to it at this stage itself is imperative.[8] There is therefore the need to look for associations and pathways which might help improve their condition to at least some extent at inception itself.
According to a study in the eastern province of Saudi Arabia, 67% of SCD children were found vitamin D deficient. This deficiency appeared commonly in the younger age group of patients and those experiencing crisis.[1] Among all vitamins in SCD patients, VDD should be carefully monitored since low amounts develop due to a combined effect of increased concentration of melanin in the skin, lesser physical activity, and inadequate food intake. A decreased calcium and vitamin D being crucial for bone metabolism lead to a reduction in the ideal bone mass peak in SCD children which determines their growth failure. Therefore, the prevalence of osteoporosis was found to be common in SCD.[9]
Vitamin D metabolism involves different organs of the body including the liver, kidney, intestines, skin, and the parathyroid. Therefore, its deficiency in SCD patients has essentially been recognized as one of the most common. Apart from nutritional impairments, SCD patients may possibly have renal impairment, which could cause hindrance in converting vitamin D to its active form. Furthermore, the ability to synthesize vitamin D from sunlight in dark-skinned individuals is compromised. Finally, the inflammatory condition in SCD could affect the vitamin D metabolic pathway.[10] Associations between vitamin D supplementation in SCD children on hydroxyurea treatment have shown a decrease in the inflammatory markers including high-sensitivity C-reactive protein (hsCRP), which supports the immune-modulatory properties of the vitamin.[11] A negative cross-sectional association between 25(OH)D and hsCRP concentrations in a Danish mother-child cohort was observed, proposing a role of the vitamin in systemic low-grade inflammation that is important for child health and future disease outcomes.[12]
We therefore conducted a small observational study on children with SCD not experiencing crisis. This would help increase the awareness among caregivers and patients for early diagnosis and management.
Methods
An observational study was performed on 40 SCD pediatric patients aged 5–16 years during the period August 2018–December 2019 at King Fahd Medical Research Center (KFMRC), King Abdulaziz University (KAU), Jeddah. The concerned Ethics Committee approved the protocol which was registered with registration number 2/36/8390. Guardians of all the subjects were given a brief of the project and their written informed consent was taken before participating in the study. Patient demographics included age, gender, diagnosis, and status.
Inclusion criteria
Male and female SCD children aged 5–16 years approximately matched for weights were included in the study. Recruitments were done at the outpatient ward and their initial readings were taken into consideration. All phenotypes were enrolled. Patients enrolled were in a steady state and had a steady hemoglobin blood level for the past 4 weeks along with an absence of fever, infection, or vaso-occlusive crisis (VOC) or transfusion before the start of the study. A repetitive painful episode/pain in bone, joints, or multiple sites/patients requiring the need for analgesics with signs of inflammation was defined as a VOC.
Exclusion criteria
As blood withdrawal is quite challenging in children below 5 years of age, we excluded them from our study. Other patients excluded were those above 16 years of age, or those having chronic diseases, endocrine problems, or on concomitant anti-coagulation treatment; previous history of overt stroke, and neurological abnormalities (visual, hearing, or motor deficits); chest wall anomalies or diagnoses other than VOC, and those having undergone blood transfusion in the past 4 weeks before start of the study. Patients taking vitamin D treatment were excluded from the study. Vitamin D values were collected from the hospital. Therefore, patients coming to the outpatient ward with multiple vitamin D tests done due to various health reasons were excluded thereby maintaining uniformity.
Blood collection
Approximately 5 mL of blood was collected (fasting was not mandatory) of which 1 ml blood was taken in EDTA tubes for hematological testing. The remaining blood was collected in non-EDTA tubes, vortexed, and centrifuged at 3000 rpm. Vortexed tubes were allowed to stand for half an hour before separating the serum obtained in the upper portion of the tubes. Proper storage of samples at −80°C was done after appropriately labeling them. While serum calcium and vitamin D were measured at KAU hospital laboratory, the Hb, white blood cell (WBC), and hsCRP values were analyzed at KFMRC, Jeddah.
Analysis
Hb and WBC were measured with a Sysmex KX-21 automated cell counter (Sysmex Corporation, Kobe Hyogo, Japan). Serum calcium was determined by reflectance spectrophotometry, using a VITROS 250 Clinical Chemistry Auto-analyzer (Ortho-Clinical Diagnostics Inc., Rochester, NY, USA). While the chemiluminescent microparticle immunoassay was used for analyzing serum 25(OH) D using a LIASON auto-analyzer (DiaSorin Inc, Stillwater, MN, USA), hsCRP was measured by an automated kit method with a SMART 700/340 analyzer (Eurolyser Diagnostica GmbH, Austria).
Classification of vitamin D was as follows: Sufficiency was termed as a level of ≥30–60 ng/mL insufficiency as 20–<30 ng/mL and deficiency as <20 ng/mL of vitamin D.[13]
hsCRP was analyzed using 20 μL serum and measured with a SMART 700/340 analyzer using an automated kit method. The standard reference range for hsCRP was <1 mg/L – low cardiovascular risk, <1–2 mg/L – moderate cardiovascular risk, and >3 mg/L – high cardiovascular risk.
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 10 software. Data were subjected to one-way ANOVA analysis. Multiple comparisons using Tukey’s test were performed with 95% CI. Means were taken with standard deviation. Significance was interpreted as a P < 0.05. The Pearson correlation test was performed using Excel to determine “r” values for correlation. The “r” values range from −1 to +1 (values from 0 to 0.4 denote weak-positive association; 0.5 to 0.8 denote a strong-positive association, and 0.8 to 1.0 denote a very strong-positive association. Likewise, values from 0 to −0.4 denote weak-negative association; −0.5 to −0.8 denote strong-negative association, and −0.8 to −1.0 denote very strong-negative association).
Results
Low vitamin D levels were observed in all the SCD children (12.337 ± 8.857 ng/mL), representing a deficiency with an average of (12.465 ± 8.812) in males and (12.164 ± 8.901) in females [Figure 1].
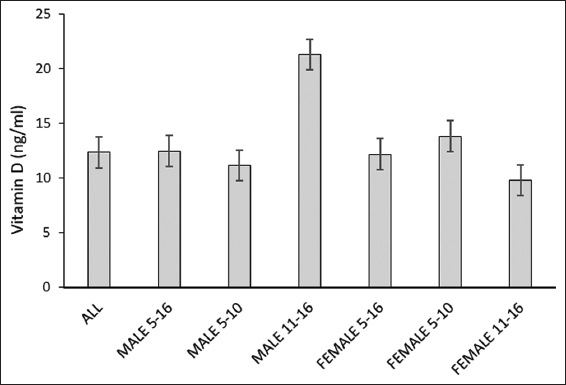
- Vitamin D levels in pediatric patients with sickle cell disease. *Age in years; All male and female 5–16, n=40; total male 5–16, n=23; male 5–10, n=20; male 11–16, n=3; total female 5–16, n=17; female 5–10, n=10; female 11–16, n=7
All patients had normal calcium levels except the females of 11–16-year age group. Low levels of Hb ranging between 7 and 8 mg/dL were observed in all patients of both genders, as is typical of SCD patients. Higher than normal hsCRP values were seen in all patients showing a risk for cardiovascular problems.
As seen in Table 1, majority of the children (80%), irrespective of their age, were vitamin D deficient. Among males, 78.26% were VDD, while 13.043% were vitamin D insufficient and only 8.695% of patients exhibited vitamin D sufficiency. Similarly, among females, 82.352% were VDD, while 5.882% were vitamin D insufficient and only 11.764% patients exhibited vitamin D sufficiency. When observed according to their ages, the males of age group 5–10 years showed 5% vitamin D sufficiency as compared to 33.333% in older males of the age group 11–16 years. On the contrary, 20% of younger females of the age group 5–10 years showed vitamin sufficiency as compared to 0% of the older females of age group 11–16 years.
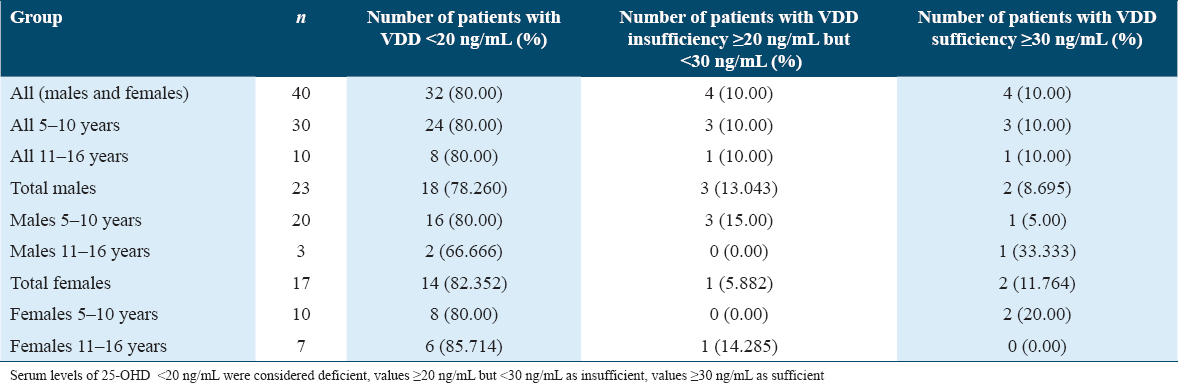
The association of age and gender with vitamin D, Ca, WBC, Hb, and hsCRP levels is depicted in Table 2. Males of only the age group 5–10 years showed a significant association with vitamin D (P = 0.0375) and WBC (P = 0.0015) levels. When correlation “r” values were considered, males of the 11–16-year age group showed a very strong-positive correlation with vitamin D (r = 0.9862) and a very strong-negative correlation with Hb (r = −0.9819) and hsCRP (r = −0.9907). Among females, only the 11–16-year age group patients exhibited a significance with vitamin D (P = 0.0487), Ca (P = 0.0118), Hb (P = 0.0007), and hsCRP (P = 0.0001) levels. This was also reflected in the r values showing a strong-negative correlation with WBC levels (r = −0.6525) as well as hsCRP (r = −0.6550) as seen in Table 2.
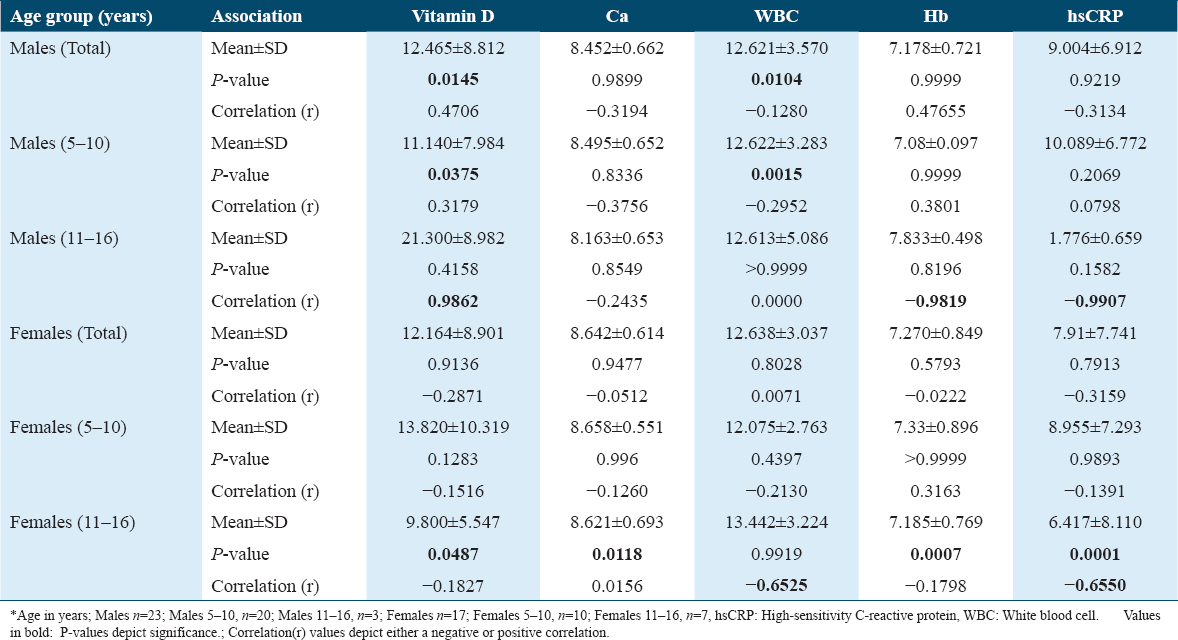
While vitamin D levels in males showed a significant association with Hb values (P = 0.0066), the females did not show any significant P-value in both the age groups. In males, a marked significance was observed only in the 11–16-year age group with a P = 0.0061 for hsCRP. The 11–16-year age group males depicted very strong-negative correlation for Hb (r = −0.9372) and hsCRP (r = −0.9546). In females, correlations presented a strong-negative association with WBC in the 5–10 age group of females (r = −0.6963) and with calcium (r = −0.6330) in the 11–16-year age group as seen in Table 3.
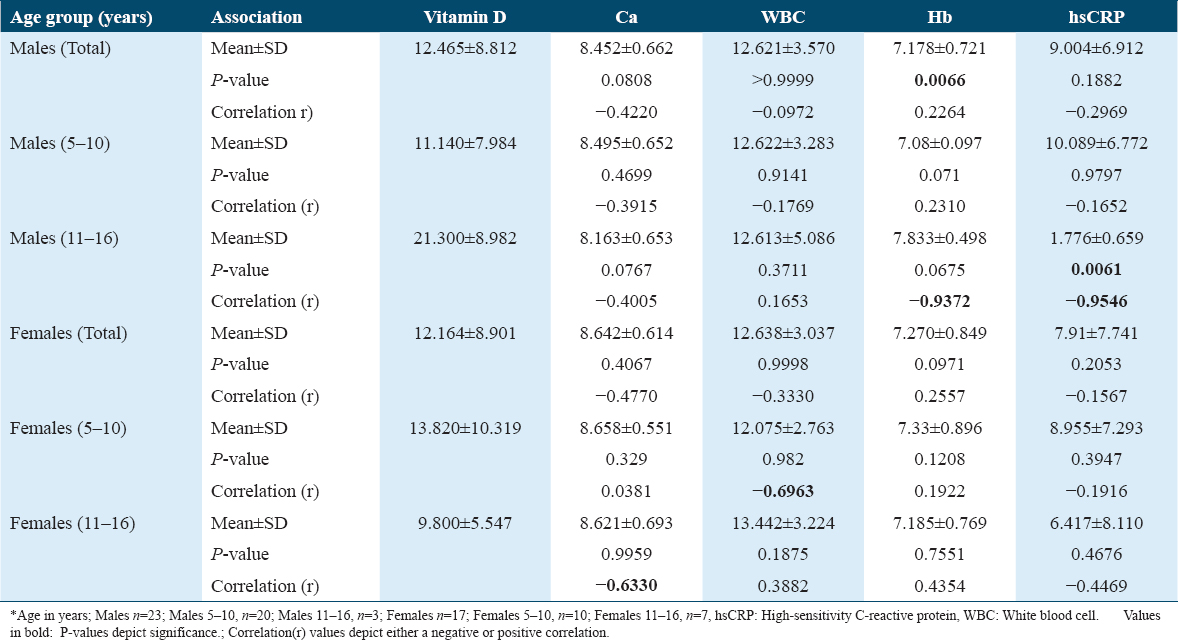
When associations of WBC with Hb and hsCRP were observed, a significant P = 0.0046 was observed with Hb in males. This association was observed only in the males of age group 5–10 years with a P = 0.0034. The r values exhibited weak-negative correlations. Among the females, only the 11–16-year age group showed an association of WBC levels with Hb exhibiting a P = 0.0052 as well as hsCRP with a P = 0.0011. Here too, the correlations were found to be very weak [Table 4].
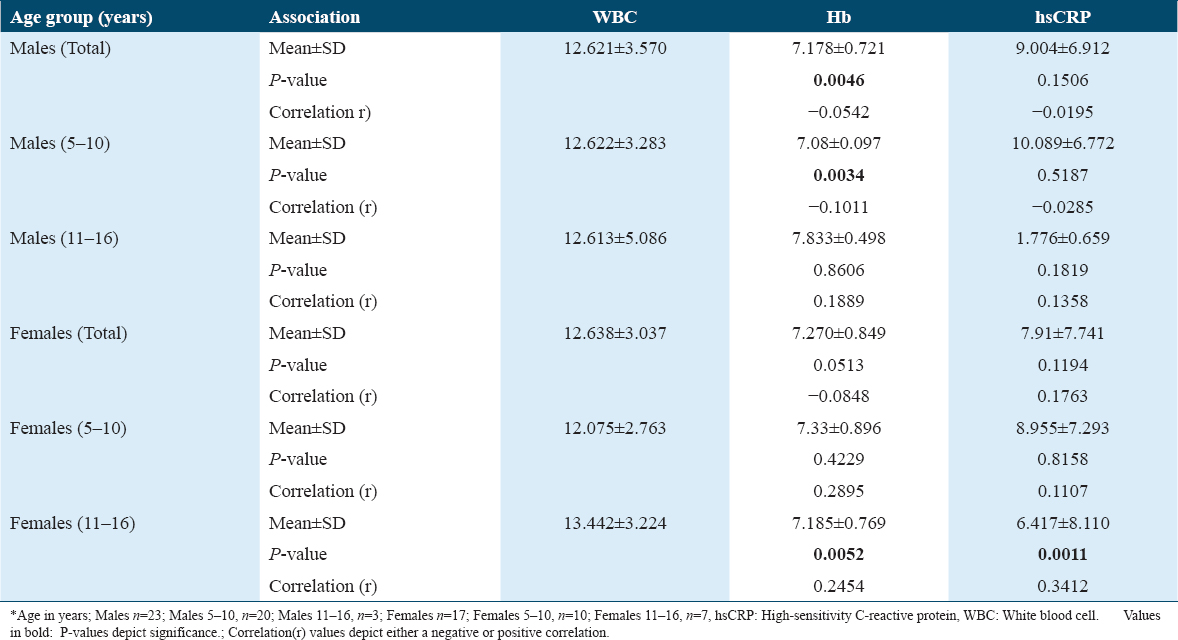
Association of Ca with WBC, Hb, and hsCRP did not show any significance. Similarly, the association of Hb with hsCRP did not show any significance.
Discussion
Hypovitaminosis of vitamin D in a sunshine-abundant area like Saudi Arabia reiterates that VDD is a global issue that is short of becoming a pandemic. Contributory factors toward VDD in SCD patients could likely be due to lesser sun exposure or/and lower consumption of vitamin D or/and malabsorption of vitamin D.[14]
In our study too, vitamin D sufficiency was found only in 3 out of the 23 males and 1 out of 17 females with SCD. In an earlier study, although low vitamin D levels were observed in SCD children younger than 5 years of age, a higher deficiency was seen during the aging process, as they aged above 5 years. This was attributed to decrease in osteocalcin which is among the most abundant of bone proteins.[9] This deficiency was observed in our study wherein almost all the children were above 5 years of age. Especially, in the females, of ages 5–10 years, there was 20% sufficiency as compared to 0% of age group 11–16 years. Values for controls for serum vitamin D and calcium in this region of Saudi Arabia have been shown to be 23.06 ± 0.25 ng/mL and 2.6 ± 0.15 nmol/L, respectively, for the age group 6–12 years according to earlier studies.[15] Although insufficient vitamin D was displayed in the control group too, their values were slightly higher than those of the sickle cell group in our study. Past research reports that the frequency of VDD in the SCD population was not much different from the control within the same ethnic group as also observed from a study from Saudi Arabia. These VDD patients also experienced frequent hospitalization during crisis showing the possibility of an association. Like this large study from the eastern province of Saudi Arabia, our work also included patients in a steady state without SCD crisis which mostly represents the SCD population.[1]
Certain characteristics, like decreased ability to absorb nutrients due to damage to the intestinal mucosa as well as an increased basal metabolic rate, appear specific to SCD patients. Apart from this, higher nutritional demands[16] resulting from higher energy expenditure to sustain normal physiologic functioning may contribute to a VDD status.
VDD has been associated with skeletal issues, cardiovascular problems, asthma nephropathy, chronic pain, etc. It also affects growth, maturation, skeletal health, and severity of the disease in SCD patients. Moreover, SCD patients have been found to be susceptible to all these complications, and VDD intervention is potentially simple and easy to improve their health.[10] Supplementation of vitamin D2 and vitamin D3 to SCD patients in a steady state for a 6-week period was effective in improving vitamin D status.[17]
Appropriate vitamin D levels may reduce inflammation, causing a lowering of the inflammatory cytokines, thereby leading to a decrease in vitamin D binding protein.[18] The low vitamin D levels are also reflected in the higher concentration of hsCRP which is an inflammatory marker in all the patients. In our present study too, all the patients irrespective of gender and age exhibited hsCRP levels in the range of >3 mg/L (mean 8.395 ± 7.296 mg/L) which is indicative of moderate-to-high cardiovascular risk.[11,19] Healthy controls aged 4–13 years exhibited hsCRP levels of 1.65±0.82 mg/L.[20]
Average calcium levels of all patients were 8.53 mg/dL which is in the normal range of 8.5–10.5 mg/dL. This could be due to the dietary intake, as milk is an integral part of a child’s meal.
Hb levels have been found to be low in SCD patients and the same was observed in our study too.[21] Earlier studies have shown that supplementation of vitamin D to sickle cell anemia patients not only reduced the pain episodes but also increased Hb levels in these patients.[22,23] Many reports also suggest the direct association of VDD with anemia related specifically to either inflammation or a chronic disease.[24,25]
WBC counts detect many disorders and evaluate the severity of the problem. It has been noted that WBC counts were found significantly increased during bacterial infection and crises among SCD children which help it being a marker for hospital admissions. In our study, we found that WBC counts were slightly raised, though not significant in almost all the patients (normal WBC range 4–11×109 cells/L), displaying a strong predisposition to acute chest syndrome and other infections common to SCD children.[26] VDD has been found related to decrease pulmonary function in SCD children but not to other morbidities of sickle cell anemia.[7]
The World Health Organization considers the VDD in Saudi Arabia surpassing the average deficiency observed globally. Past research done on children and adolescents in Saudi Arabia reflects an alarmingly high VDD prevalence of 78% and 98.1% in the urban population. Children with special health conditions like diabetes faced a similar deficiency of around 88.9%. This calls for urgent interventions addressing this issue.[27]
Another study demonstrated that the VDD in children with sickle cell anemia is so high (median being 15 ng/mL) that increasing this is a herculean task. Interventions should address this serious health issue by educating families and creating nutritional awareness.[28]
It was noted that SCD children were calorie deficient with a reduced intake of essential nutrients such as fiber and minerals such as magnesium and calcium along with vitamins D, E, and folate. This contributed to a compromised and reduced growth which is apparent during adolescence. Special care should therefore be taken to ensure appropriate micronutrient and energy intake.[29]
VDD in SCD leads to immune-compromised condition as is also the case in many infections. One study found such immune-compromised women in Saudi to be more prone to toxoplasmosis for which vitamin D could provide protection.[30] The inflammatory condition of psoriasis was also found to be correlated with the deficiency of vitamin D.[31] Even among the healthy Saudi population in the region of Qassim, the deficiency or insufficiency was found to be 67.8% which needs to be attended on a robust scale.[32]
The most accessible source of vitamin D being sunlight could keep us safe from various ailments such as cancer, cardiovascular problems, and autoimmune disorders. A potential link of the dangerously increasing cardiovascular problems was observed with vitamin D.[33] This warrants an urgent attention from governmental organizations to increase awareness in the public to regain vitamin D sufficiency.[34]
Limitations
We could not include a control group due to non-compliance from the guardians. Practicality and feasibility were considered. The sample size needs to be larger to conclusively hypothesize the associations. Furthermore, this being a single-centric study, it cannot be taken as a representative of the entire kingdom, as the results from other regions could vary. Larger multi-center studies on a national level would be interesting to also study the effect of hypo-vitaminosis on the frequency as well as severity of sickle cell crisis. The estimation of the prevalence of VDD would then be more realistic.
Conclusions
The prevalence of VDD in SCD children has gained global focus due to its manifold health implications. Our study too revealed the deficiency status in almost all SCD children in our cohort, which reiterates the need for effective governmental vitamin D screening and counseling programs. There is also a need to address the issue by the medical fraternity and health departments, for better health outcomes in this population. A close monitoring of the vitamin D status in early years of SCD is warranted. Supplementation studies for the different VDD, and insufficiency groups, in the non-crisis set of patients, would establish supplementation therapies in the early stages and avoid certain complications.
Consent to Participate
Before the start of study, consent forms were collected from all the guardians/parents of the patients participating in the study.
Data Availability
Relevant data can be made available upon reasonable request.
Competing Interests
None.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors’ Contributions
Conceptualization and design of the study – Shahida A Khan, Sarah Khan; Acquisition of data, formal analysis, and interpretation of data – Shahida A Khan, Torki Al Zughaibi, and Sarah A Khan; Manuscript writing – (review & editing) Shahida A Khan, Torki Al Zughaibi, and Sarah A Khan.
All authors critically read the manuscript and approved the final manuscript to be published, ensuring their integrity.
Acknowledgments
The authors are grateful to King Fahd Medical Research Center and King Abdulaziz University Hospital at King Abdulaziz University, Jeddah, Saudi Arabia, for the facilities provided.
References
- Vitamin D deficiency in sickle cell disease patients in the Eastern Province of Saudi Arabia. Ann Saudi Med. 2018;38:130-6.
- [Google Scholar]
- Low vitamin D status despite abundant sun exposure. J Clin Endocrinol Metab. 2007;92:2130-5.
- [Google Scholar]
- Increasing trends and significance of hypovitaminosis D:A population-based study in the Kingdom of Saudi Arabia. Arch Osteoporos. 2014;9:190.
- [Google Scholar]
- Prevalence of vitamin D deficiency rickets in adolescent school girls in Western region, Saudi Arabia. Saudi Med J. 2007;28:441-4.
- [Google Scholar]
- High prevalence of vitamin D deficiency in the sunny Eastern region of Saudi Arabia:A hospital-based study. East Mediterr Health J. 2011;17:317-22.
- [Google Scholar]
- Vitamin D:Role in chronic and acute diseases. In: Encyclopedia of Human Nutrition. Netherlands: Elsevier; 2023. p. :535-44.
- [Google Scholar]
- Vitamin D deficiency and comorbidities in children with sickle cell anemia. Pediatr Hematol Oncol. 2012;29:261-6.
- [Google Scholar]
- Impact of omega-3 fatty acids supplementation in children with sickle cell disease in Saudi Arabia. J King Saud Univ Sci. 2022;34:101942.
- [Google Scholar]
- Vitamin D in children and adolescents with sickle cell disease:An integrative review. Rev Paul Pediatr. 2015;33:350-5.
- [Google Scholar]
- Prevalence of vitamin D deficiency in sickle cell disease:A systematic review. PLoS One. 2015;10:e0119908.
- [Google Scholar]
- Vitamin D and inflammatory markers in children with sickle cell disease. Blood. 2019;134(Suppl 1):2312.
- [Google Scholar]
- Associations of 25 hydroxyvitamin D and high sensitivity C-reactive protein levels in early life. Nutrients. 2021;14:15.
- [Google Scholar]
- Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122:1142-52.
- [Google Scholar]
- Vitamin D level among patients with sickle cell anemia and its influence on bone mass. Am J Hematol. 2011;86:506-7.
- [Google Scholar]
- Vitamin D status and serum level of some elements in children with sickle cell disease in Jeddah, Saudi Arabia. Pak J Med Sci. 2003;19:295-9.
- [Google Scholar]
- Impact of omega-3 fatty acids on calorie intake and certain anthropometric measurements in children with sickle cell disease in Saudi Arabia. Bioinformation. 2019;15:189-93.
- [Google Scholar]
- Comparative effectiveness of a six-week treatment course of vitamin D2 and D3 in children with sickle cell anemia in steady state with hypovitaminosis D:A randomized clinical trial. J Hematol. 2021;10:114-22.
- [Google Scholar]
- Effect of the inflammatory response on trace element and vitamin status. Ann Clin Biochem. 2000;37:289-97.
- [Google Scholar]
- Level of 25-hydroxyvitamin D in pediatric arthritis patients. Proc Lat Acad Sci. 2019;73:425-32.
- [Google Scholar]
- The relationship between serum homocysteine and highly sensitive C-reactive protein levels in children on regular hemodialysis. Saudi J Kidney Dis Transpl. 2017;28:483-90.
- [Google Scholar]
- Prevalence of anemia among children and adolescents in rural area of Khulais in Saudi Arabia. Cureus. 2022;14:e21894.
- [Google Scholar]
- Vitamin D deficiency in adult sickle cell Patients. J Natl Med Assoc. 2017;109:36-43.
- [Google Scholar]
- Vitamin D deficiency and its association with anemia and blood transfusion requirements in Nigerian adults with sickle cell anemia. Plasmatology. 2021;15 https://doi.org/10.1177/26348535211051690
- [Google Scholar]
- A study of Vitamin D deficiency in patients of sickle cell disease and its association with severity. Int J Adv Med. 2020;7:621-25.
- [Google Scholar]
- Vitamin D deficiency and anemia risk in children:a review of emerging evidence. Pediatric Health Med Ther. 2017;8:47-55.
- [Google Scholar]
- The prevalence of abnormal leukocyte count, and its predisposing factors, in patients with sickle cell disease in Saudi Arabia. J Blood Med. 2017;8:185-91.
- [Google Scholar]
- Vitamin D deficiency in children and adolescents in Saudi Arabia:A systematic review. Cureus. 2024;16:e52040.
- [Google Scholar]
- High risk of vitamin D deficiency in children with sickle cell disease. J Am Diet Assoc. 2008;108:1512-6.
- [Google Scholar]
- Adequacy of dietary intake declines with age in children with sickle cell disease. J Am Diet Assoc. 2007;107:843-8.
- [Google Scholar]
- Toxoplasmosis in immunocompetent Saudi women:Correlation with vitamin D. Womens Health (Lond). 2021;17:17455065211043844.
- [Google Scholar]
- Association between Vitamin D deficiency and psoriasis:An exploratory study. Int J Health Sci (Qassim). 2018;12:33-9.
- [Google Scholar]
- Vitamin D status among population of Qassim Region, Saudi Arabia. Int J Health Sci (Qassim). 2011;5:116-24.
- [Google Scholar]
- Low levels of Vitamin D an emerging risk for cardiovascular diseases:A review. Int J Health Sci (Qassim). 2017;11:71-6.
- [Google Scholar]







