Zingerone mitigates metabolic dysfunction and alters pro-opiomelanocortin gene expression in offspring of high-fat diet-fed pregnant wistar rats
Address for correspondence: Dr. David Chibuike Ikwuka, Department of Medical Physiology, School of Medicine and Pharmacy, College of Medicine and Health Sciences, University of Rwanda, Rwanda. Phone: +250791374933. E-mail: davidcikwuka.ur@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Qassim Uninversity and was migrated to Scientific Scholar after the change of Publisher.
Abstract
ABSTRACT
Objectives:
In utero, exposure to maternal high-fat diet (HFD) has been identified to predispose the offspring to obesity and other metabolic dysfunctions later in life. Zingerone, a bioactive phytochemical found in ginger has potential for the treatment of metabolic diseases due to its antioxidant properties. This study investigated its potential reprogramming effect on some metabolic indices and pro-opiomelanocortin (POMC) gene in young adult offspring of Wistar rat models exposed to maternal HFD.
Methods:
30 pregnant Wistar rats were divided into five groups: Normal control group, an HFD control, and three experimental groups treated with 50, 100, or 200 mg/kg of zingerone, respectively. The treatment commenced from day 1 of pregnancy until postnatal day (PND) 21, after which the offsprings were weaned and placed on a standard diet until PND 42. On PND 42, the biochemical assays were performed on the offsprings using enzyme-linked immunosorbent assay kits and the hypothalamic POMC gene expression using reverse transcription polymerase chain reaction. Data were analyzed using analysis of variance. Values of P < 0.05 were taken as statistically significant.
Results:
Offsprings in the zingerone-treated groups showed significant (P < 0.05) decrease in body weight, glucose, insulin, cholesterol, triglycerides, and leptin levels compared to the HFD control group. Food intake and ghrelin levels increased, while POMC gene expression was inhibited with 100 and 200 mg/kg of zingerone.
Conclusion:
Maternal zingerone administration may mitigate the risk of metabolic disorders in the offspring, possibly by its influence on the anorexigenic genetic makeup of the offspring.
Keywords
High-fat diet
metabolic dysfunction
offspring
pregnancy
pro-opiomelanocortin
zingerone
Introduction
The perinatal phase has been considered as a crucial period of growth and development as a mother’s nutritional status during this period can change the developmental path of her offspring and consequently affecting their long-term health and behavior.[1-3] In line with the hypothesis formulated by Developmental Origins of Health and Disease, the paradigm of future diseases is laid during the developmental periods of pregnancy and lactation.[4,5] Evidence gathered from a variety of studies involving both humans and animals has shown that maternal consumption of a high-fat diet (HFD) during pregnancy predisposes the offspring to the development of metabolic disorders such as obesity, dyslipidemia, diabetes, insulin resistance, hypertension, etc., later in life[6,7] by malprogramming of the appetite-controlling regions of the hypothalamus which regulates energy homeostasis, specifically the pro-opiomelanocortin (POMC), influenced by epigenetics.[7,8]
Metabolic dysfunction refers to dysregulations in body’s metabolic pathways such as insulin resistance, abnormal lipid metabolism, and inflammation, which can lead to metabolic syndrome (MetS) when these dysfunctions occur together.[9] MetS, marked by hypertension, obesity, high blood glucose, high triglycerides, and low HDL cholesterol, significantly raises the risk of developing cardiovascular diseases and type 2 diabetes. Each component of MetS contributes to increased morbidity and mortality by promoting inflammation, endothelial dysfunction, and other risk factors for these diseases.[9,10] The global prevalence of MetS varied from 12.5% to 31.4% based on its definition.[11] Globally, the incidence of obesity and other associated metabolic dysfunction has reached epidemic levels, posing significant public health issues.[12-14] The imminent increase in the incidence of obesity and metabolic dysfunction with its disease burden in both adults and the children has posed significantly greater demand on our healthcare system, scientists, and its sufferers.[15] Furthermore, the sufferers of metabolic dysfunctions may however be also burdened by the expensive cost of the available treatment which poses no cure other than its management.[16]
Ginger is a powerful antioxidant with minimal side effect and often times, consumed by pregnant women due to their belief that it reduces nausea and vomiting during pregnancy.[17] Zingerone, a bioactive phytochemical fraction present in a significant amount of about 9.25% in ginger (a popularly known food spice), has been known to have potent pharmacological properties and has demonstrated antiobesity, antioxidant, and anti-inflammatory effects[18] which makes it a possible candidate that could protect against long-term adverse metabolic outcomes secondary to maternal HFD. Zingerone’s potent antioxidant properties which aid in combating various significant diseases made it the focus of this study.[17]
However, it is not known if its consumption during pregnancy can restore the malprogrammed POMC neuron in the hypothalamic feeding circuit to restore the altered energy homeostasis that forms the mechanism for the development of obesity and other metabolic dysfunctions in offspring later in life when exposed to maternal nutritional perturbations during pregnancy and lactation.[17,19] This research aimed to explore its potential in reprogramming altered hypothalamic genes, which are key to the development of obesity and subsequently contribute to other metabolic disorders, specifically in offsprings exposed to maternal nutritional perturbations during pregnancy and lactation.[17,19] The results of this study are expected to pave the way for developing therapeutic strategies to prevent obesity and promote healthier outcomes for individuals who are obese or at risk of obesity. In addition, it aims to provide a deeper understanding of whether zingerone can reverse maladaptive changes in a specific area of hypothalamic feeding circuit, thereby restoring altered energy balance – a key mechanism in the development of obesity and related metabolic disorders in offspring later in life.
This research holds potential clinical relevance for women of reproductive age who are overweight or obese and also for offspring of obese mothers. Furthermore, its findings will serve as a foundation for future studies and contribute valuable material for literature reviews.
This study therefore investigated whether or not zingerone can attenuate metabolic dysfunctions and the malprogramming of the POMC hypothalamic regulatory regions caused by maternal nutritional perturbations during pregnancy in the young adult offspring.
Materials and Methods
Experimental animals and grouping
In this study, 30 female Wistar rats weighing 170–200 g were acquired from the Animal Facility of the Faculty of Basic Medical Sciences, University of Nigeria, Enugu Campus. The rats were housed in clear plastic cages in a controlled environment set to 25 ± 1°C with a 12-h light/dark cycle. They had continuous access to standard rodent pellets and water throughout the study. The animals were allowed to acclimatize for 2 weeks before the commencement of the study.
Following acclimatization, the rats were randomly assigned to different treatment groups. The study adhered strictly to ethical standards, including the 2000 Helsinki Declaration guide for the care and use of laboratory animals, which was in line with the university’s animal rights policies.
Drugs and chemicals
Zingerone was purchased from Sigma-Aldrich, MO USA, and was given orally at doses of 50, 100, and 200 mg/kg after dissolving in sterile distilled water. All other chemicals and reagents were of high analytical grade. The route of administration and dosages used in this study were based on results obtained from previous studies.[20,21]
Mating arrangement
The estrus cycle of the female rats was monitored daily under light microscopy. The estrus cycle stages were determined using vaginal smears based on the method by Park et al.[22] Daily, subjects underwent vaginal smearing, confirming a consistent estrus cycle of 4 or 5 days, observed over at least two cycles, before being included in the study. For the smearing process, approximately 200 μL of physiological saline was introduced into the vagina with an eye dropper. Vaginal mucus and epithelial cells were collected, spread on a slide, dried on a warm plate at about 40°C for 10–20 min, then stained with 3% Giemsa solution, rinsed with water, and dried. An optical microscope was used to examine keratinocyte size, endometrial cells, presence of nuclei, and leukocytes. The stages were identified as follows:
Proestrus: Few red and white blood cells were present; cells were large and round with many nucleated cells.
Estrus: Nuclei were not visible; cells were large, primarily cornified epithelial cells, some of which appeared clustered.
Metestrus and Diestrus: High presence of red and white blood cells, few nucleated epithelial cells, and rare keratinocytes.
Male Wistar rats were introduced at proestrus at a ratio of 2 females to 1 male. These rats remained in the same cages until pregnancy was confirmed.
Confirmation of pregnancy
The mated female rats were observed daily at early hours and late evenings for vaginal plugs and pregnancy was confirmed by vaginal smear using vaginal swap on each of the mated female rats under a microscope containing normal saline. The first day of pregnancy was defined as the day spermatozoa were detected in the vaginal smear of mated female rats.[23]
Once pregnancy was confirmed, animals were randomly divided into five (5) groups of six rats each. Group I, which served as control, was fed normal rat chow (12% fat, 60% carbohydrate, and 28% protein) throughout pregnancy period of 21 days while group 2 served as negative control and was maintained on a HFD;(60% fat, 20% carbohydrate, and 20% protein) throughout pregnancy period of 21 days[24] Groups 3–5 were maintained on an HFD and received 50, 100, and 200 mg/kg b.wt of zingerone, respectively, throughout pregnancy period of 21 days.
Weaning
Throughout gestation, dams were maintained on their respective diets. At delivery, the dams were returned to the standard diet with their groupings still maintained. Litters were culled to equal sizes for the lactation period of 21 days. The offspring were studied based on maternal diets. Each of the pups was weaned on postnatal day (PND) 21[25] and placed on standard chow from PND 21 to 42. Evaluation of the offspring biochemical analysis and gene expression was done on PND 42.
Measurement of body weights
Offspring weekly body weights were measured and recorded weekly from birth to PND 42 using a digital weighing balance (DIGI 520, Japan).[26]
Measurement of food intake
The offspring daily food intake was calculated using the method of Katchy et al.,[27] by giving each of the rats a known weight of feed after which the remaining feed was weighed the next day and subtracted from the amount of feed given to the rat the previous day:
Food intake (g) = Amount given (g)–Amount remaining (g).
Biochemical measurement
At PND 42, blood samples of the offspring per group were collected by cardiac puncture into specimen bottles and allowed to clot and separated by centrifugation. The serum was used for the determination of insulin level (uIU/mL) using the enzyme-linked immunoassay (ELISA). The determination of triglyceride and cholesterol levels was by glycerol phosphate oxidase method using Enzymatic Colorimetric Diagnostic Kits from Randox Laboratories, United Kingdom.[28] To estimate glucose level, the tail vein was pricked and followed by blood collection and monitoring using glucometer (Contour®TS, India).
Serum leptin and ghrelin measurement
After an overnight fast, which lasted for 12 h, blood samples of the pups were collected into EDTA bottles from the tail vein on PND 42. They were allowed time for clotting and centrifuged. Both serum leptin and ghrelin were done using the ELISA kit.[26]
POMC gene expression in hypothalamus by reverse transcription polymerase chain reaction (RT-PCR)
The hypothalamic tissue was submerged in DNA/RNA Shield™ and homogenized. Total RNA was extracted using the Quick-RNA Miniprep Plus Kit (Zymo Research, Catalog No. R1057). The quality and quantity of the extracted RNA were measured using a NanoDrop (Thermo Scientific™ NanoDrop™ One Microvolume Ultraviolet-Visible Spectrophotometer). Complementary DNA was synthesized from 1 μg RNA using LunaScript® RT SuperMix Kit (New England Biolabs, USA) following manufacturer’s protocol. RT-quantitative PCR (qPCR) reactions for POMC were performed using SYBRLuna® Universal qPCR Master Mix (New England Biolabs, USA) on a CFX-96 real-time PCR (Bio-Rad, USA) according to the manufacturer’s instructions. Hprt was used as the reference gene and the expression of the target gene was normalized to the Hprt levels.[8]
Statistical analysis
Results were expressed as mean ± standard error of mean. Difference between all studied groups were analyzed using analysis of variance (ANOVA), followed by student Bonferroni post hoc test to compare means across groups using the Statistical Package for the Social Sciences software version 25. Values of P < 0.05 were taken as statistically significant.
Results
Effect of maternal zingerone administration on body weight in offspring of pregnant HFD
The effect of zingerone on weekly body weight in offspring of rats exposed to HFD during pregnancy from birth to PND 21 and from PND 28 to 42 is shown in Figure 1a and b. One-way ANOVA showed that HFD significantly (P < 0.05) increased body weight in the offspring when compared with normal control group. Bonferroni post hoc test showed that 50, 100, and 200 mg/kg zingerone significantly (P < 0.05) reduced the body weight relative to HFD control rats.
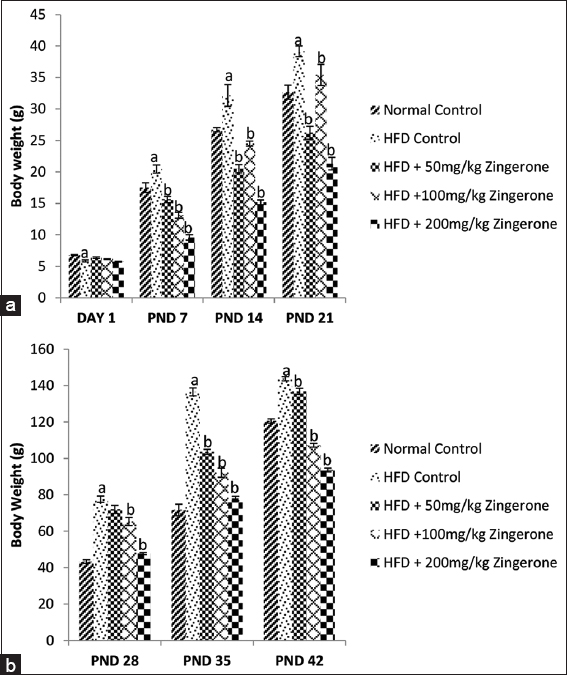
- Effect of maternal zingerone administration on body weight in offspring of pregnant HFD from birth to PND 21(a) and from PND 28 to PND 42 (b). Bars represent the mean ± standard error of mean of 6 animals per group. aP < 0.05 compared to normal control group, bP < 0.05 compared to HFD control group (one-way analysis of variance followed by Bonferroni’s post hoc test). PND: Postnatal day, HFD: High fat diet
Effect of maternal zingerone administration on food intake in offspring of pregnant HFD-fed wistar rats
The effect of zingerone on food intake in offspring of rats exposed to HFD during pregnancy is shown in Figure 2. One-way ANOVA showed that HFD significantly (P < 0.05) increased food intake in the offspring when compared with normal control group. Bonferroni post hoc test showed that 50, 100, and 200 mg/kg zingerone further significantly (P < 0.05) increased the food intake relative to HFD control rats.
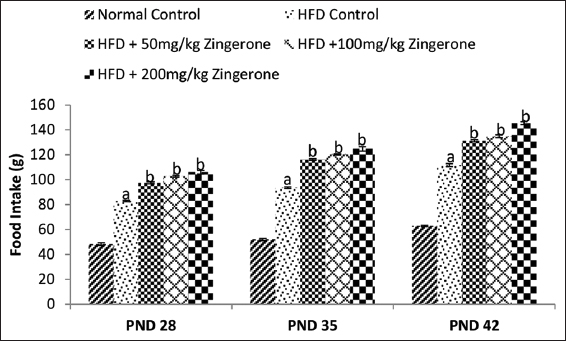
- Effect of maternal zingerone administration on weekly food intake in offspring of pregnant HFD-fed wistar rats from weaning to PND 42. Bars represent the mean ± standard error of mean of 6 animals per group. aP < 0.05 compared to normal control group, bP < 0.05 compared to HFD control group (one-way analysis of variance followed by Bonferroni’s post hoc test). PND: Postnatal day, HFD: High-fat diet
Effect of maternal consumption of zingerone on blood glucose and insulin levels in offspring of pregnant HFD-fed wistar rats
As shown in Figure 3a and b, maternal HFD significantly (P < 0.05) increased the blood glucose [Figure 3a] and insulin [Figure 3b] levels in the offspring when compared with normal control group. Maternal consumption of zingerone (50 mg/kg, 100 mg/kg, and 200 mg/kg) significantly decreased blood glucose [Figure 3a] and insulin [Figure 3b] levels when they were compared to HFD control group.
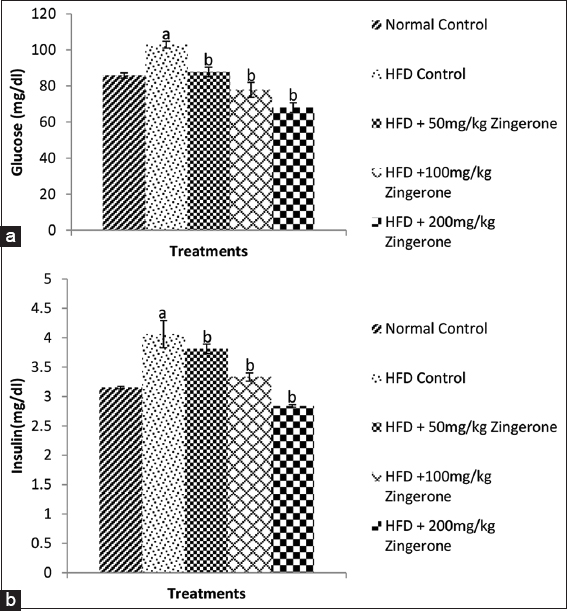
- Effect of zingerone on blood glucose (a) and insulin (b) levels in offspring of pregnant HFD-fed wistar rats. Bars represent the mean ± standard error of mean of 6 animals per group. aP < 0.05 compared to normal control group, bP < 0.05 compared to HFD control group (one-way analysis of variance followed by Bonferroni’s post hoc test). HFD: High-fat diet
Zingerone reverses the effect of maternal HFD consumption during pregnancy on total cholesterol and triglycerides concentrations in offspring
The effect of zingerone on total cholesterol and triglycerides in offspring of rats exposed to HFD during pregnancy is shown in Figure 4a and b. One-way ANOVA showed that HFD significantly (P < 0.05) increased the levels of total cholesterol [Figure 4a] and triglycerides [Figure 4b] in the offspring when compared with normal control group. Bonferroni post hoc test showed that administration of 50, 100, and 200 mg/kg zingerone significantly (P < 0.05) reduced the concentrations of total cholesterol and triglycerides in the offspring relative to HFD control rats.

- Effect of zingerone on total cholesterol (a) and triglyceride (b) concentrations in offspring of pregnant HFD-fed wistar rats. Bars represent the mean ± standard error of mean of 6 animals per group. aP < 0.05 compared to normal control group, bP < 0.05 compared to HFD control group (one-way analysis of variance followed by Bonferroni’s post hoc test). HFD: High-fat diet
Effect of maternal zingerone administration on leptin and ghrelin concentrations in offspring of pregnant HFD-fed Wistar rats
Figure 5a and b shows the effect of maternal zingerone consumption on leptin and ghrelin levels in offspring of rats exposed to HFD during pregnancy when it was compared with the normal control and HFD control groups. The result revealed that maternal HFD significantly (P < 0.05) increased leptin [Figure 5a] but reduced ghrelin [Figure 5b] levels in the offspring when compared with normal control group. Bonferroni post hoc test showed that administration of 50, 100, and 200 mg/kg zingerone significantly (P < 0.05) reduced the concentrations of leptin [Figure 5a] but increased the concentrations of ghrelin [Figure 5b] in the offspring relative to HFD control rats.
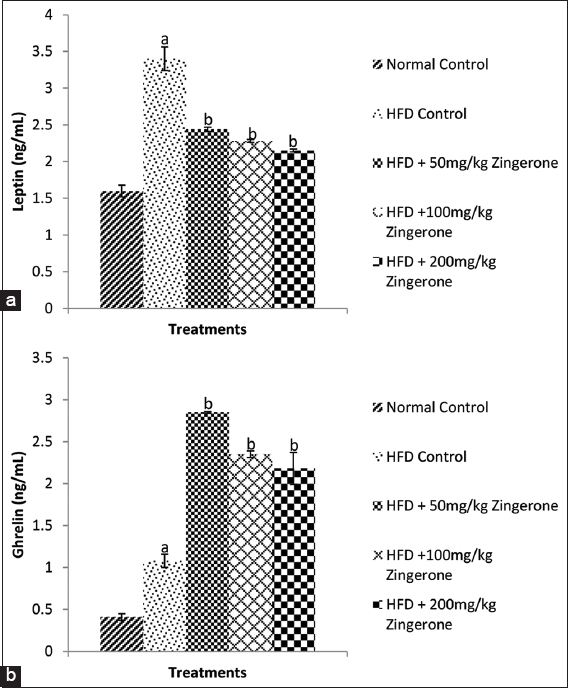
- Effect of zingerone on leptin (a) and ghrelin (b) concentrations in offspring of pregnant HFD-fed wistar rats. Bars represent the mean ± standard error of the mean of 6 animals per group. aP < 0.05 compared to normal control group, bP < 0.05 compared to HFD control group (one-way analysis of variance followed by Bonferroni’s post hoc test). HFD: High-fat diet
Effect of maternal zingerone administration on messenger RNA relative expression of POMC in offspring of pregnant HFD-fed wistar rats
The effect of zingerone on the expression of POMC in offspring of rats exposed to HFD during pregnancy is shown in Figure 6. One-way ANOVA showed that HFD significantly (P < 0.05) increased POMC expression in the offspring when compared with normal control group. Bonferroni post hoc test showed that 100 mg/kg and 200 mg/kg zingerone significantly (P < 0.05) reduced its expression relative to HFD control rats.
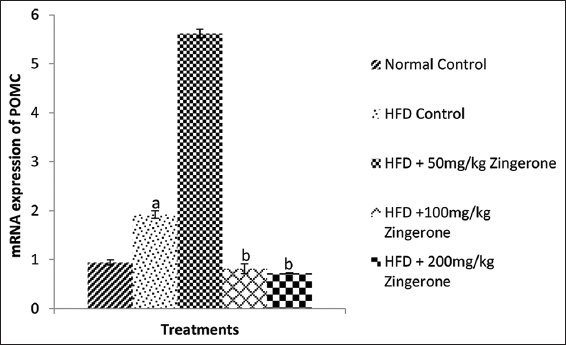
- Effect of maternal zingerone administration on messenger RNA relative expression of POMC in offspring of pregnant HFD-fed wistar rats. Bars represent the mean ± standard error of mean of 6 animals per group. aP < 0.05 compared to normal control group, bP < 0.05 compared to HFD control group (one-way analysis of variance followed by Bonferroni’s post hoc test). HFD: High-fat diet, POMA: Pro-opiomelanocortin
Discussion
This study investigated the possibility of zingerone, a dietary compound in ginger as potential therapy for attenuating metabolic disorders and reprogramming the malprogrammed hypothalamic energy regulatory circuit in offspring of high-fat-fed wistar rats during pregnancy. The composition and quantity of the maternal diet wield substantial influence over the metabolic health and growth of their offspring[29] Obesity, a widespread and pressing global public health challenge, is characterized by a high prevalence affecting populations in both developed and developing countries, significantly increasing susceptibility to chronic illnesses.[30] According to global obesity prevalence statistics, over 39% of adults worldwide were classified as overweight, and over 13% were obese. These numbers underscore the urgency of addressing this health issue.[31] Concurrently, the Food and Drug Administration-approved anti-obesity medications, although available, exhibit limited effectiveness, thus primarily serving as short-term therapeutic interventions.[32] Given these limitations, this study tried to explore the effect of zingerone in ameliorating the metabolic consequences that develop in offspring as a result of maternal nutritional perturbations during pregnancy.
The current study found that maternal consumption of an HFD during pregnancy significantly increased the body weight of the offspring compared to the normal control offspring whose dams did not consume HFD. This contradicts Wang et al.,[33] who reported lower body weights in offspring of mothers on a HFD compared to those on a control diet, regardless of when the diet was consumed. Conversely, studies by Tellechea et al.,[34] and Harmancıoğlu and Kabaran,[35] observed increased fetal weight gain due to maternal HFD, potentially caused by changes in nutrient transfer (fat and glucose) through the placenta.[36,37] These changes might lead to permanent alterations in fetal neuroendocrine pathways in the hypothalamus, affecting appetite regulation and energy metabolism in the offspring. Discrepancies between these studies and ours could be attributed to differences in the HFD components, exposure periods, and evaluation timings in the offspring.
Findings from this study showed that zingerone treatment resulted in a significant dose-dependent decrease in body weight of the offspring from birth to PND 42 compared to the untreated offspring of HFD control dams. This aligns with Han et al.,[38] who noted zingerone’s lipolytic properties in HF-fed animals. The weight decrease may be due to zingerone’s ability to enhance the breakdown of stored fat, serving as an energy reservoir by boosting basal lipolysis and isoprenaline-induced lipolysis in adipocytes.[39] This process involves hormone-sensitive lipase, which catalyzes the conversion of triglycerides (fat molecules) into free fatty acids and glycerol.[40] The resultant weight-lowering effect of zingerone may also suggest that zingerone-induced thermogenesis which enhanced energy expenditure and possibly promoted fat oxidation which aided the weight loss.[41]
In this study, maternal consumption of HFD during gestation increased food intake in the offspring, consistent with the study of Parlee and Mac Dougald.[42] They suggested that HFD consumption during gestation might promote obesity in progeny by altering hypothalamic neuropeptide production, increasing hyperphagia in offspring. Findings from this study showed that treatment with zingerone further increased the food intake in these offspring possibly by a mechanism that depends on gastric motility. Increased gastric motility speeds up digestion hence, increased hunger sensations which stimulates appetite frequently.[43] The observed hyperphagia following treatment with zingerone may also suggest the stimulatory effect of zingerone on appetite. Ginger has been reported to influence the hunger-stimulating hormone ghrelin, which reduces sensitivity to satiety signals and leads to increased cravings and appetite.[44] The increased food intake recorded in this study did not result in weight gain, possibly indicating higher excreta volume, though this was not evaluated in this study.
Findings from this study showed that maternal consumption of HFD during gestation elevated glucose and insulin concentrations in the offspring; this is consistent with previous reports.[8,45-47] The decrease in the serum levels of glucose and insulin in zingerone-treated groups may be mediated by the antioxidative and insulin-sensitizing actions of zingerone which enhances insulin sensitivity by reducing oxidative stress, a key contributor to insulin resistance.[18] The regeneration of pancreatic β-cells and stimulation of insulin release are attributed to zingerone’s strong antioxidant properties.[18,48] Zingerone’s antioxidant properties reduce oxidative stress and inflammation which are associated with insulin resistance.[18] Zingerone’s hypoglycemic effect may also be due to its stimulatory effect on AMP-activated protein kinase in the muscle cells which enhances glucose uptake independent of insulin.[49] Zingerone has also been known to improve insulin sensitivity by modulating insulin receptor function.[17] This allows cells to more efficiently uptake glucose from the bloodstream, thereby lowering blood glucose concentration.
Elevated levels of serum total cholesterol and triglycerides observed in the study are in agreement with the study of de las Heras et al.[50] indicating that maternal nutrition alterations during pregnancy and lactation can pose health risks and promote cardiovascular and metabolic diseases later in life. Remarkably, our study showed that 50 mg/kg, 100 mg/kg, and 200 mg/kg zingerone reversed the elevated levels of serum total cholesterol and triglycerides in the offspring of HFD fed dams. This reduction is consistent with findings from Geng et al.[51] and Wang et al.[52] which may be mediated by transcription factors, such as peroxisome proliferator-activated receptors, adenosine monophosphate-activated protein kinase, and nuclear factor kappa B.[52] Findings from this study indicate that zingerone supplementation increased ghrelin levels, aligning with previous research of Mansour et al.[41] suggesting ginger’s impact on ghrelin. Since zingerone is the major pungent component of ginger, we postulate that it is responsible for this effect. This may be dependent on a mechanism linked to lowering effect of oxidative stress in gastric tissues, which potentially stimulates the environment that regulates ghrelin as a result of zingerone’s strong antioxidant properties.[53]
In this study, maternal HFD led to hyperleptinemia in the offspring; this is in agreement with the studies of Ramamoorthy et al.[8] and Vasselli et al.[54] Zingerone treatment reversed this effect possibly through combined mechanisms of enhanced lipid metabolism and reduced oxidative stress and inflammation.[55] Zingerone promotes lipid oxidation and reduces fat accumulation, thereby decreasing adipose tissue, which is the primary source of leptin. As fat stores break down, leptin production from the adipose tissue also declines.[55] Chronic inflammation and oxidative stress are known to disrupt leptin signaling, resulting in elevated leptin levels and leptin resistance, especially in obesity.[56] Zingerone’s antioxidant properties help mitigate oxidative stress and reduce pro-inflammatory cytokines, which may improve leptin sensitivity and allow for a decrease in circulating leptin levels.[57]
Leptin stimulates the anorexigenic POMC gene while inhibiting the orexigenic neuropeptide Y (NPY).[58] Maternal HFD during pregnancy/lactation can impair leptin sensitivity and gene expression regulating feeding/satiety in offspring.[35] The current study found that POMC expression was increased in offspring of HFD control group in comparison with the normal control group which is in contrast with the findings of Ramamoorthy et al.[8] Leptin resistance in HFD control led to inhibited POMC expression in the ARC, resulting in weight gain,[59] consistent with this study’s findings of hyperleptinemia and leptin resistance in the offspring of HFD control group. Treatment with 100 mg/kg and 200 mg/kg zingerone reduced the expression of POMC in comparison with the offspring in the HFD control group in our study. At present, there is limited research on the impact of zingerone on POMC but findings from this study may suggest that zingerone’s effects on improving insulin and leptin sensitivity and also antioxidant properties may help optimize POMC neuron response. POMC neurons in the hypothalamus are involved in appetite and energy balance, with higher levels typically leading to reduced food intake and increased energy expenditure.[8] Zingerone’s anti-inflammatory effects may indirectly influence POMC expression by creating a healthier cellular environment, as inflammation and oxidative stress have been shown to impair POMC signaling[60] By reducing these factors, zingerone might support normalized POMC function.
Limitations
The study evaluated specific metabolic indices, possibly neglecting important metabolic effects and markers such as low-density lipoprotein, high-density lipoprotein, skeletal muscle glycogen synthase, and hormone-sensitive lipase which may increase the risk of metabolic dysfunctions; second, the study did not evaluate all the relative expression of the hypothalamic appetite-regulating genes such as NPY thereby restricting the immediate applicability of the findings to human population; the time of evaluation of the biochemical parameters in this study may also contribute to varied outcomes leading to biased conclusions. It is therefore suggested that future research could address these limitations.
Conclusion
Our study demonstrates that zingerone as a maternal nutritional supplement offers protection against metabolic disorders in offspring subjected to maternal nutritional imbalances during pregnancy. This protective effect is attributed to mechanisms involving the regulation of anorexigenic genetic pathways and enhanced antioxidant defense. These results further reinforce the potential role of antioxidants and adaptogens in mitigating the developmental programming of metabolic disorders.
Ethical Approval
The ethical clearance for this study was sought and obtained from the Health Research and Ethics Committee of the University of Nigeria Teaching Hospital, Ituku-Ozalla, Enugu, with protocol number NHREC/05/01/2008B-FWA00002458-1RB00002323.
Patient Consent Statement
Not applicable.
Consent to Participate
Not Applicable.
Availability of Data
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Consent for Publication
Not applicable.
Competing Interests
The authors declared no conflicts of interest in conducting this research.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author’s Contributions
The authors D.B.A. and B.U.A did the conceptualization and designed the study. D.BA., E.S., C.S.J., and N.A.K. conducted the experiments. D.B.A., A.U.K, and D.C.I. did the formal analysis. D.B.A., C.S.J., E.S., B.U.A., and D.C.I. wrote the manuscript. B.U.A. supervised the study. All the authors contributed to drafting the work, critically revised the manuscript, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Acknowledgment
The authors acknowledge the inputs of the Late Prof. Eghosa Iyare before his demise and dedicate the manuscript to his remembrance.
References
- Maternal dietary patterns are associated with susceptibility to a depressive-like phenotype in rat offspring. Dev Cogn Neurosci. 2021;47:100879.
- [Google Scholar]
- Maternal effects in mammals:Broadening our understanding of offspring programming. Front Neuroendocrinol. 2021;62:100924.
- [Google Scholar]
- The influence of the dietary exposome on oxidative stress in pregnancy complications. Mol Aspects Med. 2022;87:101098.
- [Google Scholar]
- The developmental origins of health and disease (DOHaD) Am J Lifestyle Med. 2019;14:47-50.
- [Google Scholar]
- Maternal flavonoids intake reverts depression-like behaviour in rat female offspring. Nutrients. 2019;11:572.
- [Google Scholar]
- Female and male C57BL/6J offspring exposed to maternal obesogenic diet develop altered hypothalamic energy metabolism in adulthood. Am J Physiol Endocrinol Metab. 2022;323:E448-66.
- [Google Scholar]
- Maternal overnutrition programs epigenetic changes in the regulatory regions of hypothalamic Pomc in the offspring of rats. Int J Obes (Lond). 2018;42:1431-44.
- [Google Scholar]
- Metabolic syndrome:Updates on pathophysiology and management in 2021. Int J Mol Sci. 2022;23:786.
- [Google Scholar]
- Metabolic syndrome:Risk factors, diagnosis, pathogenesis, and management with natural approaches. Food Chemi Adv. 2023;3:100335.
- [Google Scholar]
- Geographic distribution of metabolic syndrome and its components in the general adult population:A meta-analysis of global data from 28 million individuals. Diabetes Res Clin Pract. 2022;188:109924.
- [Google Scholar]
- Prevalence and epidemiological determinants of metabolically obese but normal-weight in Chinese population. BMC Public Health. 2020;20:487.
- [Google Scholar]
- Epidemiological impact of metabolic syndrome in overweight and obese European children and adolescents:A systematic literature review. Nutrients. 2023;15:3895.
- [Google Scholar]
- Obesity and type 2 diabetes mellitus:Connections in epidemiology, pathogenesis, and treatments. Front Endocrinol (Lausanne). 2023;14:1161521.
- [Google Scholar]
- Disease burden and associated risk factors for metabolic syndrome among adults in Ethiopia. BMC Cardiovasc Disord. 2019;19:236.
- [Google Scholar]
- New advances in metabolic syndrome, from prevention to treatment:The role of diet and food. Nutrients. 2023;15:640.
- [Google Scholar]
- A review on pharmacological properties of zingerone (4-(4-Hydroxy-3-methoxyphenyl)-2-butanone) ScientificWorldJournal. 2015;2015:816364.
- [Google Scholar]
- Zingerone (4-(4-hydroxy-3-methylphenyl) butan-2-one) protects against alloxan-induced diabetes via alleviation of oxidative stress and inflammation:Probable role of NF-kB activation. Saudi Pharm J. 2018;26:1137-45.
- [Google Scholar]
- Structural alterations in Pseudomonas aeruginosa by zingerone contribute to enhanced susceptibility to antibiotics, serum and phagocytes. Life Sci. 2014;117:24-32.
- [Google Scholar]
- Nephroprotective effect of zingerone against ccl4-induced renal toxicity in swiss albino mice:Molecular mechanism. Oxid Med Cell Longev. 2018;2018:2474831.
- [Google Scholar]
- Hepatoprotective potential of zingerone against nonalcoholic fatty liver disease in rats fed with fructose-enriched diet. Gen Physiol Biophys. 2016;35:185-94.
- [Google Scholar]
- Estrus cycles of the female Tscherskia triton (Mammalia:Rodentia:Cricetidae) according to the photoperiod. Korean J Environ Biol. 2017;35:160-8.
- [Google Scholar]
- Reproductive characteristics of the female laboratory rat. Afr J Biotechnol. 2013;12:2510-4.
- [Google Scholar]
- Metabolic consequences of flavonoid and saponin extracts from Gongronema Latifolium leaves in offspring of rats that consumed sucrose during lactation. Int J Health Sci (Qassim). 2024;18:4-9.
- [Google Scholar]
- Prenatal and early postnatal food restrictions cause changes in brain oxidative status and orexigenic/anorexigenic hormones in the offspring of rats:Prevention by quercetin and kaempferol. Curr Res Pharmacol Drug Discov. 2020;1:39-52.
- [Google Scholar]
- Consumption of Gongronema latifolium aqueous leaf extract during lactation may improve metabolic homeostasis in young adult offspring. Pak J Biol Sci. 2020;23:1201-9.
- [Google Scholar]
- Determination of blood glucose using an oxidase-peroxidase system with a non-carcinogenic chromogen. J Clin Pathol. 1969;22:158-61.
- [Google Scholar]
- Effects of maternal malnutrition and supplementation on the embryonic and placental development of rats. Reprod Toxicol. 2019;90:31-40.
- [Google Scholar]
- Obesity genes and leptin. In: Handbook of Obesity. United States:CRC Press; 2020. p. :231-52.
- [Google Scholar]
- Zingerone:A review of its analytical methods, pharmacological properties, and molecular mechanisms. Phytother Res. 2020;34:223-38.
- [Google Scholar]
- Association between High-Fat Diet during Pregnancy and heart weight of the offspring:A multivariate and mediation analysis. Nutrients. 2022;14:4237.
- [Google Scholar]
- The association between high fat diet around gestation and metabolic syndrome-related phenotypes in rats:A systematic review and meta-analysis. Sci Rep. 2017;7:5086-118.
- [Google Scholar]
- Maternal high fat diets:Impacts on offspring obesity and epigenetic hypothalamic programming. Front Genet. 2023;14:1158089.
- [Google Scholar]
- Maternal “junk-food“feeding of rat dams alters food choices and development of the mesolimbic reward pathway in the offspring. FASEB J. 2011;25:2167-79.
- [Google Scholar]
- Perinatal overnutrition and the programming of food preferences:Pathways and mechanisms. J Dev Orig Health Dis. 2012;3:299-308.
- [Google Scholar]
- Effects of zingerone on fat storage in ovariectomized rats. Yakugaku Zasshi. 2008;128:1195-201.
- [Google Scholar]
- Lipolytic Effects of Zingerone in Isolated rat Adipocytes Proceedings of the. Annual Northeast Pharmacy Research Conference 2012
- [Google Scholar]
- A systematic review of the anti-obesity and weight lowering effect of ginger (Zingiber officinale Roscoe) and its mechanisms of action. Phytother Res. 2018;32:577-85.
- [Google Scholar]
- Ginger consumption enhances the thermic effect of food and promotes feelings of satiety without affecting metabolic and hormonal parameters in overweight men:A pilot study. Metabolism. 2012;61:1347-52.
- [Google Scholar]
- Maternal nutrition and risk of obesity in offspring:The Trojan horse of developmental plasticity. Biochim Biophys Acta. 2014;1842:495-506.
- [Google Scholar]
- Gastrointestinal hormones and regulation of gastric emptying. Curr Opin Endocrinol Diabetes Obes. 2019;26:3-10.
- [Google Scholar]
- The effectiveness of Nigella sativa and ginger as appetite suppressants:An experimental study on healthy wistar rats. Vasc Health Risk Manag. 2023;19:1-11.
- [Google Scholar]
- Programmed metabolic syndrome:Prenatal undernutrition and postweaning overnutrition. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2306-14.
- [Google Scholar]
- The importance of catch-up growth after early malnutrition for the programming of obesity in male rat. Obesity (Silver Spring). 2006;14:1330-43.
- [Google Scholar]
- Prenatal and postnatal pathways to obesity:Different underlying mechanisms, different metabolic outcomes. Endocrinology. 2007;148:2345-54.
- [Google Scholar]
- Antagonistic effects of Zingerone, a phenolic alkanone against radiation-induced cytotoxicity, genotoxicity, apoptosis and oxidative stress in Chinese hamster lung fibroblast cells growing in vitro . Mutagenesis. 2010;25:577-87.
- [Google Scholar]
- Zingerone ameliorates non-alcoholic fatty liver disease in rats by activating AMPK. J Food Biochem. 2022;46:e14149.
- [Google Scholar]
- Molecular factors involved in the hypolipidemic- and insulin-sensitizing effects of a ginger (Zingiber officinale Roscoe) extract in rats fed a high-fat diet. Appl Physiol Nutr Metab. 2017;42:209-15.
- [Google Scholar]
- Protective effects of zingerone on high cholesterol diet-induced atherosclerosis through lipid regulatory signaling pathway. Hum Exp Toxicol. 2021;40:1732-45.
- [Google Scholar]
- Beneficial effects of ginger Zingiber officinale Roscoe on obesity and metabolic syndrome:A review. Ann N Y Acad Sci. 2017;1398:83-98.
- [Google Scholar]
- Lowering oxidative stress in ghrelin cells stimulates ghrelin secretion. Am J Physiol Endocrinol Metab. 2020;319:E330-7.
- [Google Scholar]
- Dietary components in the development of leptin resistance. Adv Nutr. 2013;4:164-75.
- [Google Scholar]
- Role of leptin in obesity, cardiovascular disease, and type 2 diabetes. Int J Mol Sci. 2024;25:2338.
- [Google Scholar]
- Zingerone (4-(four-hydroxy-3-methylphenyl) butane-two-1) modulates adjuvant-induced rheumatoid arthritis by regulating inflammatory cytokines and antioxidants. Redox Rep. 2021;26:62-70.
- [Google Scholar]
- Leptin modulates the intrinsic excitability of AgRP/NPY neurons in the arcuate nucleus of the hypothalamus. J Neurosci. 2014;34:5486-96.
- [Google Scholar]
- Challenges and opportunities of defining clinical leptin resistance. Cell Metab. 2012;15:150-6.
- [Google Scholar]
- Perinatal metabolic inflammation in the hypothalamus impairs the development of homeostatic feeding circuitry. Metabolism. 2023;147:155677.
- [Google Scholar]







